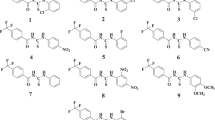
Abstract
Despite the significant development of diagnostic procedures and therapeutic options in past few decades, glaucoma is still highly prevalent and represents one of the leading causes of blindness in the world due to progressive and irreversible changes in optic nerve. Detection of carbonic anhydrase as a suitable target for the control of intraocular pressure indicated the beginning of carbonic anhydrase inhibitors application for the antiglaucoma treatment. Considering the multitude of proven and potential therapeutic applications of carbonic anhydrase inhibitors, the discovery of new chemotypes with carbonic anhydrase inhibitory activity will continue to be a significant aim. In this article we review the literature on synthetic chalcones as human carbonic anhydrase inhibitors, discussing their possible application focusing on chemical structure and Ki experimental values. From currently available experimental data and from the results we have obtained performing in silico calculations, we generated data collection on the basis of which we proposed 14 compounds of particular interest for further lead optimization and drug CAI development. Having previously experimentally determined excellent hCA II selectivity and strong inhibition effect and in our study predicted favorable physicochemical, pharmacokinetic, and toxicological profiles, a benzoxazolone chalcone derivative (139) stands out among the selected compounds. To examine potential therapeutic application, this candidate may be taken for further evaluation in in vivo studies.

Graphical abstract













Similar content being viewed by others
References
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. https://doi.org/10.1016/j.ophtha.2014.05.013.
Boland MV, Quigley HA. Risk factors and open-angle glaucoma: classification and application. J Glaucoma. 2007;16:406–18. https://doi.org/10.1097/ijg.0b013e31806540a1.
Marcus MW, de Vries MM, Junoy Montolio FG, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011;118:1989–94.e2. https://doi.org/10.1016/j.ophtha.2011.03.012.
Ko F, Boland MV, Gupta P, Gadkaree SK, Vitale S, Guallar E, et al. Diabetes, triglyceride levels, and other risk factors for glaucoma in the national health and nutrition examination survey 2005−2008. Invest Ophthalmol Vis Sci. 2016;57:2152–7. https://doi.org/10.1167/iovs.15-18373.
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–11. https://doi.org/10.1001/jama.2014.3192.
Carta F, Supuran CT, Scozzafava A. Novel therapies for glaucoma: a patent review 2007–2011. Expert Opin Ther Pat. 2012;22:79–88. https://doi.org/10.1517/13543776.2012.649006.
Scozzafava A, Supuran CT. Glaucoma and the applications of carbonic anhydrase inhibitors. In: Frost S, McKenna R, editors. Carbonic anhydrase: mechanism, regulation, links to disease, and industrial applications. Dordrecht: Springer; 2014. p. 349–59. https://doi.org/10.1007/978-94-007-7359-2_17.
Alterio V, Di Fiore A, D’Ambrosio K, Supuran CT, De Simone G. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms. Chem Rev. 2012;112:4421–68. https://doi.org/10.1021/cr200176r.
Lindskog S. Structure and mechanism of carbonic anhydrase. Pharmcol Ther. 1997;74:1–20. https://doi.org/10.1016/s0163-7258(96)00198-2.
Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem. 2007;15:4336–50. https://doi.org/10.1016/j.bmc.2007.04.020.
Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett. 2010;20:3467–74. https://doi.org/10.1016/j.bmcl.2010.05.009.
Friedenwald JS. The formation of the intraocular fluid. Am J Ophthalmol. 1949;32:9–27. https://doi.org/10.1016/s0002-9394(14)78354-0.
Kinsey VE. Comparative chemistry of aqueous humor in posterior and anterior chambers of rabbit eye, its physiologic significance. AMA Arch Ophthalmol. 1953;50:401–17. https://doi.org/10.1001/archopht.1953.00920030409001.
Kinsey VE, Barany E. The rate flow of aqueous humor. II. Derivation of rate of flow and its physiologic significance. Am J Ophthalmol. 1949;32:189–202. https://doi.org/10.1016/S0002-9394(14)78372-2.
Wistrand PJ. Carbonic anhydrase in the anterior uvea of the rabbit. Acta Physiol Scand. 1951;24:145–8. https://doi.org/10.1111/j.1748-1716.1951.tb00833.x.
