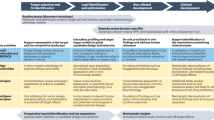
Abstract
In this review, we trace origins of the joint initiative of the pharmaceutical industry and major regulatory authorities to provide a framework for the identification, quantification, and testing of drug metabolites (i.e., Metabolites in Safety Testing; MIST). Dr. Tom Baillie was hugely instrumental in initiating and guiding this process and continues to be influential in this area up to present day. Current industry approaches to MIST are described, including evolution in techniques for metabolite identification, measurement, and characterization, plus a survey of contemporary technologies used to assess whether human metabolites are disproportionate and thus may require standalone safety assessment, clinical pharmacology, and PK/PD studies. The multiple steps involved with nonclinical safety assessment of metabolites formed in humans to a greater extent than animals are covered, which leads to frequently unnecessary safety assessment of stable circulating human metabolites. Two recent case studies of marketed drugs are included, where it is shown that additional nonclinical safety assessment of disproportionate human metabolite(s) did not appear to provide useful information relevant to human safety. This retrospective also addresses impact of MIST guidance on overall drug safety, including relative contributions of on- vs. off-target activity of parent drug vs. reactive human metabolites leading to idiosyncratic toxicity for a series drugs withdrawn from the US market since 1980. The special case of metabolite involvement in developmental and reproductive toxicity profile is described. The manuscript concludes with discussion of how MIST guidelines over the last 20 years have likely impacted on industry productivity for new molecular entities.



Similar content being viewed by others
References
Zhu M, Zhang H, Humphreys WG. Drug metabolite profiling and identification by high-resolution mass spectrometry. J Biol Chem. 2011;286:25419–25. https://doi.org/10.1074/jbc.R110.200055.
Anderson S, Luffer-Atlas D, Knadler MP. Predicting circulating human metabolites: how good are we? Chem Res Toxicol. 2009;22:243–56. https://doi.org/10.1021/tx8004086.
Loi C-M, Smith DA, Dalvie DK. Which metabolites circulate? Drug Metab Dispos. 2013;41:933–51. https://doi.org/10.1124/dmd.112.050278.
Baillie TA, Cayen MN, Fouda H, Gerson RJ, Green JD, Grossman SJ, et al. Drug metabolites in safety testing. Toxicol Appl Pharm. 2002;182:188–96. https://doi.org/10.1006/taap.2002.9440.
Hastings KL, El-Hage J, Jacobs A, Leighton J, Morse D, Osterberg RE. Letter to the editor. Toxicol Appl Pharm. 2003;190:91–92. https://doi.org/10.1016/s0041-008x(03)00150-9.
Baillie TA, Cayen MN, Fouda H, Gerson RJ, Green JD, Grossman SJ, et al. Drug metabolites in safety testing. Toxicol Appl Pharm. 2003;190:93–94. https://doi.org/10.1016/S0041-008X(03)00151-0.
US Food and Drug Administration (FDA). Guidance for industry: safety testing of drug metabolites. 2020. https://www.fda.gov/media/72279/download. Accessed February 28, 2023.
Prueksaritanont T, Lin JH, Baillie TA. Complicating factors in safety testing of drug metabolites: kinetic differences between generated and preformed metabolites. Toxicol Appl Pharm. 2006;217:143–52. https://doi.org/10.1016/j.taap.2006.08.009.
ICH M3(R2). Guidance on nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals. 2010. https://www.fda.gov/media/71542/download. Accessed February 28, 2023.
ICH M3(R2). Questions and answers (R2) on nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals. 2011. https://www.ema.europa.eu/en/documents/other/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en.pdf. Accessed February 28, 2023.
Robison TW, Jacobs A. Metabolites in safety testing. Bioanalysis. 2009;1:1193–1200. https://doi.org/10.4155/bio.09.98.
Luffer-Atlas D. The early estimation of circulating drug metabolites in humans. Expert Opin Drug Metab Toxicol. 2012;8:985–97. https://doi.org/10.1517/17425255.2012.693159.
Luffer-Atlas D, Atrakchi A. A decade of drug metabolite safety testing: industry and regulatory shared learning. Expert Opin Drug Metab Toxicol. 2017;13:897–900. https://doi.org/10.1080/17425255.2017.1364362.
Schadt S, Bister B, Chowdhury SK, Funk C, Hop CECA, Humphreys WG. et al. A decade in the MIST: learnings from investigations of drug metabolites in drug development under the “metabolites in safety testing” regulatory guidance. Drug Metab Dispos. 2018;46:865–78. https://doi.org/10.1124/dmd.117.079848.
