Abstract
Small molecule drugs that covalently bind irreversibly to their target proteins have several advantages over conventional reversible inhibitors. They include increased duration of action, less-frequent drug dosing, reduced pharmacokinetic sensitivity, and the potential to target intractable shallow binding sites. Despite these advantages, the key challenges of irreversible covalent drugs are their potential for off-target toxicities and immunogenicity risks. Incorporating reversibility into covalent drugs would lead to less off-target toxicity by forming reversible adducts with off-target proteins and thus reducing the risk of idiosyncratic toxicities caused by the permanent modification of proteins, which leads to higher levels of potential haptens. Herein, we systematically review electrophilic warheads employed during the development of reversible covalent drugs. We hope the structural insights of electrophilic warheads would provide helpful information to medicinal chemists and aid in designing covalent drugs with better on-target selectivity and improved safety.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The history of covalent drugs spans more than one hundred years. The covalent inhibition mechanism of early covalent drugs, such as acetylsalicylic acid (aspirin), was not elucidated until much later [1]. It turns out that aspirin covalently modifies the cyclooxygenase (COX) enzyme by acetylation of Ser530 near its active site. This covalent modification blocks proper binding of the native substrate and leads to irreversible inhibition. Before 2000, it was generally perceived that covalent warheads such as acrylamides would lead to non-specific irreversible inhibition, resulting in idiosyncratic toxicities. However, covalent drugs have garnered much attention in drug discovery in the last two decades and the approach has now become a common modality for protein target inhibition.
Currently more than 40 covalent drugs are in the market and a myriad of them are in clinical trials. Unlike small molecule non-covalent drugs, covalent drugs bind reversibly or irreversibly via a covalent bond to the target protein, which results in slow off rates and sustained inhibition [2, 3]. This tight binding affinity (slow Koff) of an irreversible covalent drug for its target protein allows for targeting sites with low ligand ability (e.g., protein with shallow, or highly charged binding sites). Irreversible covalent inhibitors exhibit prolonged duration of action as compared to non-covalent drugs, which could lead to lower and less frequent dosing. Exquisite selectivity can be achieved within a target protein family if a poorly conserved cysteine is specifically targeted with a covalent warhead from a drug [2, 4]. Despite of all these advantages, one of the key challenges of irreversible covalent drugs is the potential for immunogenicity or toxicity [5]. Overly reactive electrophilic warheads can react non-specifically with nucleophilic residues on other proteins, which could result in off-target toxicities and allergies. Furthermore, irreversible covalent inhibitors tend to undergo extrahepatic clearance more readily than non-covalent inhibitors, which can result in lower bioavailability [6].
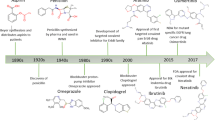
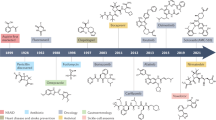
Compounds containing an electrophilic warhead which reversibly binds to the target protein may offer a solution to the aforementioned challenges of irreversible covalent inhibitors [7]. Reversible covalent inhibitors are not permanently bound and can be released from off-target proteins, thereby reducing the chances of undesirable activation of the immune system and off-target toxicity. A number of reversible covalent inhibitors are marketed drugs (Fig. 1). Saxagliptin (1) is a reversible covalent drug containing a nitrile group as an electrophile warhead which target Ser630 residue of dipeptidyl peptidase-4 (DPP-4) [8]. It was approved by the FDA in 2009 for the treatment of diabetes mellitus (Type 2). Telaprevir (2) and boceprevir (3) are ɑ-ketoamide-based reversible covalent drugs (both approved by FDA in 2011) which react with the catalytic serine residue of hepatitis C NS3 serine protease [9]. Narlaprevir (4) is a potent second-generation inhibitor of hepatitis C virus (HCV) NS3 protease with a Ki of 7 nM [10]. It is ɑ-ketoamide-based reversible covalent drug developed by R-Pharm and was approved for the treatment of hepatitis C in Russia in 2016. The first-in-class 26S proteasome inhibitor, bortezomib (5) contains a boronic acid moiety as the electrophilic warhead which targets the N-terminal threonine of 26S proteasome. It was approved by the FDA in 2003 for the treatment of multiple melanoma [11].
Another 26S proteasome inhibitor ixazomib (6) is an orally bioavailable, reversible covalent inhibitor binding to the β5 subunit of the 20S proteasome. It was approved by the FDA in 2015 for use in combination with dexamethasone and lenalidomide to treat patients with multiple myeloma [12]. Voxelotor (7) was approved by the FDA in 2019 to treat sickle cell disease. Its aldehyde warhead reversibly binds to the N-terminal valine of hemoglobin via imine formation [13]. Nirmatrelvir (8) is also a reversible covalent inhibitor with a nitrile warhead targeting a cysteine in the main protease (Mpro) of SARS-CoV-2. It was approved by the FDA in 2022 for the treatment of COVID-19 [14].
The successful application of reversible covalent bond formation in drug discovery inspired medicinal chemists to expand this research area to find new electrophilic warheads which reversibly bind to cysteine and non-cysteine amino acid residues. One of the goals in the development of reversible covalent inhibitors is the extension of the residence time of the covalent inhibitor with the target protein to improve the potency. For this purpose, the medicinal chemists are tuning the reactivity of the reversible covalent warhead and making structural modifications around the electrophilic warhead to stabilize the reversible covalent adduct in order to extend its half-life. Herein we will discuss the recent progress made in the search of reversible covalent inhibitors.
α-Cyanoacrylamide
Incorporation of an electron-withdrawing nitrile group at the α-position of acrylamide greatly accelerates the thia-Michael addition and the acidic proton at the α-position facilitates a retro-Michael reaction. This retro-Michael reaction will decrease the on-target residence time of reversible covalent inhibitors in comparison to irreversible covalent inhibitors. The proposed reaction mechanism of nucleophilic Cys481 of Bruton’s tyrosine kinase (BTK) reacting with the electrophilic α-cyanoacrylamide of compound 9 (PDB ID: 4YHF) [15] is shown in Fig. 2.
Bradshaw et al. tuned the residence time of the α-cyanoacrylamide-based reversible covalent inhibitors (9, 10) of BTK by modifying the size of the β-capping substituent which resulted in sustained BTK occupancy in vivo after oral administration in rats (Fig. 3) [15]. The target occupancy for compound 9 with bulky β-capping substituent is 50% at 20 h while the target occupancy for compound 10 is just 5% at 20 h. Rilzabrutinib (PRN1008, 11) and PRN473 (12) are orally bioavailable, reversible covalent inhibitors of BTK [16]. Rilzabrutinib (11) is in phase 3 clinical trial for treating pemphigus vulgaris and immune thrombocytopenia. The Phase1–2 clinical trials data of rilzabrutinib (11) revealed that it is active and associated with only low-level toxic effects at all dose levels. The dose of 400 mg twice daily was identified as a safe dose for further testing in Phase 3. Overall, rilzabrutinib (11) showed a rapid and durable clinical efficacy that improved with length of treatment in patients with immune thrombocytopenia, who had received multiple therapies previously [17, 18]. PRN473 (12) with doses ranging from 8 to 21 mg/kg was investigated in a clinical study of canine pemphigus foliaceous. Initially all nine dogs had positive clinical response to treatment, resulting in reduction of lesions by the end of week 2 of treatment. At week 4, four dogs continued to improve while three dogs sustained near complete remission by the end of study. PRN473 (12) is well absorbed in dogs in both toxicology and canine clinical studies, but it is poorly absorbed orally in humans [17]. PRN473 (12) has completed a Phase 1 clinical trial as a topical agent. Smith et al. reported a series of α-cyanoacrylamide-based reversible covalent inhibitors of epidermal growth factor receptor (EGFR) [19]. They identified compound 13, which inhibited EGFRL858R and EGFRL858R/T790M with IC50 of 10 and 20 nM, respectively and was shown to be over 5-fold more specific for mutant over wild-type EGFR (IC50 = 96 nM). Thus, inhibitor 13 provides a promising starting point for developing a more selective mutant EGFR inhibitor. London et al. conducted covalent docking of large chemical libraries and discovered a potent Janus tyrosine kinase 3 (JAK3) reversible covalent inhibitor 14 which targets Cys909 (IC50 = 49 nM) [20]. Besides JAK3, compound 14 also potently binds to multiple off-targets, such as ERB-BR (IC50 = 44 nM) and B-lymphoid tyrosine kinase (BLK) (IC50 = 22 nM).
