Abstract
Carboxylated chalcones and other related flavonoids were synthesized and evaluated as inhibitors of xanthine oxidase, which is a known target for synthetic and herbal drugs used against hyperuricemia, gout, and other diseases. The 4-carboxylated chalcones with hydroxy, methoxy, and ethoxy groups at ring A were found to exhibit in vitro inhibitory activities with IC50 values in the range of 0.057 to 0.26 μM, being 10–60-fold more potent than allopurinol. Structurally related carboxylic acids with Δ3,9-homoisoflavonoid and flavone scaffolds also showed micromolar activity towards xanthine oxidase. At the same time, dihydrochalcone and Δ2,3-homoisoflavonoid carboxylic acids as well as their oxa-analogues were more than two orders of magnitude less effective inhibitors. Kinetic and molecular docking studies indicated that the carboxylated chalcones and Δ3,9-homoisoflavonoids are mixed-type inhibitors, which mostly bind to free enzyme occupying the active site of xanthine oxidase.
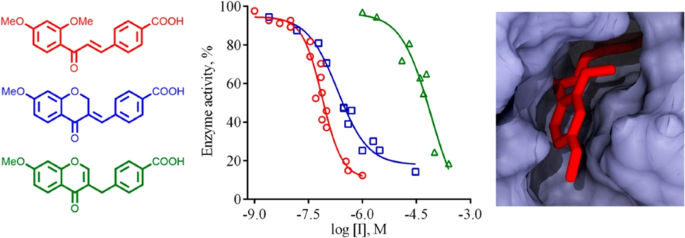
Graphical Abstract









Similar content being viewed by others
References
Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. https://doi.org/10.1017/jns.2016.41
Rozmer Z, Perjési P. Naturally occurring chalcones and their biological activities. Phytochem Rev. 2016;15:87–120. https://doi.org/10.1007/s11101-014-9387-8
Zhuang C, Zhang W, Sheng C, Zhang W, Xing C, Miao Z. Chalcone: a privileged structure in medicinal chemistry. Chem Rew. 2017;117:7762–810. https://doi.org/10.1021/acs.chemrev.7b00020
Castelli MV, López SN Homoisoflavonoids: occurrence, biosynthesis, and biological activity. In: Atta-ur-Rahman, editor. Studies in Natural Products Chemistry. Amsterdam: Elsevier. 2017. p. 315–354. https://doi.org/10.1016/B978-0-444-63929-5.00009-7
Rocha S, Ribeiro D, Fernandes E, Freitas M. A systematic review on anti-diabetic properties of chalcones. Curr Med Chem. 2020;27:2257–321. https://doi.org/10.2174/0929867325666181001112226
Aboul-Enein NM, El-Azzouny AA, Saleh AO, Maklad AY. On chemical structures with potent antiepileptic/anticonvulsant profile. Mini-Rev Med Chem. 2012;12:671–700. https://doi.org/10.2174/138955712800626665
Zhou B, Xing C. Diverse molecular targets for chalcones with varied bioactivities. Med Chem (Los Angeles). 2015;5:388–404. https://doi.org/10.4172/2161-0444.1000291
Nile SH, Keum YS, Nile AS, Jalde SS, Patel RV. Antioxidant, anti-inflammatory, and enzyme inhibitory activity of natural plant flavonoids and their synthesized derivatives. J Biochem Mol Toxicol. 2018;32:e22002. https://doi.org/10.1002/jbt.22002
Mahapatra DK, Bharti SK, Asati V. Anti-cancer chalcones: structural and molecular target perspectives. Eur J Med Chem. 2015;98:69–114. https://doi.org/10.1016/j.ejmech.2015.05.004
Ouyang Y, Li J, Chen X, Fu X, Sun S, Wu Q. Chalcone derivatives: role in anticancer therapy. Biomolecules. 2021;11:894 https://doi.org/10.3390/biom11060894
Liu W, He M, Li Y, Peng Z, Wang G. A review on synthetic chalcone derivatives as tubulin polymerisation inhibitors. J Enzyme Inhib Med Chem. 2022;37:9–38. https://doi.org/10.1080/14756366.2021.1976772
Gliozzi M, Malara N, Muscoli S, Mollace V. The treatment of hyperuricemia. Int J Cardiol. 2016;213:23–27. https://doi.org/10.1016/j.ijcard.2015.08.087
Battelli MG, Polito L, Bortolotti M, Bolognesi A. Xanthine oxidoreductase in cancer: more than a differentiation marker. Cancer Med. 2016;5:546–57. https://doi.org/10.1002/cam4.601
