Abstract
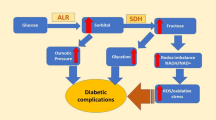
In this work, isatin was employed as the scaffold to design aldose reductase inhibitors with antioxidant activity. Most of the isatin derivatives were proved to be excellent in the inhibition of aldose reductase (ALR2) with IC50 values at submicromolar level, and (E)-2-(5-(4-methoxystyryl)-2,3-dioxoindolin-1-yl) acetic acid (9g) was identified as the most effective with an IC50 value of 0.015 μM. Moreover, compounds 9a–h with styryl side chains at the C5 position of isatin showed potent antioxidant activity. Particularly, the phenolic compound 9h demonstrated similar antioxidant activity with the well-known antioxidant Trolox. Structure-activity relationship and molecular docking studies showed that the acetic acid group at N1 and C5 p-hydroxystyryl side chain were the key structures to increase the aldose reductase inhibitory activity and antioxidant activity.






Similar content being viewed by others
References
Hao X, Han Z, Li Y, Li C, Wang X, Zhang X, et al. Synthesis and structure–activity relationship studies of phenolic hydroxyl derivatives based on quinoxalinone as aldose reductase inhibitors with antioxidant activity. Bioorg Med Chem Lett. 2017;27:887–92
Maccari R, Ottanà R. Targeting aldose reductase for the treatment of diabetes complications and inflammatory diseases: new insights and future directions. J Med Chem. 2015;58:2047–67
Alexiou P, Pegklidou K, Chatzopoulou M, Nicolaou I, Demopoulos VJ. Aldose reductase enzyme and its implication to major health problems of the 21st century. Curr Med Chem. 2009;16:734–52
Chen H, Zhang X, Zhang X, Fan Z, Liu W, Lei Y, et al. Dihydrobenzoxazinone derivatives as aldose reductase inhibitors with antioxidant activity. Bioorg Med Chem. 2020;28:115699
Quattrini L, La Motta C. Aldose reductase inhibitors: 2013-present. Expert Opin Ther Pat. 2019;29:199–213
Kador PF. The role of aldose reductase in the development of diabetic complications. Med Res Rev. 1988;8:325–52
La Motta C, Sartini S, Mugnaini L, Simorini F, Taliani S, Salerno S, et al. Pyrido[1,2-a]pyrimidin-4-one derivatives as a novel class of selective aldose reductase inhibitors exhibiting antioxidant activity. J Med Chem. 2007;50:4917–27
Han Z, Hao X, Ma B, Zhu C. A series of pyrido[2,3-b]pyrazin-3(4H)-one derivatives as aldose reductase inhibitors with antioxidant activity. Eur J Med Chem. 2016;121:308–17
Da Settimo F, Primofiore G, La Motta C, Sartini S, Taliani S, Simorini F, et al. Naphtho [1, 2-d] isothiazole acetic acid derivatives as a novel class of selective aldose reductase inhibitors. J Med Chem. 2005;48:6897–907
Maccari R, Vitale RM, Ottanà R, Rocchicciol M, Marrazzo A, Cardile V, et al. Structure-activity relationships and molecular modelling of new 5-arylidene-4-thiazolidinone derivatives as aldose reductase inhibitors and potential anti-inflammatory agents. Eur J Med Chem. 2014;81:1–14
Wiernsperger NF. Oxidative stress as a therapeutic target in diabetes: revisiting the controversy. Diabetes Metab. 2003;29:579–85
Farooqui AA. Inflammation and oxidative stress in neurological disorders. Springer International Publishing, 2014
Tang WH, Martin KA, Hwa J. Aldose reductase, oxidative stress, and diabetic mellitus. Front Pharm. 2012;3:87
Drel VR, Pacher P, Ali TK, Shin J, Julius U, El-Remessy AB, et al. Aldose reductase inhibitor fidarestat counteracts diabetes-associated cataract formation, retinal oxidative-nitrosative stress, glial activation, and apoptosis. Int J Mol Med. 2008;21:667–76
Drel VR, Pacher P, Stevens MJ, Obrosova IG. Aldose reductase inhibition counteracts nitrosative stress and poly (ADP-ribose) polymerase activation in diabetic rat kidney and high-glucose-exposed human mesangial cells. Free Radic Biol Med. 2006;40:1454–65
Ji Y, Chen X, Chen H, Zhang X, Fan Z, Xie L, et al. Designing of acyl sulphonamide based quinoxalinones as multifunctional aldose reductase inhibitors. Bioorg Med Chem. 2019;27:1658–69