Forster and his coworkers [21] reported two selective reversible covalent inhibitors 15 and 16 of JAK3 (Fig. 4A) with IC50 of 127 pM and 154 pM, respectively. Compound 15 showed high selectivity of 409-, 2724- and 3614-fold over JAK1, JAK2, and tyrosine kinase 2 (TYK2) respectively, while 16 showed selectivity over JAK1 (416-fold), JAK2 (1753-fold), and TYK2 (5831-fold). They determined the complex crystal structure of 16 with JAK3 and found that the α-cyanoacrylamide electrophilic warhead formed a reversible covalent bond with Cys909. The crystal complex of 16 and the target protein displayed the coexistence of both covalent and the non-covalent binding modes (Fig. 4B). The presence of both binding modes of 16 with JAK3 support the reversible character of the covalent interaction. Serafimova’s group [22] working on development of reversible covalent chemical probes also disclosed reversible covalent inhibitor 17 against RSK2. The co-crystal structure of 17 showed that Cys436 on the β2 sheet of RSK2 formed a covalent C‒S bond with α-cyanoacrylate warhead of 17 (Fig. 4D). The reversible nature of 17 was confirmed by proteolysis experiments.
Using the electrophilic fragment-based screening strategy, Miller et al. [23] discovered 18 and 19 with strong binding affinity for RSK2 (IC50 = 12 nM and 15 nM, respectively). The co-crystal structure of 19 with RSK2 shows that covalent bond is formed between the electrophilic α-cyanoacrylamide and Cys436 of T493M RSK2 (Fig. 5B). Targeting focal adhesion kinase (FAK) is a potential approach for the treatment of human malignant glioblastoma. Recently, Li and colleagues [24] reported compounds 20 and 21 (Fig. 5C) as reversible covalent inhibitors of FAK with good binding affinity with IC50(s) ranging from 1.0 to 2.5 nM. Another research group [25, 26] reported cyanoacrylate and Boc-protected cyanoarylamide derivatives 22 and 23 as reversible covalent inhibitors of 3CL protease showing effective antiviral activity against EV71. These two compounds showed broad antiviral effects, acting on 293T, RD and Vero cell lines. Moreover, both 22 and 23 showed remarkable antiviral activities against EV71 A, B, C, CVA16, and CVB3 viral strains.
Nitriles
The human cathepsin K inhibitors, odanacatib (24) [27] and balicatib (25) [28] containing a nitrile warhead, have advanced into phase III and phase II clinical trials, respectively. In the development of reversible covalent drugs, the proliferation of nitriles as an electrophilic warhead is generally due to their lower electrophilicity relative to other more reactive groups, reducing the possibility of unwanted reactions with off-target proteins that would hinder the development of safe drugs. The proposed reaction mechanism [29] of a nitrile-based inhibitor such as nirmatrelvir (8) (PDB ID: 7TLL) [14] reacting with SARS-CoV-2 Mpro Omicron P132H is shown in Fig. 6. The nitrile moiety of nirmatrelvir (8) acts as the electrophilic warhead targeting Cys145 in the main protease (Mpro) of SARS-CoV-2.
Herein we further highlight the potential use of nitriles in the discovery of reversible covalent inhibitors. Cianni et al. reported two odanacatib (24) derivatives Neq0659 (26) and Neq0820 (27) [30], as potent reversible covalent inhibitors of cruzain (Cz). Cz is the major cysteine protease expressed in the Trypanosoma cruzi parasite, which is the etiological agent causing Chagas disease. Furthermore, they designed and synthesized a different series of nitrile-based reversible covalent inhibitors of Cz represented by 28 (Ki = 6.8 nM) with nano-molar range binding affinity (Fig. 7) [31]. In another example, Cortez et al. [32] discovered a bicalutamide derivative (29) as a reversible covalent androgen receptor (AR) antagonist, via introduction of a nitrogen atom into the 4-cyano-benzene ring of bicalutamide to activate the nitrile group. The resulting activation allowed the formation of a reversible covalent bond with the proximal conserved Cys784 of AR. Interestingly, 5N-bicalutamide (29) (Ki = 0.15 nM) binds to AR with ∼150-fold greater affinity than the parent compound bicalutamide (Ki = 22.3 nM). Benson et al. [33] at Pfizer also reported the use of an azanitrile warhead in their reversible covalent inhibitor PF-303 (30) (Fig. 7), which was employed as a chemical probe to investigate the phenotype of BTK inhibition in mice. PF-303 (30) is a highly potent (IC50 = 0.64 nM) and orally bioavailable inhibitor of BTK.
In 2021, Bridenbach and colleagues [34] reported an azanitrile-based reversible covalent inhibitor 31 for Mpro of SARS-CoV-2. Compound 31 was shown to covalently bind (Ki = 24 nM) to the catalytic cysteine of Mpro. Meanwhile, Bai et al. [35] also discovered a series of inhibitors against the 3CL protease of SARS-CoV-2. The typical example 32 (IC50 = 24 nM) is shown in Fig. 7. These inhibitors also covalently bind to the Mpro catalytic cysteine. Cysteine protease B (CPB) is an attractive biological target for therapeutic intervention in the treatment of leishmaniasis. CPB can also be inhibited by reversible covalent inhibitors. Matos and co-workers [36] designed a series of dipeptidyl nitrile derivatives against CPB. They discovered that the most potent CPB inhibitor 33 (pKi = 6.82) was also selective for human cathepsin B (pKi < 5). Nitrile-based inhibitors like compound 34 (Fig. 8) showed promising activity against CPB of L. mexicana. Further optimization of 34 resulted in compound 35 is shown in Fig. 8 [37]. The electrophilic nitrile reacts with the catalytic residue Cys26 at the S1 subsite and forms a covalent adduct. Another group in the same year reported 36 [38] with almost similar structure of 34 (Fig. 8) showing similar inhibition of LmCPB.
Rhodesain, a cysteine protease of Trypanosoma brucei rhodesiense which can cause Human African Trypanosomiasis, has been validated as a drug target [39]. A series of dipeptide nitriles were synthesized by Schirmeister’s group and evaluated for inhibition of rhodesain. From their study they identified compound 37 (Ki = 5.3 nM) as a potent reversible covalent inhibitor of rhodesain [40]. Giroud et al. [41] also reported dipeptide nitrile-based reversible covalent inhibitor 38 (Ki = 7.4 nM) that exhibited both potent and selective activity against rhodesain in vivo against the parasite with acceptable pharmacokinetic properties. The cathepsin protease L (TgCPL) of Toxoplasma gondii, which causes chronic infection in the CNS, is critical to parasite survival during the chronic phase [42, 43]. The currently available inhibitors are ineffective against the chronic form. Therefore, there is an urgent need to develop effective and brain-penetrant inhibitors which could lead to development of new therapeutics to treat this parasitic infection. Zwicker et al. reported nitrile-based inhibitors 39 and 40 of TgCPL [44]. In further development of their work, the peptidomimetic scaffold with a triazine ring resulted in the synthesis of compound 41 which exhibits good brain exposure in mice after IP administration and showed efficacy in an in vitro model of bradyzoite stage parasites [45].