Spracklin DK, Chen D, Bergman AJ, Callegari E, Obach RS. Comprehensive drug disposition knowledge generated in the modern human radiolabeled ADME study. CPT Pharmacomet Syst Pharm. 2020;9:428–34. https://doi.org/10.1002/psp4.12540.
Young GC, Spracklin DK, James AD, Hvenegaard MG, Scarfe G, Wagner DS, et al. Considerations for human ADME strategy and design paradigm shift(s) – an industry white paper. Clin Pharm Ther. 2023. https://doi.org/10.1002/cpt.2691.
Zelesky V, Schneider R, Janiszewski J, Zamora I, Ferguson J, Troutman M. Software automation tools for increased throughput metabolic soft-spot identification in early drug discovery. Bioanalysis. 2013;5:1165–79. https://doi.org/10.4155/bio.13.89.
Sharma R, Walker GS. Practical applications of NMR spectroscopy in preclinical drug metabolism studies. Everett JR, editor. In: NMR in Pharmaceutical Sciences. New York: John Wiley & Sons; 2015. pp. 267–80. https://doi.org/10.1002/9780470034590.emrstm1412.
Gillam EMJ, Kramlinger VM. Opportunities for accelerating drug discovery and development by using engineered drug-metabolizing enzymes. Drug Metab Dispos. 2023;51:392–402. https://doi.org/10.1124/dmd.121.000743.
Gao H, Deng S, Obach RS. A simple liquid chromatography-tandem mass spectrometry method to determine relative plasma exposures of drug metabolites across species for metabolite safety assessments. Drug Metab Dispos. 2010;38:2147–56. https://doi.org/10.1124/dmd.112.050278.
Ma S, Li Z, Lee K-J, Chowdhury SK. Determination of exposure multiples of human metabolites for MIST assessment in preclinical safety species without using reference standards or radiolabeled compounds. Chem Res Toxicol. 2010;23:1871–3. https://doi.org/10.1021/tx100363k.
Takahashi RH, Khojasteh C, Wright M, Hop CECA, Ma S. Mixed matrix method provides a reliable metabolite exposure comparison for assessment of metabolites in safety testing (MIST). Drug Metab Lett. 2017;11:21–28. https://doi.org/10.2174/1872312811666170710193229.
Yu CP, Chen CL, Gorycki FL, Neiss TG. A rapid method for quantitatively estimating metabolites in human plasma in the absence of synthetic standards using a combination of liquid chromatography/mass spectrometry and radiometric detection. Rapid Commun Mass Spectrom. 2007;21:497–502. https://doi.org/10.1002/rcm.2863.
Zhang D, Raghavan N, Chando T, Gambardella J, Fu Y, Zhang D, et al. LC-MS/MS-based approach for obtaining exposure estimates of metabolites in early clinical trials using radioactive metabolites as reference standards. Drug Metab Lett. 2007;1:293–8. https://doi.org/10.2174/187231207783221411.
Cuyckens F, Pauwels N, Koppen V, Leclercq L. Use of relative 12C/14C isotope ratios to estimate metabolite concentrations in the absence of authentic standards. Bioanalysis. 2012;4:143–56. https://doi.org/10.4155/bio.11.302.
Hatsis P, Waters NJ, Argikar UA. Implications for metabolite quantification by mass spectrometry in the absence of authentic standards. Drug Metab Dispos. 2017;45:492–6. https://doi.org/10.1124/dmd.117.075259.
Dear GJ, Roberts AD, Beaumont C, North SE. Evaluation of preparative high performance liquid chromatography and cryoprobe-nuclear magnetic resonance spectroscopy for the early quantitative estimation of drug metabolites in human plasma. J Chromatogr B Anal Technol Biomed Life Sci. 2008;876:182–90. https://doi.org/10.1016/j.jchromb.2008.10.040.
Walker GS, Ryder TF, Sharma R, Smith EB, Freund A. Validation of isolated metabolites from drug metabolism studies as analytical standards by quantitative NMR. Drug Metab Dispos. 2011;39:433–40. https://doi.org/10.1124/dmd.110.036343.
Yi P, Luffer-Atlas D. A radiocalibration method with pseudo internal standard to estimate circulating metabolite concentrations. Bioanalysis. 2010;2:1195–210. https://doi.org/10.4155/bio.10.81.