Luna G, Dolzhenko AV, Mancera RL. Inhibitors of xanthine oxidase: scaffold diversity and structure-based drug design. ChemMedChem. 2019;14:714–43. https://doi.org/10.1002/cmdc.201900034
Rashad AY, Kassab SE, Daabees HG, Moneim AEA, Rostom SAF. Febuxostat-based amides and some derived heterocycles targeting xanthine oxidase and COX inhibition. Synthesis, in vitro and in vivo biological evaluation, molecular modeling and in silico ADMET studies. Bioorg Chem. 2021;113:104948 https://doi.org/10.1016/j.bioorg.2021.104948
Song JU, Choi SP, Kim TH, Jung C-K, Lee J-Y, Jung S-H, et al. Design and synthesis of novel 2-(indol-5-yl)thiazole derivatives as xanthine oxidase inhibitors. Bioorg Med Chem Lett. 2015;25:1254–8. https://doi.org/10.1016/j.bmcl.2015.01.055
Guan Q, Cheng Z, Ma X, Wang L, Feng D, Cui Y, et al. Synthesis and bioevaluation of 2-phenyl-4-methyl-1,3-selenazole-5-carboxylic acids as potent xanthine oxidase inhibitors. Eur J Med Chem. 2014;85:508–16. https://doi.org/10.1016/j.ejmech.2014.08.014
Hofmann E, Webster J, Do T, Kline R, Snider L, Hauser Q, et al. Hydroxylated chalcones with dual properties: xanthine oxidase inhibitors and radical scavengers. Bioorg Med Chem. 2016;24:578–87. https://doi.org/10.1016/j.bmc.2015.12.024
Bui TH, Nguyen NT, Dang PH, Hguyen HX, Nguyen MTT. Design and synthesis of chalcone derivatives as potential non-purine xanthine oxidase inhibitors. SpringerPlus. 2016;5:1–8. https://doi.org/10.1186/s40064-016-3485-6
Xie Z, Luo X, Zou Z, Zhang X, Huang F, Li R, et al. Synthesis and evaluation of hydroxychalcones as multifunctional non-purine xanthine oxidase inhibitors for the treatment of hyperuricemia. Bioorg Med Chem Lett. 2017;27:3602–6. https://doi.org/10.1016/j.bmcl.2017.01.053
Yang C, Liu Y, Tu Y, Li L, Du J, Yu D, et al. Chalcone derivatives as xanthine oxidase inhibitors: synthesis, binding mode investigation, biological evaluation, and ADMET prediction. Bioorg Chem. 2023;131:106320. https://doi.org/10.1016/j.bioorg.2022.106320
Mehmood A, Ishaq M, Zhao L, Safdar B, Rehman A, Munir M, et al. Natural compounds with xanthine oxidase inhibitory activity: a review. Chem Biol Drug Des. 2019;93:387–418. https://doi.org/10.1111/cbdd.13437
Finch A, Kubler P. The management of gout. Aust Prescr. 2016;39:119–22. https://doi.org/10.18773/austprescr.2016.047
White WB, Saag KG, Becker MA, Borer JS, Gorelick PB, Whelton A, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. 2018;387:1200–10. https://doi.org/10.1056/NEJMoa1710895
Nielsen SF, Boesen T, Larsen M, Schønning K, Kromann H. Antibacterial chalcones–bioisosteric replacement of the 4′-hydroxy group. Bioorg Med Chem. 2004;12:3047–54. https://doi.org/10.1016/j.bmc.2004.03.071
Dong X, Wang L, Huang X, Liu T, Wei E, Du L, et al. Pharmacophore identification, synthesis, and biological evaluation of carboxylated chalcone derivatives as CysLT1 antagonists. Bioorg Med Chem. 2010;18:5519–27. https://doi.org/10.1016/j.bmc.2010.06.047
Sharma H, Patil S, Sanchez TW, Neamati N, Schinazi RF, Buolamwini JK. Synthesis, biological evaluation and 3D-QSAR studies of 3-keto salicylic acid chalcones and related amides as novel HIV-1 integrase inhibitors. Bioorg Med Chem. 2011;19:2030–45. https://doi.org/10.1016/j.bmc.2011.01.047