Becker B. The mechanism of the fall in intraocular pressure induced by the carbonic anhydrase inhibitor, diamox. Am J Ophthalmol. 1955;39:177–84. https://doi.org/10.1016/0002-9394(55)90022-2.
Kinsey VE, Reddy DV. Turnover of total carbon dioxide in the aqueous humors and the effect thereon of acetazolamide. AMA Arch Ophthalmol. 1959;62:78–83. https://doi.org/10.1001/archopht.1959.04220010082009.
Supuran CT. How many carbonic anhydrase inhibition mechanisms exist. J Enzyme Inhib Med Chem. 2016;31:345–60. https://doi.org/10.3109/14756366.2015.1122001.
Kumar S, Rulhania S, Jaswal S, Monga V. Recent advances in the medicinal chemistry of carbonic anhydrase inhibitors. Eur J Med Chem. 2021;209:112923 https://doi.org/10.1016/j.ejmech.2020.112923
Carta F, Supuran CT, Scozzafava A. Sulfonamides and their isosters as carbonic anhydrase inhibitors. Future Med Chem. 2014;6:1149–65. https://doi.org/10.4155/fmc.14.68.
Ghorai S, Pulya S, Ghosh K, Panda P, Ghosh B, Gayen S. Structure-activity relationship of human carbonic anhydrase-II inhibitors: Detailed insight for future development as anti-glaucoma agents. Bioorg Chem. 2020;95:103557 https://doi.org/10.1016/j.bioorg.2019.103557.
Karioti A, Carta F, Supuran CT. Phenols and polyphenols as carbonic anhydrase inhibitors. Molecules. 2016;21:1649 https://doi.org/10.3390/molecules21121649.
Tsai JC. Innovative IOP-independent neuroprotection and neuroregeneration strategies in the pipeline for glaucoma. J Ophthalmol. 2020;2020:9329310 https://doi.org/10.1155/2020/9329310.
Boia R, Ruzafa N, Aires ID, Pereiro X, Ambrósio AF, Vecino E, et al. Neuroprotective strategies for retinal ganglion cell degeneration: current status and challenges ahead. Int J Mol Sci. 2020;21:2262 https://doi.org/10.3390/ijms21072262.
Nowakowska Z. A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem. 2007;42:125–37. https://doi.org/10.1016/j.ejmech.2006.09.019.
Chu HW, Sethy B, Hsieh PW, Horng JT. Identification of potential drug targets of broad-spectrum inhibitors with a Michael acceptor moiety using shotgun proteomics. Viruses. 2021;13:1756 https://doi.org/10.3390/v13091756.
Constantinescu T, Lungu CN. Anticancer activity of natural and synthetic chalcones. Int J Mol Sci. 2021;22:11306 https://doi.org/10.3390/ijms222111306.
Salehi B, Quispe C, Chamkhi I, El Omari N, Balahbib A, Sharifi-Rad J, et al. Pharmacological properties of chalcones: A review of preclinical including molecular mechanisms and clinical evidence. Front Pharmcol. 2021;11:592654 https://doi.org/10.3389/fphar.2020.592654.
Zhuang C, Zhang W, Sheng C, Zhang W, Xing C, Miao Z. Chalcone: a privileged structure in medicinal chemistry. Chem Rev. 2017;117:7762–810. https://doi.org/10.1021/acs.chemrev.7b00020.
de Freitas Silva M, Pruccoli L, Morroni F, Sita G, Seghetti F, Viegas C, et al. The Keap1/Nrf2-ARE pathway as a pharmacological target for chalcones. Molecules. 2018;23:1803 https://doi.org/10.3390/molecules23071803.
Ur Rashid H, Xu Y, Ahmad N, Muhammad Y, Wang L. Promising anti-inflammatory effects of chalcones via inhibition of cyclooxygenase, prostaglandin E2, inducible NO synthase and nuclear factor κb activities. Bioorg Chem. 2019;87:335–65. https://doi.org/10.1016/j.bioorg.2019.03.033.
Adelusi TI, Akinbolaji GR, Yin X, Ayinde KS, Olaoba OT. Neurotrophic, anti-neuroinflammatory, and redox balance mechanisms of chalcones. Eur J Pharmcol. 2021;891:173695 https://doi.org/10.1016/j.ejphar.2020.173695.