Ketones
The ketone moiety activated by an adjacent electron-withdrawing group also works as a reversible electrophilic warhead and shows broad-range reactivity toward nucleophilic amino acid residues, such as cysteine and serine. Ketone-based peptidomimetics serve as reversible covalent inhibitors of serine and cysteine proteases mimicking a tetrahedral transition state through the formation of hemiketal and thiohemiketal complex respectively. For instance, the α-ketoamide-based inhibitors telaprevir (2) and boceprevir (3) react with the catalytic serine (Ser1139) of hepatitis C virus NS3/4A. The proposed reaction mechanism and the X-ray crystal structure of 2 in complex with the NS3/4A protease (PDB ID: 3SV6) [46] is illustrated in Fig. 9.
The successful application of the ketone group as a reversible covalent warhead inspired many pharmaceutical companies to develop the antiviral agents for the pathogen SARS-CoV-2. The α-hydroxymethylketone compound PF-00835231 (42) showed potent inhibition against SARS-CoV-2 Mpro with an IC50 of 6.9 nM [47]. The co-crystal structure (PDB ID: 6XHM) of 42 complexed with SARS-CoV-2 Mpro reveals that the carbonyl group of the hydroxymethyl ketone is covalently bonded to Cys145 forming a thiohemiketal (Fig. 10). Further optimization of solubility and the pharmacokinetic profile led to its phosphate pro-drug, PF-07304814 (43) (Fig. 10) [48].
Zhang et al. reported a broad-spectrum, α-ketoamide-based inhibitor 44 of coronavirus and enterovirus replication [49]. This inhibitor was further optimized to a more selective inhibitor 45 against SARS-CoV-2 Mpro, with better pharmacokinetic profile by inserting a pyridone ring and replacing the cyclohexyl and cinnamoyl moieties by a smaller cyclopropyl group and a hydrophobic tert-butyloxycarbonyl group, respectively [50]. The crystal structure of SARS-CoV-2 Mpro in complex with 45 (PDB ID: 6Y2F) confirmed that the ketoamide moiety of 45 is involved in covalent adduct formation with Cys145 (Fig. 10) [50]. Sacco’s group also disclosed α-ketoamide reversible covalent inhibitors of SARS-CoV-2 Mpro, UAWJ246 (46) with Ki = 36 nM and UAWJ248 (47) with Ki = 13 nM [51]. Voss’s research group discovered tripeptidic α-ketoamide 48 as a potent reversible covalent inhibitor of the protease with IC50 of 38 nM [52]. Based on compound 48, Wang et al. developed a more potent derivative 49 with IC50 of 25.3 nM against the protease together with excellent anticancer activity [53].
The trifluoromethyl ketone moiety was found to be an effective reversible covalent warhead targeting cysteine residues. Zhang et al. discovered a series of selective fibroblast growth factor receptor 4 (FGFR4) inhibitors with a low nM IC50 value. The reversible covalent bonding mode of these inhibitors was confirmed by using MALDI-TOF mass spectrometry and X-ray crystallographic studies. The X-ray crystal structure of compound 50 in complex with FGFR4 (PDB ID: 7VJL) shows that the trifluoromethyl ketone warhead is covalently bonded to Cys552 (Fig. 11). The trifluoromethyl ketone moiety was also successfully used to design new JAK3 inhibitors. One example 51 of a JAK3 inhibitor with IC50 = 87.2 nM is shown in Fig. 11 [54].
Aldehyde
Analogously to electrophilic ketones, aldehydes can also serve as reversible covalent warheads. The aldehyde moiety reacts reversibly with cysteine or lysine to form the thiohemiacetal or Schiff base, respectively. Generally, the aldehyde moiety should be avoided in drug design due to its metabolic liability and potential toxicity [55]. However, in some cases aldehydes are stable as highlighted in voxelotor (6). The Schiff base formed from the aldehyde of voxelotor (6) and the amino group of Val1 of hemoglobin is stabilized by a strong intramolecular H-bond as shown in the crystal structure (PDB ID: 5E83) Fig. 12.
In another example of the stabilization of lysine Schiff base, Cal et al. [56] used the strategy of coordination of the nitrogen lone-pair electrons of the imine to a boronic acid in the reversible protein modification. In this strategy, boronic acid is introduced to the ortho-position of a benzaldehyde (52) moiety to obtain an iminoboronate upon Schiff base formation where the latter is stabilized by an intramolecular dative bond between the electrophilic boron center and the nucleophilic nitrogen lone-pair electrons. It has been confirmed that this reaction is reversible under physiological conditions [57, 58]. The chemistry of iminoboronates was applied by Akçay et al. [59] to develop the reversible covalent inhibitors of the induced myeloid leukemia cell differentiation protein (MCL-1). The incorporation of 2-carbonylphenylboronic acid with pharmacophores of MCL-1 inhibitors were designed to reversibly target the surface-exposed Lys234 side chain of the target protein. 2-Carbonylphenylboronic acids 53‒55 (Fig. 13) showed low nanomolar IC50 values in a TR-FRET assay. On the other hand, derivatives lacking either the boronic acid or the ortho-carbonyl group were significantly less active. Mass spectrometry analysis showed that compound 53 was approximately 50% attached to the target protein after 1 h, while the reaction with acetophenone 55 was slightly slower. Reversibility of these inhibitors was confirmed by surface plasmon resonance (SPR) experiments.
In 2021, Quach’s group [60] also utilized the chemistry of iminoboronates to develop BCR-ABL reversible covalent inhibitors to target the catalytic lysine (Lys271) with excellent potency against both wild-type and mutant ABL kinases. The representative example 56 is highlighted here. As expected, 56 showed time-dependent inhibition of ABL with IC50 values from 13 nM at 0 h to 1.7 nM after 12 h. Inhibitor 56 also showed excellent time-dependent potency against both mutants, ABLT315I from 25 nM (T = 0 h) to 0.1 nM (T = 6 h) and ABLE255K from 43 nM (T = 0 h) to 0.5 nM (T = 12 h). The X-ray co-crystal structures of 56 with the ABL kinase domain (PDB ID; 7DT2) did not show the expected dative bond between the imine nitrogen and the boron atom. However, from the enzymatic assay data and MALDI-TOF analysis, the boronic acid was believed to still play a significant role in the formation of the adduct. Therefore, they proposed that the obtained cocrystal structure of 56 with the ABL kinase domain (PDB ID; 7DT2) may be the key intermediate during the formation of the iminoboronates.
Furthermore, imine products were found to be stabilized by incorporating a nitrogen atom close to the aldehyde instead of a boronic acid, such as the benzaldehyde derivative of o-aminomethyl phenylboronic acid (57) [61]. Excitingly, the o-aminomethyl phenylboronic acid-derived imines are much more stable with half-lives of approximately 5–11 hours than the imines that were stabilized only by a boronic acid. The o-aminomethyl phenylboronic acid group was introduced into the cyclic peptide that non-covalently binds to the sortase A enzyme of Staphylococcus aureus, a potential target for combating S. aureus infections. The resulting compound 58 (Fig. 13) potently binds to sortase A through covalent modification of a lysine residue (Lys173) in the sortase A enzyme. Thus, they discovered the o-aminomethyl phenylboronic acid group as a promising motif for targeting lysine by reversible covalent inhibitors with long residence times.