Davis-Bruno KL, Atrakchi A. A regulatory perspective on issues and approaches in characterizing human metabolites. Chem Res Toxicol. 2006;19:1561–3. https://doi.org/10.1021/tx060203m.
Atrakchi AH. Interpretation and considerations on the safety evaluation of human drug metabolites. Chem Res Toxicol. 2009;22:1217–20. https://doi.org/10.1021/tx900124j.
Surapaneni S, Yerramilli U, Bai A, Dalvie D, Brooks J, Wang X, et al. Absorption, metabolism, and excretion, in vitro pharmacology, and clinical pharmacokinetics of ozanimod, a novel sphingosine 1-phosphate receptor modulator. Drug Metab Dispos. 2021;49:405–19. https://doi.org/10.1124/dmd.120.000220.
US FDA Center For Drug Evaluation and Research. Summary Review: Ozanimod. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/209899Orig1s000SumR.pdf. Accessed February 28, 2023.
Highlights of Prescribing Information in US: Zeposia. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209899s000lbl.pdf. Accessed February 28, 2023.
Stamatellos V-P, Rigas A, Stamoula E, Lallas A, Papadopoulou A, Papazisis G. S1P receptor modulators in multiple sclerosis: detecting a potential skin cancer safety signal. Mult Scler Relat Disord. 2022;59:1–10. https://doi.org/10.1016/j.msard.2022.103681.
European Medicines Agency (EMA). Committee for Medicinal Products for Human Use (CHMP) assessment report: Verzenios. 2022. https://www.ema.europa.eu/en/documents/variation-report/verzenios-h-c-004302-ii-0013-epar-assessment-report-variation_en.pdf. Accessed February 28, 2023.
Humphreys WG, Unger SE. Safety assessment of drug metabolites: characterization of chemically stable metabolites. Chem Res Toxicol. 2006;19:1564–9. https://doi.org/10.1021/tx6002547.
Smith DA, Obach RS. Metabolites and safety: what are the concerns, and how should we address them? Chem Res Toxicol. 2006;19:1570–9. https://doi.org/10.1021/tx0602012.
Baillie TA. Approaches to the assessment of stable and chemically reactive drug metabolites in early clinical trials. Chem Res Toxicol. 2009;22:263–6. https://doi.org/10.1021/tx800439k.
US FDA. Guidance for industry: clinical drug interaction studies — cytochrome P450 enzyme- and transporter-mediated drug interactions. 2020. https://www.fda.gov/media/134581/download. Accessed February 28, 2023.
Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N. Engl J Med. 2002;346:539–40. https://doi.org/10.1056/nejm200202143460721.
Hondeghem LM, Dujardin K, Hoffmann P, Dumotier B, De Clerck F, Drug-induced QTC. prolongation dangerously underestimates proarrhythmic potential: lessons from terfenadine. J Cardiovasc Pharm. 2011;57:589–97. https://doi.org/10.1097/fjc.0b013e3182135e91.
Smith DA, Obach RS. Metabolites in safety testing (MIST): considerations of mechanisms of toxicity with dose, abundance, and duration of treatment. Chem Res Toxicol. 2009;22:267–79. https://doi.org/10.1021/tx800415j.
Loughlin J, Quinn S, Rivero E, Wong J, Huang J, Kralstein J, et al. Tegaserod and the risk of cardiovascular ischemic events: an observational cohort study. J Cardiovasc Pharm Ther. 2010;15:151–7. https://doi.org/10.1177/1074248409360357.
Sharretts J, Galescu O, Gomatam S, Andraca-Carrera E, Hampp C, Yanoff L. Cancer risk associated with lorcaserin — The FDA’s review of the CAMELLIA-TIMI 61 trial. N. Engl J Med. 2020;383:1000–2. https://doi.org/10.1056/NEJMp2003873.
Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Reg Toxicol Pharm. 2000;32:56–67. https://doi.org/10.1006/rtph.2000.1399.
Monticello TM, Jones TW, Dambach DM, Potter DM, Bolt MW, Liu M, et al. Current nonclinical testing paradigm enables safe entry to First-In-Human clinical trials: the IQ consortium nonclinical to clinical translational database. Toxicol Appl Pharm. 2017;334:100–9. https://doi.org/10.1016/j.taap.2017.09.006.
Jaeschke H. Troglitazone hepatotoxicity: are we getting closer to understanding idiosyncratic liver injury? Toxicol Sci. 2007;97:1–3. https://doi.org/10.1093/toxsci/kfm021.