Kontogiorgis C, Mantzanidou M, Hadjipavlou-Litina D. Chalcones and their potential role in inflammation. Mini Rev Med Chem. 2008;8:1224–42. https://doi.org/10.2174/138955708786141034.
Katsori AM, Hadjipavlou-Litina D. Recent progress in therapeutic applications of chalcones. Expert Opin Ther Pat. 2011;21:1575–96. https://doi.org/10.1517/13543776.2011.596529.
Zhou B, Xing C. Diverse molecular targets for chalcones with varied bioactivities. Med Chem. 2015;5:388–404. https://doi.org/10.4172/2161-0444.1000291.
Jasim HA, Nahar L, Jasim MA, Moore SA, Ritchie KJ, Sarker SD. Chalcones: synthetic chemistry follows where nature leads. Biomolecules. 2021;11:1203 https://doi.org/10.3390/biom11081203.
Dizdaroglu Y, Albay C, Arslan T, Ece A, Turkoglu EA, Efe A, et al. Design, synthesis and molecular modelling studies of some pyrazole derivatives as carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem. 2020;35:289–97. https://doi.org/10.1080/14756366.2019.1695791.
Tuğrak M, Yamalı C, Gül HI, Demir Y. Inhibitory effects of the chalcones towards carbonic anhydrase I, II and acetylcholinesterase enzymes. Erzincan Univ J Sci Technol. 2020;13:1138–46. https://doi.org/10.18185/erzifbed.748798.
Burmaoglu S, Kazancioglu EA, Kaya R, Kazancıoglu M, Karaman M, Algul O, et al. Synthesis of novel organohalogen chalcone derivatives and screening of their molecular docking study and some enzymes inhibition effects. J Mol Struct. 2020;1208:127868 https://doi.org/10.1016/j.molstruc.2020.127868.
Gürdere MB, Budak Y, Kocyigit UM, Taslimi P, Tüzün B, Ceylan M. ADME properties, bioactivity and molecular docking studies of 4-amino-chalcone derivatives: new analogues for the treatment of Alzheimer, glaucoma and epileptic diseases. Silico Pharmcol. 2021;9:34 https://doi.org/10.1007/s40203-021-00094-x.
Burmaoglu S, Kazancioglu EA, Kazancioglu MZ, Sağlamtaş R, Yalcin G, Gulcin I, et al. Synthesis, molecular docking and some metabolic enzyme inhibition properties of biphenyl-substituted chalcone derivatives. J Mol Struct. 2022;1254:132358 https://doi.org/10.1016/j.molstruc.2022.132358.
Yamali C, Gul H, Çakır T, Demir Y, Gülçin I. Aminoalkylated phenolic chalcones: Investigation of biological effects on acetylcholinesterase and carbonic anhydrase I and II as potential lead enzyme inhibitors. Lett Drug Des Discov. 2020;17:1283–92. https://doi.org/10.2174/1570180817999200520123510.
Arslan T, Türkoğlu EA, Şentürk M, Supuran CT. Synthesis and carbonic anhydrase inhibitory properties of novel chalcone substituted benzenesulfonamides. Bioorg Med Chem Lett. 2016;26:5867–70. https://doi.org/10.1016/j.bmcl.2016.11.017.
Bilginer S, Anil B, Koca M, Demir Y, Gülçin İ. Novel Mannich bases with strong carbonic anhydrases and acetylcholinesterase inhibition effects: 3-(aminomethyl)-6-{3-[4-(trifluoromethyl)phenyl]acryloyl}-2(3H)-benzoxazolones. Turk J Chem. 2021;45:805–18. https://doi.org/10.3906/kim-2101-25.
Kocyigit UM, Budak Y, Gürdere MB, Tekin Ş, Köprülü TK, Ertürk F, et al. Synthesis, characterization, anticancer, antimicrobial and carbonic anhydrase inhibition profiles of novel (3aR,4S,7R,7aS)-2-(4-((E)-3-(3-aryl)acryloyl) phenyl)-3a,4,7,7a-tetrahydro-1H-4,7-methanoisoindole-1,3(2H)-dione derivatives. Bioorg Chem. 2017;70:118–25. https://doi.org/10.1016/j.bioorg.2016.12.001.