In a drug discovery program carried out at Novartis, Knoepfel et al. [62] identified 2-formylquinoline amide 59 (IC50 = 57 nM) as a potent inhibitor of FGFR4 with a good selectivity in the FGFR family. FGFR4 is considered as a potential target for the treatment of hepatocellular carcinoma (HCC) because of its important role in HCC development and progression. Targeting the unique cysteine (Cys522) in the FGFR4 hinge region with the formyl group provided an opportunity for high FGFR family selectivity. Scaffold morphing of quinolone-amide 59 resulted in several 2-formyl tetrahydronaphthyridine urea analogs like 60 with better FGFR4 activity, selectivity, and improved physicochemical properties. Further extensive optimization of the 2-formyl tetrahydronaphthyridine urea series resulted in a drug candidate, roblitinib (61) (Fig. 14) which is being evaluated in phase III clinical trials [63, 64]. In a Phase 1–2 study, roblitinib (61) demonstrated favorable pharmacokinetic characteristics with evidence of FGFR4 inhibition. Roblitinib (61) alone or in combination with spartalizumab had a manageable safety profile with AEs that are considered on-target effects of pathway inhibition. Clinical efficacy was observed in patients with hepatocellular carcinoma.
As previously mentioned, the aldehyde moiety is also susceptible to the nucleophilic addition by the cysteine-SH which leads to the formation of a reversible thiohemiacetal adduct with relatively high stability and a longer residence time. Aldehyde-based reversible covalent inhibitors targeting cysteine have been reported before [65, 66]. This warhead received increased attention during the development of potent inhibitors of SARS-CoV-2 Mpro after the WHO declared COVID-19 as a global health emergency in 2020. Dai et al. [67] rationally designed two of the first peptidomimetic derivatives 62 and 63, targeting SARS-CoV-2 Mpro. Both exhibited excellent inhibitory activity (IC50 of 53 nM and 40 nM, respectively) and potent anti-SARS-CoV-2 infection activity (EC50 of 530 nM and 720 nM, respectively). The X-ray co-crystal structure of 62 complexed with SARS-CoV-2 Mpro (PDB ID: 6LZE) showed that the aldehyde group is covalently bound to Cys145 of Mpro (Fig. 15). Both compounds exhibited favorable pharmacokinetic properties in vivo and low toxicity. Qiao et al. [68] synthesized a series of new bicycloproline-containing Mpro inhibitors derived from either telaprevir (2) or boceprevir (3). All synthesized compounds inhibited SARS-CoV-2 Mpro activity in vitro, with IC50 values ranging from 7.6 to 748.5 nM. The two lead compounds MI-09 (64) and MI-30 (65) showed excellent antiviral activity in cell-based assays. Both compounds 64 and 65 displayed favorable pharmacokinetic properties and safety in rats and significantly reduced lung viral loads and lung lesions in a transgenic mouse model of SARS-CoV-2 infection. Another research group [69] synthesized UAWJ9-36-1 (66) and UAWJ9-36-3 (67) with very similar structures to 64. Both compounds showed comparable inhibitory activity against SARS-CoV-2 Mpro to 64.
GC-373 (68) and its prodrug GC-376 (69) in which the aldehyde was masked as its bisulfite adduct to increase solubility and allow release under physiological conditions, were initially designed as inhibitors of feline coronavirus (FCoV) 3CL protease for use of veterinary medications [70]. In the search of anti-COVID-19 agents recently, the two compounds 68 and 69 were tested for the inhibition of SARS-CoV-2 Mpro. Both 68 and 69 inhibit the SARS-CoV-2 Mpro in vitro with IC50 of 0.40 μM and 0.19 μM, respectively [71, 72]. The X-ray crystallographic studies of both compounds in complex with SARS-CoV-2 Mpro displayed similar binding modes of 68 (PDB ID: 6WTK) and 69 (PDB ID: 6WTJ) which confirms that the bisulfite adduct of 69 rapidly releases the aldehyde (Fig. 16). Further in vitro investigation using a model of SARS-CoV-2 infection in Vero E6 cells showed high inhibitory activity of both 68 and 69 with EC50 of 1.5 and 0.92 μM, respectively with low cytotoxicity even at high concentrations.
The research group of Vuong [73] also synthesized a series of analogs of GC376 (69) and identified compound 70 with excellent inhibitory activity against Mpro (IC50 = 0.07 μM) and antiviral efficacy (EC50 = 0.57 μM). The improved activity of 70 in comparison to the parent compound 69 is attributed to the more compact cyclopropyl group of 70 which can penetrate deeper into the S2 pocket of the target protein. Additionally, introduction of a fluorine on the Cbz group allows this moiety to move away from the solvent exposed region. Further structural modification of GC-376 (69) by replacing the isopropyl moiety with a phenyl group resulted to UAWJ247 (71) with IC50 of 0.045 μM [51]. Recently, Liu et al. reported a series of analogs of 69. They discovered NK01-63 (72) which is more active than the lead compound with excellent inhibitory activity (IC50 = 16 nM) against SARS-CoV-2. Compound 72 also showed excellent antiviral effect in cell assay (EC50 = 6 nM in Huh-7ACE2 infected cells) with high selectivity against other human proteases [74]. Yang and his colleagues reported tripeptidyl inhibitors MPI3 (73) and MPI8 (74) (Fig. 16) as SARS-CoV-2 Mpro inhibitors with high activity (8.5 and 105 nM respectively). Despite the weaker enzymatic activity of 74 in comparison to 73, it completely inhibits the SARS-CoV-2 infection in Vero E6 and A549/ACE2 cell lines with an IC50 of 0.31 μM [75]. Moreover, other aldehyde-based reversible covalent tripeptidyl inhibitors of SARS-CoV-2 Mpro were explored [76,77,78].
Boronic acids
After approval of bortezomib (4) by the FDA in 2003, boronic acids have garnered much attention by the pharmaceutical industry and now various boron-containing drugs are available on the market and many more are currently being evaluated in clinical trials. Comprehensive reviews are available on the use of boronic acids in drug discovery and as covalent inhibitors [79‒82]. Here we will briefly describe the application of the boronic acid moiety as a reversible electrophilic warhead in the discovery of reversible covalent drugs. Boronic acids can form covalent adducts with nucleophiles such as serine, lysine, threonine, tyrosine, and cysteine residues in target proteins. As described before, bortezomib (4), containing a boronic acid as an electrophilic warhead which targets the N-terminal threonine of 26S proteasome, is the first-in-class reversible covalent inhibitor for the treatment of multiple melanomas [10]. The X-ray structure of human 20 S proteasome complex with bortezomib (4) (PDB ID: 5LF3) indicated that the boronic acid formed a tetrahedral adduct with a threonine residue of the target protein [83]. Ixazomib (5) is a second-generation proteasome inhibitor that also possesses a boronic acid group. It was approved by the FDA in 2015 for the treatment of multiple myeloma. Delanzomib (75) composed of a boronic acid as the electrophilic warhead is a novel orally-active inhibitor of the chymotrypsin-like activity of the proteasome that down-modulates the nuclear factor-κB (NF-κB) activity [83]. Vaborbactam (76), approved for the treatment of various bacterial infections, consists of a cyclic boronic acid as the electrophilic warhead targeting the serine residue of the β-lactamase as illustrated in Fig. 17 (PDB ID: 4XUZ) [84]. Caselli et al. [85] synthesized a library of 26 α-triazolylmethaneboronic acids. These compounds showed Ki values ranging from 0.09 μM to 38 μM against the clinically concerning Acinetobacter-derived cephalosporinase, ADC-7. In this series the most potent compound 77 (Ki = 90 nM) is covalently bound to the catalytic serine.
It has been reported that the replacement of carboxylic acid (78) and amide (79) by boronic acid (80) at the C-terminal of dipeptidic inhibitors against Zika, West Nile, and dengue viral proteases resulted in a thousand-fold affinity gain [86]. The most active compound 80 had Ki values of 51, 82 and 40 nM for dengue, West Nile and Zika viral proteases, respectively. The crystal structure of 80 in complex with ZIKV NS2B-NS3 protease (PDB ID: 5LC0) [87] showed that a six-membered boronate structure was formed between glycerol and boronic acid, in which the boron atom was covalently bonded to the Ser135 residue of the target protein (Fig. 17).