Hamada Y, Araki N, Horiuchi S, Hotta N. Role of polyol pathway in nonenzymatic glycation. Nephrol, Dial, Transpl. 1996;11:95–98
Chung SSM, Chung SK. Genetic analysis of aldose reductase in diabetic complications. Curr Med Chem. 2003;10:1375–87
Van Zandt MC, Jones ML, Gunn DE, Geraci LS, Jones JH, Sawicki DR, et al. Discovery of 3-[(4, 5, 7-trifluorobenzothiazol-2-yl) methyl] indole-N-acetic acid (lidorestat) and congeners as highly potent and selective inhibitors of aldose reductase for treatment of chronic diabetic complications. J Med Chem. 2005;48:3141–52
Mylari BL, Armento SJ, Beebe DA, Conn EL, Coutcher JB, Dina MS, et al. A highly selective, non-hydantoin, non-carboxylic acid inhibitor of aldose reductase with potent oral activity in diabetic rat models: 6-(5-chloro-3-methylbenzofuran-2-sulfonyl)-2-H-pyridazin-3-one. J Med Chem. 2003;46:2283–6
Steele JW, Faulds D, Goa KL. Epalrestat. Drugs Aging. 1993;3:532–55
Maccari R, Ottanà R. 2, 4-Thiazolidinedione and 2-thioxo-4-thiazolidinone derivatives as aldose reductase inhibitors in the search for drugs to prevent long-term diabetes complications. Adv Mol Mech Pharmacol Diabetic Complications. 2010;219–45
Qin X, Hao X, Han H, Zhu S, Yang Y, Wu B, et al. Design and synthesis of potent and multifunctional aldose reductase inhibitors based on quinoxalinones. J Med Chem. 2015;58:1254–67
Barski OA, Gabbay KH, Grimshaw CE, Bohren KM. Mechanism of human aldehyde reductase: characterization of the active site pocket. Biochemistry. 1995;34:11264–75
Wang J, Yun D, Yao J, Fu W, Huang F, Chen L, et al. Design, synthesis and QSAR study of novel isatin analogues inspired michael acceptor as potential anticancer compounds. Eur J Med Chem. 2018;144:493–503
Zhang Q, Teng Y, Yuan Y, Ruan T, Wang Q, Gao X, et al. Synthesis and cytotoxic studies of novel 5-phenylisatin derivatives and their anti-migration and anti-angiogenic evaluation. Eur J Med Chem. 2018;156:800–14
Andreani A, Burnelli S, Granaiola M, Leoni A, Locatelli A, Morigi R, et al. New isatin derivatives with antioxidant activity. Eur J Med Chem. 2010;45:1374–8
Zuo A-R, Dong H-H, Yu Y-Y, Shu Q-L, Zheng L-X, Yu X-Y, et al. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin Med. 2018;13:1–12
Wang C, White A, Schwartz S, Alluri S, Cattabiani T, Zhang L, et al. Novel synthesis and functionalization of ortho–ortho disubstituted biphenyls and a highly condensed novel heterocycle using radical cyclization reaction. Tetrahedron. 2012;68:9750–62
Joule JA. Thiophenes from viktor meyer to poly (thiophene) some reactions and synthesis. Phosphorus Sulfur Silicon Relat Elem. 2013;188:287–316
Zhou N, Polozov AM, O’Connell M, Burgeson J, Yu P, Zeller W, et al. 1,7-Disubstituted oxyindoles are potent and selective EP 3 receptor antagonists. Bioorg Med Chem Lett. 2010;20:2658–64
El‐Kabbani O, Ramsland P, Darmanin C, Chung RPT, Podjarny A. Structure of human aldose reductase holoenzyme in complex with Statil: An approach to structure‐based inhibitor design of the enzyme. Proteins: Struct, Funct, Bioinf. 2003;50:230–8
Hayman S, Kinoshita JH. Isolation and properties of lens aldose reductase. J Biol Chem. 1965;240:877–82
Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958;181:1199–200
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8
Liu L, Liu Y, Cui J, Liu H, Liu Y-B, Qiao W-L, et al. Oxidative stress induces gastric submucosal arteriolar dysfunction in the elderly. World J Gastroenterol. 2013;19:9439–46
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 21572021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, W., Chen, H., Zhang, X. et al. Isatin derivatives as a new class of aldose reductase inhibitors with antioxidant activity. Med Chem Res 30, 1588–1602 (2021). https://doi.org/10.1007/s00044-021-02751-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02751-4