Kocyigit UM, Budak Y, Eligüzel F, Taslimi P, Kılıç D, Gulçin İ, et al. Synthesis and carbonic anhydrase inhibition of tetrabromo chalcone derivatives. Arch Pharmcol. 2017;350:e1700198 https://doi.org/10.1002/ardp.201700198.
Singh P, Swain B, Thacker PS, Sigalapalli DK, Purnachander Yadav P, Angeli A, et al. Synthesis and carbonic anhydrase inhibition studies of sulfonamide based indole-1,2,3-triazole chalcone hybrids. Bioorg Chem. 2020;99:103839 https://doi.org/10.1016/j.bioorg.2020.103839.
Singh P, Purnachander Yadav P, Swain B, Thacker PS, Angeli A, Supuran CT, et al. Discovery of a novel series of indolylchalcone-benzenesulfonamide hybrids acting as selective carbonic anhydrase II inhibitors. Bioorg Chem. 2021;108:104647 https://doi.org/10.1016/j.bioorg.2021.104647.
Burmaoglu S, Yilmaz AO, Polat MF, Kaya R, Gulcin İ, Algul O. Synthesis and biological evaluation of novel tris-chalcones as potent carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase and α-glycosidase inhibitors. Bioorg Chem. 2019;85:191–7. https://doi.org/10.1016/j.bioorg.2018.12.035.
Burmaoglu S, Yilmaz AO, Polat MF, Kaya R, Gulcin İ, Algul O. Synthesis of novel tris-chalcones and determination of their inhibition profiles against some metabolic enzymes. Arch Physiol Biochem. 2021;127:153–61. https://doi.org/10.1080/13813455.2019.1623265.
SwissADME, http://www.swissadme.ch/. Accessed Jun 2022.
OSIRIS Property Explorer, http://www.organic-chemistry.org/prog/peo/. Accessed June 2022.
Stellenboom N. Comparison of the inhibitory potential towards carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase of chalcone and chalcone epoxide. J Biochem Mol Toxicol. 2019;33:e22240 https://doi.org/10.1002/jbt.22240.
Aslan HE, Demir Y, Özaslan MS, Türkan F, Beydemir Ş, Küfrevioğlu ÖI. The behavior of some chalcones on acetylcholinesterase and carbonic anhydrase activity. Drug Chem Toxicol. 2019;42:634–40. https://doi.org/10.1080/01480545.2018.1463242.
Bayrak Ç, Taslimi P, Gülçin İ, Menzek A. The first synthesis of 4-phenylbutenone derivative bromophenols including natural products and their inhibition profiles for carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase enzymes. Bioorg Chem. 2017;72:359–66. https://doi.org/10.1016/j.bioorg.2017.03.001.
Mahar J, Saeed A, Belfield KD, Ali Larik F, Ali Channar P, Ali Kazi M, et al. 1-(2-Hydroxy-5-((trimethylsilyl)ethynyl)phenyl)ethanone based α,β-unsaturated derivatives an alternate to non-sulfonamide carbonic anhydrase II inhibitors, synthesis via Sonogashira coupling, binding analysis, Lipinsk’s rule validation. Bioorg Chem. 2019;84:170–6. https://doi.org/10.1016/j.bioorg.2018.11.031.
Koçyiğit ÜM, Gezegen H, Taslimi P. Synthesis, characterization, and biological studies of chalcone derivatives containing Schiff bases: Synthetic derivatives for the treatment of epilepsy and Alzheimer’s disease. Arch Pharmcol. 2020;353:e2000202 https://doi.org/10.1002/ardp.202000202.
Kocyigit UM, Budak Y, Gürdere MB, Ertürk F, Yencilek B, Taslimi P, et al. Synthesis of chalcone-imide derivatives and investigation of their anticancer and antimicrobial activities, carbonic anhydrase and acetylcholinesterase enzymes inhibition profiles. Arch Physiol Biochem. 2018;124:61–8. https://doi.org/10.1080/13813455.2017.1360914.
Gençer N, Bilen Ç, Demir D, Atahan A, Ceylan M, Küçükislamoğlu M. In vitro inhibition effect of some chalcones on erythrocyte carbonic anhydrase I and II. Artif Cells Nanomed Biotechnol. 2013;41:384–8. https://doi.org/10.3109/21691401.2012.761226.