Begum S, Begum SKA, Mallika A, Bharathi K. Synthesis, evaluation and in silico studies of 4-N,N-dimethylamino and 4-carboxy chalcones as promising antinociceptive agents. In: Jyothi S, Mamatha D, Satapathy S, Raju K, Favorskaya M, editors. International conference on computational and bio-engineering. CBE 2019. Learning and analytics in intelligent systems. Springer, Cham; 2020;15. p. 481–90. https://doi.org/10.1007/978-3-030-46939-9_42
De Souza ACA, Mori M, Sens L, Rocha RF, Tizziani T, de Souza LFS, et al. A chalcone derivative binds a putative allosteric site of YopH: inhibition of a virulence factor of Yersinia. Bioorg Med Chem Lett. 2020;30:127350. https://doi.org/10.1016/j.bmcl.2020.127350
Siddaiah V, Rao CV, Venkateswarlu S, Krishnaraju AV, Subbaraju GV. Synthesis, stereochemical assignments, and biological activities of homoisoflavonoids. Bioorg Med Chem. 2006;14:2545–51. https://doi.org/10.1016/j.bmc.2005.11.031
Regenass P, Abboud D, Daubeuf F, Lehalle C, Gizzi P, Riché PS, et al. Discovery of a locally and orally active CXCL12 neutraligand (LIT-927) with anti-inflammatory effect in a murine model of allergic airway hypereosinophilia. J Med Chem. 2018;61:7671–86. https://doi.org/10.1021/acs.jmedchem.8b00657
Frasinyuk MS. Synthesis and aminomethylation of 3-substituted 6-hydroxy-1,2-benzisoxazoles. Chem Heterocycl Compd. 2014;50:1616–23. https://doi.org/10.1007/s10593-014-1631-z
Enroth C, Eger BT, Okamoto K, Nishino T, Nishino T, Pai EF. Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: structure-based mechanism of conversion. Proc Natl Acad Sci USA. 2000;97:10723–8. https://doi.org/10.1073/pnas.97.20.10723
Muzychka OV, Kobzar OL, Popova AV, Frasinyuk MS, Vovk AI. Carboxylated aurone derivatives as potent inhibitors of xanthine oxidase. Bioorg Med Chem. 2017;25:3606–13. https://doi.org/10.1016/j.bmc.2017.04.048
Aruoma OI. Deoxyribose assay for detecting hydroxyl radicals. Methods Enzymol. 1994;233:57–66. https://doi.org/10.1016/S0076-6879(94)33008-5
Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. 2010;31:455–61. https://doi.org/10.1002/jcc.21334
Huber R, Hof P, Duarte RO, Moura JJ, Moura I, Liu MY, et al. A structure-based catalytic mechanism for he xanthine oxidase family of molybdenum enzymes. Proc Natl Acad Sci. 1996;93:8846–51. https://doi.org/10.1073/pnas.93.17.8846
Gowthaman R, Miller SA, Rogers S, Khowsathit J, Lan L, Bai N, et al. DARC: Mapping surface topography by Ray-Casting for effective virtual screening at protein interaction sites. J Med Chem. 2016;59:4152–70. https://doi.org/10.1021/acs.jmedchem.5b00150
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–42. https://doi.org/10.1093/nar/28.1.235
MarvinSketch was used for drawing chemical structures, MarvinSketch version 5.2.4, ChemAxon (https://www.chemaxon.com)
Hanwell MD, Curtis DE, Lonie DC, Vandermeersch T, Zurek E, Hutchison GR. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform. 2012;4:1–17. https://doi.org/10.1186/1758-2946-4-17
Sanner MF. Python: a programming language for software integration and development. J Mol Graph Model. 1999;17:57–61
Acknowledgements
This work was supported by the National Academy of Sciences of Ukraine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kobzar, O.L., Tatarchuk, A.V., Mrug, G.P. et al. Carboxylated chalcones and related flavonoids as inhibitors of xanthine oxidase. Med Chem Res 32, 1804–1815 (2023). https://doi.org/10.1007/s00044-023-03109-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03109-8