Miscellaneous warheads
A reversible-covalent approach was recently pursued by Jakob et al. to engage a histidine (His315) in wild-type isocitrate dehydrogenase (IDH)1 [88]. A high-throughput screen and subsequent optimization identified covalent reversible inhibitor 81 with IC50 of 110 nM and favorable permeability. Covalent bond formation of the α-cyanoenone moiety with His315 in the binding pocket of NADPH via aza-Michael addition was confirmed by X-ray crystallography. The X-ray crystal structure of 81 complexed in IDH1 (PDB ID: 6BL1) is shown in the Fig. 18. Reversibility of 81 was confirmed by wash-out and jump-dilution experiments.
Shindo et al. [89] reported α-chlorofluoroacetamide as a novel warhead for the development of covalent inhibitors. Regardless of the weak intrinsic reactivity, α-chlorofluoroacetamide-appended quinazoline exhibited high reactivity toward Cys797 of EGFR. For example, NS-062 (82) had higher target specificity for EGFR than the corresponding Michael acceptors in a wide range of concentrations (0.1–10 μM) in cells. The covalent adduct formed between cysteine and the α-chlorofluoroacetamide derivative was susceptible to hydrolysis and reversibly released the intact thiol but remained stable in the solvent-sequestered ATP-binding pocket of EGFR. This characteristic environment-dependent hydrolysis can potentially reduce off-target protein modification by α-chlorofluoroacetamide-based drugs. Oral administration of NS-062 (82) significantly suppressed tumor growth in a mouse xenograft model (Fig. 19).
In 2016, Schirmeister and co-workers [90] developed α-halovinylsulfones as a new class of covalent reversible cysteine protease inhibitors. Structure optimization of α-fluorovinylsulfones/-sulfonates by rhodesain-based molecular modeling approaches resulted in compound 83, the most potent and selective reversible covalent inhibitor in the series with single-digit nanomolar affinity and high selectivity toward mammalian cathepsins B and L (Fig. 20). Enzymatic dilution assays and MS experiments indicated that 83 is a potent tight binder (Ki = 3 nM) and slowly reversible inhibitor of trypanosomal cysteine protease rhodesain [91].
Conclusions
Covalent drugs through formation of a covalent bond with their target proteins exhibit a favorable pharmacological profile, such as enhanced potency and prolonged duration of action over non-covalent drugs, leading to a higher possibility of durable efficacy in the treatment of hard-to-treat human diseases. However, one of the key challenges associated with covalent drugs is how to mitigate selectivity and toxicities. The electrophilic warheads could react with nucleophilic residues of proteins other than the intended target protein, which could lead to off-target effects and further idiosyncratic toxicity and haptenization. This issue of covalent therapeutics can be potentially de-risked by tuning the chemistry of electrophilic warhead to form a reversible covalent bond with the intended target protein. This would allow for more sustained target engagement with lower immunogenicity risks and fewer off-target effects by readily releasing from unintended proteins. This strategy has been successfully applied in the development of reversible covalent drugs. Herein we have described the current status of reversible covalent inhibitors. We believe that the cumulative structural data of reversible covalent inhibitors presented in this review will provide a valuable guidance for future research in this area to further explore the chemistry of reversible covalent warheads to develop life-saving drugs.
Abbreviations
- COX::
-
Cyclooxygenase
- COVID-19::
-
Coronavirus disease of 2019
- SARS-CoV-2::
-
Severe acute respiratory syndrome-corona virus—2
- PDB::
-
Protein data bank
- BTK::
-
Bruton’s tyrosine kinase
- EGFR::
-
Epidermal growth factor receptor
- JAK3::
-
Janus kinase 3
- TYK2::
-
Tyrosine kinase 2
- RSK2::
-
Serine/threonine kinase 2
- EV71::
-
Enterovirus 71
- CVA16::
-
Coxsackievirus A16
- CVB3::
-
Coxsackievirus B3
- CPB::
-
Cysteine protease B
- TgCPL::
-
Toxoplasma gondii cathepsin protease L
- (MALDI) TOF MS::
-
Matrix assisted laser desorption ionization mass spectrometry
- FGFR4::
-
Fibroblast growth factor receptor 4
- TR-FRET::
-
Time-resolved fluorescence resonance energy transfer
- SPR::
-
Surface plasmon resonance
- NADPH::
-
Nicotinamide adenine dinucleotide phosphate hydrogen
- ATP::
-
Adenosine tri-phosphate
- ERB-BR::
-
Epidermal growth factor receptor
- BLK::
-
B-Lymphoid tyrosine kinase
- Cbz::
-
Carbobenzoxy
- RSK2::
-
P90 ribosomal S6 kinase 2
- DPP-4::
-
Dipeptidyl peptidase-4
- IP::
-
Intraperitoneal
- BCR-ABL::
-
Breakpoint cluster region- Abelson
References
Roth GJ, Stanford N, Majerus PW. Acetylation of prostaglandin synthase by aspirin. PNAS. 1975;72:3073–6. https://doi.org/10.1073/pnas.72.8.307
Singh J, Petter RC, Baillie TA, Whitty A. The resurgence of covalent drugs. Nat Rev Drug Discov. 2011;10:307–17. https://doi.org/10.1038/nrd3410
Vita ED. 10 years into the resurgence of covalent drugs. Future Med Chem. 2021;13:193–210. https://doi.org/10.4155/fmc-2020-0236
Lonsdale R, Ward RA. Structure-based design of targeted covalent inhibitors. Chem Soc Rev. 2018;47:3816–30. https://doi.org/10.1039/C7CS00220C
Singh J. The ascension of targeted covalent inhibitors. J Med Chem. 2022;65:5886–901. https://doi.org/10.1021/acs.jmedchem.1c02134
Shibata Y, Chiba M. The role of extrahepatic metabolism in the pharmacokinetics of the targeted covalent inhibitors afatinib, ibrutinib, and neratinib. Drug Metab Dispos. 2015;43:375–84. https://doi.org/10.1124/dmd.114.061424
Bandyopadhyay A, Gao J. Targeting biomolecules with reversible covalent chemistry. Curr Opin Chem Biol. 2016;34:110–6. https://doi.org/10.1016/j.cbpa.2016.08.011
Augeri DJ, Robl JA, Betebenner DA, Magnin DR, Khanna A, Robertson JG, et al. Discovery and preclinical profile of saxagliptin (BMS-477118): a highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem. 2005;48:5025–37. https://doi.org/10.1021/jm050261p
O’Meara JA, Lemke CT, Godbout C, Kukolj G, Lagacé L, Moreau B, et al. Molecular mechanism by which a potent hepatitis C virus NS3-NS4A protease inhibitor overcomes emergence of resistance. J Biol Chem. 2013;288:5673–81. https://doi.org/10.1074/jbc.M112.439455