Bilginer S, Gul HI, Erdal FS, Sakagami H, Levent S, Gulcin I, et al. Synthesis, cytotoxicities, and carbonic anhydrase inhibition potential of 6-(3-aryl-2-propenoyl)-2(3H)-benzoxazolones. J Enzyme Inhib Med Chem. 2019;34:1722–9. https://doi.org/10.1080/14756366.2019.1670657.
Bilginer S, Gul HI, Erdal FS, Sakagami H, Gulcin I. New halogenated chalcones with cytotoxic and carbonic anhydrase inhibitory properties: 6-(3-Halogenated phenyl-2-propen-1-oyl)-2(3H)-benzoxazolones. Arch Pharmcol. 2020;353:e1900384 https://doi.org/10.1002/ardp.201900384.
Kuday H, Sonmez F, Bilen C, Yavuz E, Gençer N, Kucukislamoglu M. Synthesis and in vitro inhibition effect of new pyrido[2,3-d]pyrimidine derivatives on erythrocyte carbonic anhydrase I and II. Biomed Res Int. 2014;2014:594879 https://doi.org/10.1155/2014/594879.
Peerzada MN, Khan P, Ahmad K, Hassan MI, Azam A. Synthesis, characterization and biological evaluation of tertiary sulfonamide derivatives of pyridyl-indole based heteroaryl chalcone as potential carbonic anhydrase IX inhibitors and anticancer agents. Eur J Med Chem. 2018;155:13–23. https://doi.org/10.1016/j.ejmech.2018.05.034.
Arslan T, Çelik G, Çelik H, Şentürk M, Yaylı N, Ekinci D. Synthesis and biological evaluation of novel bischalcone derivatives as carbonic anhydrase inhibitors. Arch Pharmcol. 2016;349:741–8. https://doi.org/10.1002/ardp.201600122.
Tutar U, Koçyiğit ÜM, Gezegen H. Evaluation of antimicrobial, antibiofilm and carbonic anhydrase inhibition profiles of 1,3-bis-chalcone derivatives. J Biochem Mol Toxicol. 2019;33:e22281 https://doi.org/10.1002/jbt.22281.
Özen F, Günel A, Baran A. DNA-binding, enzyme inhibition, and photochemical properties of chalcone-containing metallophthalocyanine compounds. Bioorg Chem. 2018;81:71–8. https://doi.org/10.1016/j.bioorg.2018.08.002.
Arslan T. Design, synthesis of novel peripherally tetra-chalcone substituted phthalocyanines and their inhibitory effects on acetylcholinesterase andcarbonic anhydrases (hCA I and II). J Organomet Chem. 2021;951:122021. https://doi.org/10.1016/j.jorganchem.2021.122021.
Supuran CT. Carbon- versus sulphur-based zinc binding groups for carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem. 2018;33:485–95. https://doi.org/10.1080/14756366.2018.1428572.
Bonardi A, Nocentini A, Bua S, Combs J, Lomelino C, Andring J, et al. Sulfonamide inhibitors of human carbonic anhydrases designed through a three-tails approach: improving ligand/isoform matching and selectivity of action. J Med Chem. 2020;63:7422–44. https://doi.org/10.1021/acs.jmedchem.0c00733.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. https://doi.org/10.1016/s0169-409x(00)00129-0.
Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45:2615–23. https://doi.org/10.1021/jm020017n.
https://www.glaucomaassociates.com/glaucoma/types-of-glaucoma/ Accessed 26 Jul 2022.
Mincione F, Scozzafava A, Supuran CT. The development of topically acting carbonic anhydrase inhibitors as antiglaucoma agents. Curr Pharmcol Des. 2008;14:649–54. https://doi.org/10.2174/138161208783877866.
Funding
The work was funded by the Ministry of Science and Technological Development of Serbia (Project 451-03-68/2022-14/200113) and Faculty of Medicine, University of Niš Internal project No. 40.
Author information
Authors and Affiliations
Contributions
All authors contributed to each stage of the manuscript preparation. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Authors know of no conflict of interest associated with this publication and there has been no financial support of this work that could have influenced its outcome.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gocić, V., Marković, A. & Lazarević, J. The potential of chalcone derivatives as human carbonic anhydrase inhibitors in the therapy of glaucoma. Med Chem Res 31, 2103–2118 (2022). https://doi.org/10.1007/s00044-022-02978-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02978-9