Evans DC, Watt AP, Nicoll-Griffith DA, Baillie TA. Drug-protein adducts: an industry perspective on minimizing the potential for drug bioactivation in drug discovery and development. Chem Res Toxicol. 2003;17:3–16. https://doi.org/10.1021/tx034170b.
Nakayama S, Atsumi R, Takakusa H, Kobayashi Y, Kurihara A, Nagai Y, et al. A zone classification system for risk assessment of idiosyncratic drug toxicity using daily dose and covalent binding. Drug Metab Dispos. 2009;37:1970–7. https://doi.org/10.1124/dmd.109.027797.
Bauman JN, Kelly JM, Tripathy S, Zhao SX, Lam WW, Kalgutkar AS. et al. Can in vitro metabolism-dependent covalent binding data distinguish hepatotoxic from nonhepatotoxic drugs? An analysis using human hepatocytes and liver S-9 fraction. Chem Res Toxicol. 2009;22:332–40. https://doi.org/10.1021/tx800407w.
Ito T, Ando H, Handa H. Teratogenic effects of thalidomide: molecular mechanisms. Cell Mol Life Sci. 2011;68:1569–79. https://doi.org/10.1007/s00018-010-0619-9.
Hirose Y, Kitazono T, Sezaki M, Abe M, Sakimura K, Funato H. et al. Hypnotic effect of thalidomide is independent of teratogenic ubiquitin/proteasome pathway. Proc Natl Acad Sci USA. 2020;117:23106–12. https://doi.org/10.1073/pnas.1917701117.
De Santis M, Straface G, Carducci B, Cavaliere AF, De Santis L, Lucchese A, et al. Risk of drug-induced congenital defects. Eur J Obstet Gynecol Reprod Biol. 2004;117:10–19. https://doi.org/10.1016/j.ejogrb.2004.04.022.
Fathe K, Palacios A, Finnell RH. Brief report novel mechanism for valproate‐induced teratogenicity. Birth Defects Res A: Clin Mol Teratol. 2014;100:592–597. https://doi.org/10.1002/bdra.23277.
Collins MD, Mao GE. Teratology of retinoids. Annu Rev Pharm Toxicol. 1999;39:399–430. https://doi.org/10.1146/annurev.pharmtox.39.1.399.
Lepper ER, Smith NF, Cox MC, Scripture CD, Figg WD. Thalidomide metabolism and hydrolysis: mechanisms and implications. Curr Drug Metab. 2006;7:677–85. https://doi.org/10.2174/138920006778017777.
Diamond S, Boer J, Maduskuie TP Jr, Falahatpisheh N, Li Y, Yeleswaram S. Species-specific metabolism of SGX523 by aldehyde oxidase and the toxicological implications. Drug Metab Dispos. 2010;38:1277–85. https://doi.org/10.1124/dmd.110.032375.
Schmid EF, Smith DA. Keynote review: is declining innovation in the pharmaceutical industry a myth? Drug Discov Today. 2005;10:1031–9. https://doi.org/10.1016/s1359-6446(05)03524-5.
Pammolli F, Magazzini L, Riccaboni M. The productivity crisis in pharmaceutical R&D. Nat Rev Drug Discov. 2011;10:428–38. https://doi.org/10.1038/nrd3405.
Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov. 2012;11:191–200. https://doi.org/10.1038/nrd3681.
Smith D Postmarketing attrition. In: Attrition in the pharmaceutical industry: reasons, implications, and pathways forward. Alex A, Harris CJ, Smith DA, editors. New York: John Wiley & Sons; 2015. pp.128–157. https://doi.org/10.1002/9781118819586.ch5.
Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer. 2006;6:714–27. https://doi.org/10.1038/nrc1913.
Moore TJ, Zhang H, Anderson G, Alexander GC. Estimated costs of pivotal trials for novel therapeutic agents approved by the US Food and Drug Administration, 2015–2016. JAMA Intern Med. 2018;178:1451–7. https://doi.org/10.1001/jamainternmed.2018.3931.
Acknowledgements
The authors gratefully acknowledge our individual and collaborative interactions with Dr. Tom Baillie over the course of his highly productive and impactful career. Debra Luffer-Atlas is an employee of Eli Lilly and Company. R. Scott Obach is an employee of Pfizer Inc. Dennis A. Smith is unaffiliated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luffer-Atlas, D., Obach, R.S. & Smith, D.A. A MIST conception: what has been learned from twenty years of human metabolite safety assessment?. Med Chem Res 32, 1933–1949 (2023). https://doi.org/10.1007/s00044-023-03089-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03089-9