Arasappan A, Bennett F, Bogen SL, Venkatraman S, Blackman M, Chen KX, et al. Discovery of narlaprevir (SCH 900518): a potent, second generation HCV NS3 serine protease inhibitor. ACS Med Chem Lett. 2010;1:64–69. https://doi.org/10.1021/ml9000276
Greener BS, Millan DS. Bortezomib (velcade): a first-in-class proteasome inhibitor. Mod Drug Synth. 2010. p. 99–110. https://doi.org/10.1002/9780470768594.ch8
Shirley M. Ixazomib: first global approval. Drugs. 2016;76:405–11. https://doi.org/10.1007/s40265-016-0548-5
Oksenberg D, Dufu K, Patel MP, Chuang C, Li Z, Xu Q, et al. GBT440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half-life in a murine model of sickle cell disease. Br J Haematol. 2016;175:141–53. https://doi.org/10.1111/bjh.14214
Greasley SE, Noell S, Plotnikova O, Ferre R, Liu W, Bolanos B, et al. Structural basis for the in vitro efficacy of nirmatrelvir against SARS-CoV-2 variants. J Biol Chem. 2022;298:101972 https://doi.org/10.1016/j.jbc.2022.101972
Bradshaw JM, McFarland JM, Paavilainen VO, Bisconte A, Tam D, Phan VT, et al. Prolonged and tunable residence time using reversible covalent kinase inhibitors. Nat Chem Biol. 2015;11:525–31. https://doi.org/10.1038/nchembio.1817
Owens TD, Brameld KA, Verner EJ, Ton T, Li X, Zhu J, et al. Discovery of reversible covalent Bruton’s tyrosine kinase inhibitors PRN473 and PRN1008 (rilzabrutinib). J Med Chem. 2022;65:5300–16. https://doi.org/10.1021/acs.jmedchem.1c01170
Smith PF, Krishnarajah J, Nunn PA, Hill RJ, Karr D, Tam D, Masjedizadeh M, Funk JO, Gourlay SG. A phase I trial of PRN1008, a novel reversible covalent inhibitor of Bruton’s tyrosine kinase, in healthy volunteers. Br J Clin Pharm. 2017;83:2367–76. https://doi.org/10.1111/bcp.13351
Kuter DJ, Efraim M, Mayer J, Trněný M, McDonald V, Bird R, et al. Rilzabrutinib, an oral BTK inhibitor, in immune thrombocytopenia. N Engl J Med. 2022;386:1421–31. https://doi.org/10.1056/NEJMoa2110297
Smith S, Keul M, Engel J, Basu D, Eppmann S, Rauh D. Characterization of covalent-reversible EGFR inhibitors. ACS Omega. 2017;2:1563–75. https://doi.org/10.1021/acsomega.7b00157
London N, Miller RM, Krishnan S, Uchida K, Irwin JJ, Eidam O, et al. Covalent docking of large libraries for the discovery of chemical probes. Nat Chem Biol. 2014;10:1066–72. https://doi.org/10.1038/nchembio.1666
Forster M, Chaikuad A, Bauer Silke M, Holstein J, Robers Matthew B, Corona Cesear R, et al. Selective JAK3 inhibitors with a covalent reversible binding mode targeting a new induced fit binding pocket. Cell Chem Biol. 2016;23:1335–40. https://doi.org/10.1016/j.chembiol.2016.10.008
Serafimova IM, Pufall MA, Krishnan S, Duda K, Cohen MS, Maglathlin RL, et al. Reversible targeting of noncatalytic cysteines with chemically tuned electrophiles. Nat Chem Biol. 2012;8:471–6. https://doi.org/10.1038/nchembio.925
Miller RM, Paavilainen VO, Krishnan S, Serafimova IM, Taunton J. Electrophilic fragment-based design of reversible covalent kinase inhibitors. J Am Chem Soc. 2013;135:5298–301. https://doi.org/10.1021/ja401221b
Li B, Li Y, Tomkiewicz-Raulet C, Dao P, Lietha D, Yen-Pon E, et al. Design, synthesis, and biological evaluation of covalent inhibitors of focal adhesion kinase (FAK) against human malignant glioblastoma. J Med Chem. 2020;63:12707–24. https://doi.org/10.1021/acs.jmedchem.0c01059
Ma Y, Li L, He S, Shang C, Sun Y, Liu N, et al. Application of dually activated Michael acceptor to the rational design of reversible covalent inhibitor for enterovirus 71 3C protease. J Med Chem. 2019;62:6146–62. https://doi.org/10.1021/acs.jmedchem.9b00387
Liu M, Xu B, Ma Y, Shang L, Ye S, Wang Y. Reversible covalent inhibitors suppress enterovirus 71 infection by targeting the 3C protease. Antivir Res. 2021;192:105102. https://doi.org/10.1016/j.antiviral.2021.105102
Gauthier JY, Chauret N, Cromlish W, Desmarais S, Duong LT, Falgueyret JP, et al. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg Med Chem Lett. 2008;18:923–8. https://doi.org/10.1016/j.bmcl.2007.12.047
Mukherjee K, Chattopadhyay N. Pharmacological inhibition of cathepsin K: a promising novel approach for postmenopausal osteoporosis therapy. Biochem Pharm. 2016;117:10–9. https://doi.org/10.1016/j.bcp.2016.04.010
Quesne MG, Ward RA, de Visser SP. Cysteine protease inhibition by nitrile-based inhibitors: a computational study. Front Chem. 2013;1:39. https://doi.org/10.3389/fchem.2013.00039
Cianni L, Rocho FDR, Rosini F, Bonatto V, Ribeiro JFR, Lameira J, et al. Optimization strategy of single-digit nanomolar cross-class inhibitors of mammalian and protozoa cysteine proteases. Bioorg Chem. 2020;101:104039. https://doi.org/10.1016/j.bioorg.2020.104039
Alves L, Santos DA, Cendron R, Rocho FR, Matos TKB, Leitão A, et al. Nitrile-based peptoids as cysteine protease inhibitors. Bioorg Med Chem. 2021;41:116211. https://doi.org/10.1016/j.bmc.2021.116211
de Jesus Cortez F, Nguyen P, Truillet C, Tian B, Kuchenbecker KM, Evans MJ, et al. Development of 5N-bicalutamide, a high-affinity reversible covalent antiandrogen. ACS Chem Biol. 2017;12:2934–9. https://doi.org/10.1021/acschembio.7b00702
Benson MJ, Rodriguez V, von Schack D, Keegan S, Cook TA, Edmonds J, et al. Modeling the clinical phenotype of BTK inhibition in the mature murine immune system. J Immunol. 2014;193:185–97. https://doi.org/10.4049/jimmunol.1302570
Breidenbach J, Lemke C, Pillaiyar T, Schäkel L, Al Hamwi G, Diett M, et al. Targeting the main protease of SARS-CoV-2: from the establishment of high throughput screening to the design of tailored inhibitors. Angew. Chem Int Ed Engl. 2021;60:10423–9. https://doi.org/10.1002/anie.202016961
Bai B, Arutyunova E, Khan MB, Lu J, Joyce MA, Saffran HA, et al. Peptidomimetic nitrile warheads as SARS-CoV-2 3CL protease inhibitors. RSC Med Chem. 2021;12:1722–30. https://doi.org/10.1039/D1MD00247C
Matos TKB, Batista PHJ, dos Reis Rocho F, de Vita D, Pearce N, Kellam B, et al. Synthesis and matched molecular pair analysis of covalent reversible inhibitors of the cysteine protease CPB. Bioorg Med Chem Lett. 2020;30:127439. https://doi.org/10.1016/j.bmcl.2020.127439
Ribeiro JFR, Cianni L, Li C, Warwick TG, de Vita D, Rosini F, et al. Crystal structure of Leishmania mexicana cysteine protease B in complex with a high-affinity azadipeptide nitrile inhibitor. Bioorg Med Chem. 2020;28:115743. https://doi.org/10.1016/j.bmc.2020.115743
Cianni L, Lemke C, Gilberg E, Feldmann C, Rosini F, Rocho FDR, et al. Mapping the S1 and S1’ subsites of cysteine proteases with new dipeptidyl nitrile inhibitors as trypanocidal agents. PLoS Negl Trop Dis. 2020;14:e0007755. https://doi.org/10.1371/journal.pntd.0007755
Dos Santos Nascimento IJ, de Aquino TM, da Silva-Júnior EF. Cruzain and rhodesain inhibitors: last decade of advances in seeking for new compounds against American and African Trypanosomiases. Curr Top Med Chem. 2021;21:1871–99. https://doi.org/10.2174/1568026621666210331152702
Schirmeister T, Schmitz J, Jung S, Schmenger T, Krauth-Siegel RL, Gütschow M. Evaluation of dipeptide nitriles as inhibitors of rhodesain, a major cysteine protease of Trypanosoma brucei. Bioorg Med Chem Lett. 2017;27:45–50. https://doi.org/10.1016/j.bmcl.2016.11.036
Giroud M, Kuhn B, Saint-Auret S, Kuratli C, Martin RE, Schuler F, et al. 2H-1,2,3-Triazole-based dipeptidyl nitriles: potent, selective, and trypanocidal rhodesain inhibitors by structure-based design. J Med Chem. 2018;61:3370–88. https://doi.org/10.1021/acs.jmedchem.7b01870
Pittman KJ, Aliota MT, Knoll LJ. Dual transcriptional profiling of mice and Toxoplasma gondii during acute and chronic infection. BMC Genom. 2014;15:806–24. https://doi.org/10.1186/1471-2164-15-806
Larson ET, Parussini F, Huynh MH, Giebel JD, Kelley AM, Zhang L, et al. Toxoplasma gondii cathepsin L is the primary target of the invasion-inhibitory compound morpholinurea-leucyl-homophenyl-vinyl sulfone phenyl. J Biol Chem. 2009;284:26839–50. https://doi.org/10.1074/jbc.M109.003780
Zwicker JD, Diaz NA, Guerra AJ, Kirchhoff PD, Wen B, Sun D, et al. Optimization of dipeptidic inhibitors of cathepsin L for improved Toxoplasma gondii selectivity and CNS permeability. Bioorg Med Chem Lett. 2018;28:1972–80. https://doi.org/10.1016/j.bmcl.2018.03.020
Zwicker JD, Smith D, Guerra AJ, Hitchens JR, Haug N, Vander Roest S, et al. Discovery and optimization of triazine nitrile inhibitors of Toxoplasma gondii cathepsin L for the potential treatment of chronic toxoplasmosis in the CNS. ACS Chem Neurosci. 2020;11:2450–63. https://doi.org/10.1021/acschemneuro.9b00674
Romano KP, Ali A, Aydin C, Soumana D, Ozen A, Deveau LM, et al. The molecular basis of drug resistance against hepatitis C virus NS3/4A protease inhibitors. PLOS Pathog. 2012;8:e1002832. https://doi.org/10.1371/journal.ppat.1002832
Hoffman RL, Kania RS, Brothers MA, Davies JF, Ferre RA, Gajiwala KS, et al. Discovery of ketone-based covalent inhibitors of coronavirus 3CL proteases for the potential therapeutic treatment of COVID-19. J Med Chem. 2020;63:12725–47. https://doi.org/10.1021/acs.jmedchem.0c01063
Boras B, Jones RM, Anson BJ, Arenson D, Aschenbrenner L, Bakowski MA, et al. Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID19. Nat Commun. 2021;12:6055. https://doi.org/10.1038/s41467-021-26239-2
Zhang L, Lin D, Kusov Y, Nian Y, Ma Q, Wang J, et al. α-Ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication: structure-based design, synthesis, and activity assessment. J Med Chem. 2020;63:4562–78. https://doi.org/10.1021/acs.jmedchem.9b01828
Zhang L, Lin D, Sun X, Curth U, Drosten C, Sauerhering L, et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368:409–12. https://doi.org/10.1126/science.abb3405
Sacco MD, Ma C, Lagarias P, Gao A, Townsend JA, Meng X, et al. Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against M(pro) and cathepsin L. Sci Adv. 2020;6:eabe0751. https://doi.org/10.1126/sciadv.abe0751
Voss C, Scholz C, Knorr S, Beck P, Stein ML, Zall A, et al. α-Keto phenylamides as P1′-extended proteasome inhibitors. ChemMedChem. 2014;9:2557–64. https://doi.org/10.1002/cmdc.201402244
Wang J, Liang B, Chen Y, Fuk-Woo Chan J, Yuan S, Ye H, et al. A new class of α-ketoamide derivatives with potent anticancer and anti-SARS-CoV-2 activities. Eur J Med Chem. 2021;215:113267. https://doi.org/10.1016/j.ejmech.2021.113267
Zhang Z, Wang Y, Chen X, Song X, Tu Z, Chen Y, et al. Characterization of an aromatic trifluoromethyl ketone as a new warhead for covalently reversible kinase inhibitor design. Bioorg Med Chem. 2021;50:116457. https://doi.org/10.1016/j.bmc.2021.116457
Malátková P, Wsól V. Carbonyl reduction pathways in drug metabolism. Drug Metab Rev. 2014;46:96–123. https://doi.org/10.3109/03602532.2013.853078
Cal PMSD, Vicente JB, Pires E, Coelho AV, Veiros LSF, Cordeiro C, et al. Iminoboronates: a new strategy for reversible protein modification. J Am Chem Soc. 2012;134:10299–305. https://doi.org/10.1021/ja303436y
Bandyopadhyay A, McCarthy KA, Kelly MA, Gao J. Targeting bacteria via iminoboronate chemistry of amine-presenting lipids. Nat Commun. 2015;6:6561. https://doi.org/10.1038/ncomms7561
Bandyopadhyay A, Gao J. Iminoboronate formation leads to fast and reversible conjugation chemistry of α-nucleophiles at neutral pH. Chem Eur J. 2015;21:14748–52. https://doi.org/10.1002/chem.201502077
Akçay G, Belmonte MA, Aquila B, Chuaqui C, Hird AW, Lamb ML, et al. Inhibition of Mcl-1 through covalent modification of a noncatalytic lysine side chain. Nat Chem Biol. 2016;12:931–6. https://doi.org/10.1038/nchembio.2174
Quach D, Tang G, Anantharajan J, Baburajendran N, Poulsen A, Wee JLK, et al. Strategic design of catalytic lysine-targeting reversible covalent BCR-ABL inhibitors. Angew Chem Int Ed. 2021;60:17131–7. https://doi.org/10.1002/anie.202105383
Reja RM, Wang W, Lyu Y, Haeffner F, Gao J. Lysine-targeting reversible covalent inhibitors with long residence time. J Am Chem Soc. 2022;144:1152–7. https://doi.org/10.1021/jacs.1c12702
Knoepfel T, Furet P, Mah R, Buschmann N, Leblanc C, Ripoche S, et al. 2-Formylpyridyl ureas as highly selective reversible-covalent inhibitors of fibroblast growth factor receptor 4. ACS Med Chem Lett. 2018;9:215–20. https://doi.org/10.1021/acsmedchemlett.7b00485
Fairhurst RA, Knoepfel T, Buschmann N, Leblanc C, Mah R, Todorov M, et al. Discovery of roblitinib (FGF401) as a reversible-covalent inhibitor of the kinase activity of fibroblast growth factor receptor 4. J Med Chem. 2020;63:12542–73. https://doi.org/10.1021/acs.jmedchem.0c01019
Weiss A, Adler F, Buhles A, Stamm C, Fairhurst RA, Kiffe M, et al. FGF401, a first-in-class highly selective and potent FGFR4 inhibitor for the treatment of FGF19-driven hepatocellular cancer. Mol Cancer Ther. 2019;18:2194–206. https://doi.org/10.1158/1535-7163.MCT-18-1291
Fukiage C, Azuma M, Nakamura Y, Tamada Y, Nakamura M, Shearer TR. SJA6017, a newly synthesized peptide aldehyde inhibitor of calpain: amelioration of cataract in cultured rat lenses. Biochim Biophys Acta Mol Basis Dis. 1997;1361:304–12. https://doi.org/10.1016/S0925-4439(97)00043-4
Guan N, Korukonda R, Hurh E, Schmittgen TD, Donkor IO, Dalton JT. Apoptosis induced by novel aldehyde calpain inhibitors in human tumor cell lines. Int J Oncol. 2006;29:655–63. https://doi.org/10.3892/ijo.29.3.655
Dai W, Zhang B, Jiang X-M, Su H, Li J, Zhao Y, et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–5. https://doi.org/10.1126/science.abb4489
Qiao J, Li Y-S, Zeng R, Liu F-L, Luo R-H, Huang C, et al. SARS-CoV-2 Mpro inhibitors with antiviral activity in a transgenic mouse model. Science. 2021;371:1374–8. https://doi.org/10.1126/science.abf1611
Xia Z, Sacco M, Hu Y, Ma C, Meng X, Zhang F, et al. Rational design of hybrid SARS-CoV-2 main protease inhibitors guided by the superimposed cocrystal structures with the peptidomimetic inhibitors gc-376, telaprevir, and boceprevir. ACS Pharm Transl Sci. 2021;4:1408–21. https://doi.org/10.1021/acsptsci.1c00099
Kim Y, Lovell S, Tiew K-C, Mandadapu SR, Alliston KR, Battaile KP, et al. Broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, noroviruses, and coronaviruses. J Virol. 2012;86:11754–62. https://doi.org/10.1128/JVI.01348-12
Vuong W, Khan MB, Fischer C, Arutyunova E, Lamer T, Shields J, et al. Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat Commun. 2020;11:4282. https://doi.org/10.1038/s41467-020-18096-2
Ma C, Sacco MD, Hurst B, Townsend JA, Hu Y, Szeto T, et al. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020;30:678–92. https://doi.org/10.1038/s41422-020-0356-z
Vuong W, Fischer C, Khan MB, van Belkum MJ, Lamer T, Willoughby KD, et al. Improved SARS-CoV-2 Mpro inhibitors based on feline antiviral drug GC376: Structural enhancements, increased solubility, and micellar studies. Eur J Med Chem. 2021;222:113584. https://doi.org/10.1016/j.ejmech.2021.113584
Liu H, Iketani S, Zask A, Khanizeman N, Bednarova E, Forouhar F, et al. Development of optimized drug-like small molecule inhibitors of the SARS-CoV-2 3CL protease for treatment of COVID-19. Nat Commun. 2022;13:1891. https://doi.org/10.1038/s41467-022-29413-2
Yang KS, Ma XR, Ma Y, Alugubelli YR, Scott DA, Vatansever EC, et al. A quick route to multiple highly potent SARS-CoV-2 main protease inhibitors. ChemMedChem. 2021;16:942–8. https://doi.org/10.1002/cmdc.202000924
Costanzi E, Kuzikov M, Esposito F, Albani S, Demitri N, Giabbai B, et al. Structural and biochemical analysis of the dual inhibition of MG-132 against SARS-CoV-2 main protease (Mpro/3CLpro) and human Cathepsin-L. Int J Mol Sci. 2021;22:11779. https://doi.org/10.3390/ijms222111779
Kuzikov M, Costanzi E, Reinshagen J, Esposito F, Vangeel L, Wolf M, et al. Identification of inhibitors of SARS-CoV-2 3CL-pro enzymatic activity using a small molecule in vitro repurposing screen. ACS Pharm Transl Sci. 2021;4:1096–110. https://doi.org/10.1021/acsptsci.0c00216
Dampalla CS, Kim Y, Bickmeier N, Rathnayake AD, Nguyen HN, Zheng J, et al. Structure-guided design of conformationally constrained cyclohexane inhibitors of severe acute respiratory syndrome coronavirus-2 3CL protease. J Med Chem. 2021;64:10047–58. https://doi.org/10.1021/acs.jmedchem.1c00319
Song S, Gao P, Sun L, Kang D, Kongsted J, Poongavanam V, et al. Recent developments in the medicinal chemistry of single boron atom-containing compounds. Acta Pharm Sin B. 2021;11:3035–59. https://doi.org/10.1016/j.apsb.2021.01.010
Plescia J, Moitessier N. Design and discovery of boronic acid drugs. Eur J Med Chem. 2020;195:112270. https://doi.org/10.1016/j.ejmech.2020.112270
Smoum R, Rubinstein A, Dembitsky VM, Srebnik M. Boron containing compounds as protease inhibitors. Chem Rev. 2012;112:4156–220. https://doi.org/10.1021/cr608202m
De Cesco S, Kurian J, Dufresne C, Mittermaier AK, Moitessier N. Covalent inhibitors design and discovery. Eur J Med Chem. 2017;138:96–114. https://doi.org/10.1016/j.ejmech.2017.06.019
Schrader J, Henneberg F, Mata RA, Tittmann K, Schneider TR, Stark H, et al. The inhibition mechanism of human 20S proteasomes enables next-generation inhibitor design. Science. 2016;353:594–8. https://doi.org/10.1126/science.aaf8993
Hecker SJ, Reddy KR, Totrov M, Hirst GC, Lomovskaya O, Griffith DC, et al. Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility vs class a serine carbapenemases. J Med Chem. 2015;58:3682–92. https://doi.org/10.1021/acs.jmedchem.5b00127
Caselli E, Fini F, Introvigne ML, Stucchi M, Taracila MA, Fish ER, et al. 1,2,3-Triazolylmethaneboronate: a structure activity relationship study of a class of β-lactamase inhibitors against Acinetobacter baumannii cephalosporinase. ACS Infect Dis. 2020;6:1965–75. https://doi.org/10.1021/acsinfecdis.0c00254
Nitsche C, Zhang L, Weigel LF, Schilz J, Graf D, Bartenschlager R, et al. Peptide–boronic acid inhibitors of flaviviral proteases: medicinal chemistry and structural biology. J Med Chem. 2017;60:511–6. https://doi.org/10.1021/acs.jmedchem.6b01021
Lei J, Hansen G, Nitsche C, Klein CD, Zhang L, Hilgenfeld R. Crystal structure of Zika virus NS2B-NS3 protease in complex with a boronate inhibitor. Science. 2016;353:503–5. https://doi.org/10.1126/science.aag2419
Jakob CG, Upadhyay AK, Donner PL, Nicholl E, Addo SN, Qiu W, et al. Novel modes of inhibition of wild-type isocitrate dehydrogenase 1 (IDH1): direct covalent modification of His315. J Med Chem. 2018;61:6647–57. https://doi.org/10.1021/acs.jmedchem.8b00305
Shindo N, Fuchida H, Sato M, Watari K, Shibata T, Kuwata K, et al. Selective and reversible modification of kinase cysteines with chlorofluoroacetamides. Nat Chem Biol. 2019;15:250–8. https://doi.org/10.1038/s41589-018-0204-3
Schirmeister T, Kesselring J, Jung S, Schneider TH, Weickert A, Becker J, et al. Quantum chemical-based protocol for the rational design of covalent inhibitors. J Am Chem Soc. 2016;138:8332–5. https://doi.org/10.1021/jacs.6b03052
Jung S, Fuchs N, Johe P, Wagner A, Diehl E, Yuliani T, et al. Fluorovinylsulfones and -sulfonates as potent covalent reversible inhibitors of the trypanosomal cysteine protease rhodesain: structure–activity relationship, inhibition mechanism, metabolism, and in vivo studies. J Med Chem. 2021;64:12322–58. https://doi.org/10.1021/acs.jmedchem.1c01002
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Faridoon, Ng, R., Zhang, G. et al. An update on the discovery and development of reversible covalent inhibitors. Med Chem Res 32, 1039–1062 (2023). https://doi.org/10.1007/s00044-023-03065-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03065-3