Abstract
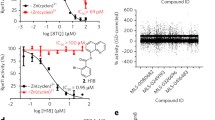
In recent years, the human immunoproteasome has emerged as an attractive therapeutic target for various diseases, leading to a growing interest in the discovery of immunoproteasome inhibitors that selectively target specific subunits. Herein we report the design, synthesis, and evaluation of a new immunoproteasome inhibitor that feature a macrocyclic ring containing an internal α-ketoamide warhead. This compound is a selective and reversible inhibitor of immunoproteasome subunits β1i and β5i and shows essentially no inhibition of constitutive proteasome subunits.






Similar content being viewed by others
Abbreviations
- UPS:
-
ubiquitin-proteasome system;
- CP:
-
core particle;
- RP:
-
regulatory particle;
- LMP2:
-
low-molecular mass protein-2;
- MECL1:
-
multicatalytic endopeptidase complex-like 1;
- LMP7:
-
low-molecular mass protein-7;
- DMP:
-
Dess-Martin periodinane;
- HBTU:
-
N,N,N′,N′-tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate;
- IIDQ:
-
2-isobutoxy-1-isobutoxycarbonyl-1,2-dihydroquin-oline;
- NOE:
-
nuclear overhauser effect;
- EDC:
-
N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide.
References
Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82(2):373–428. https://doi.org/10.1152/physrev.00027.2001
Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem. 2009;78:477–513. https://doi.org/10.1146/annurev.biochem.78.081507.101607
Ebstein F, Kloetzel PM, Kruger E, Seifert U. Emerging roles of immunoproteasomes beyond MHC class I antigen processing. Cell Mol Life Sci. 2012;69(15):2543–58. https://doi.org/10.1007/s00018-012-0938-0
Groettrup M, Khan S, Schwarz K, Schmidtke G. Interferon-gamma inducible exchanges of 20S proteasome active site subunits: why? Biochimie. 2001;83(3–4):367–72
Manasanch EE, Orlowski RZ. Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol. 2017;14(7):417–33. https://doi.org/10.1038/nrclinonc.2016.206
Basler M, Mundt S, Bitzer A, Schmidt C, Groettrup M. The immunoproteasome: a novel drug target for autoimmune diseases. Clin Exp Rheumatol. 2015;33(4 Suppl 92):S74–9
Kaur G, Batra S. Emerging role of immunoproteasomes in pathophysiology. Immunol Cell Biol. 2016;94(9):812–20. https://doi.org/10.1038/icb.2016.50
Ettari R, Previti S, Bitto A, Grasso S, Zappala M. Immunoproteasome-Selective Inhibitors: a Promising Strategy to Treat Hematologic Malignancies, Autoimmune and Inflammatory Diseases. Curr Med Chem. 2016;23(12):1217–38
Kisselev AF, Groettrup M. Subunit specific inhibitors of proteasomes and their potential for immunomodulation. Curr Opin Chem Biol. 2014;23:16–22. https://doi.org/10.1016/j.cbpa.2014.08.012
Huber EM, Basler M, Schwab R, Heinemeyer W, Kirk CJ, Groettrup M, et al. Immuno- and constitutive proteasome crystal structures reveal differences in substrate and inhibitor specificity. Cell. 2012;148(4):727–38. https://doi.org/10.1016/j.cell.2011.12.030
de Bruin G, Huber EM, Xin BT, van Rooden EJ, Al-Ayed K, Kim KB, et al. Structure-based design of beta1i or beta5i specific inhibitors of human immunoproteasomes. J Med Chem. 2014;57(14):6197–209. https://doi.org/10.1021/jm500716s
Bakas NA, Schultz CR, Yco LP, Roberts CC, Chang CA, Bachmann AS, et al. Immunoproteasome inhibition and bioactivity of thiasyrbactins. Bioorg Med Chem. 2018;26(2):401–12. https://doi.org/10.1016/j.bmc.2017.11.048
Dubiella C, Baur R, Cui H, Huber EM, Groll M. Selective Inhibition of the Immunoproteasome by Structure-Based Targeting of a Non-catalytic Cysteine. Angew Chem Int Ed Engl. 2015;54(52):15888–91. https://doi.org/10.1002/anie.201506631
Singh PK, Fan H, Jiang X, Shi L, Nathan CF, Lin G. Immunoproteasome beta5i-Selective Dipeptidomimetic Inhibitors. ChemMedChem. 2016;11(19):2127–31. https://doi.org/10.1002/cmdc.201600384
Fan H, Angelo NG, Warren JD, Nathan CF, Lin G. Oxathiazolones Selectively Inhibit the Human Immunoproteasome over the Constitutive Proteasome. ACS Med Chem Letters. 2014;5(4):405–10. https://doi.org/10.1021/ml400531d
Sosic I, Gobec M, Brus B, Knez D, Zivec M, Konc J, et al. Nonpeptidic Selective Inhibitors of the Chymotrypsin-Like (beta5 i) Subunit of the Immunoproteasome. Angew Chem Int Ed Engl. 2016;55(19):5745–8. https://doi.org/10.1002/anie.201600190
Ho YK, Bargagna-Mohan P, Wehenkel M, Mohan R, Kim KB. LMP2-specific inhibitors: chemical genetic tools for proteasome biology. Chem Biol. 2007;14(4):419–30. https://doi.org/10.1016/j.chembiol.2007.03.008
Kuhn DJ, Hunsucker SA, Chen Q, Voorhees PM, Orlowski M, Orlowski RZ. Targeted inhibition of the immunoproteasome is a potent strategy against models of multiple myeloma that overcomes resistance to conventional drugs and nonspecific proteasome inhibitors. Blood. 2009;113(19):4667–76. https://doi.org/10.1182/blood-2008-07-171637
Basler M, Lauer C, Moebius J, Weber R, Przybylski M, Kisselev AF, et al. Why the structure but not the activity of the immunoproteasome subunit low molecular mass polypeptide 2 rescues antigen presentation. J Immunol. 2012;189(4):1868–77. https://doi.org/10.4049/jimmunol.1103592
Huber EM, de Bruin G, Heinemeyer W, Paniagua Soriano G, Overkleeft HS, Groll M. Systematic Analyses of Substrate Preferences of 20S Proteasomes Using Peptidic Epoxyketone Inhibitors. J Am Chem Soc. 2015;137(24):7835–42. https://doi.org/10.1021/jacs.5b03688
Johnson HWB, Anderl JL, Bradley EK, Bui J, Jones J, Arastu-Kapur S, et al. Discovery of Highly Selective Inhibitors of the Immunoproteasome Low Molecular Mass Polypeptide 2 (LMP2) Subunit. ACS Med Chem Letters. 2017;8(4):413–7. https://doi.org/10.1021/acsmedchemlett.6b00496
Schrader J, Henneberg F, Mata RA, Tittmann K, Schneider TR, Stark H, et al. The inhibition mechanism of human 20S proteasomes enables next-generation inhibitor design. Science. 2016;353(6299):594–8. https://doi.org/10.1126/science.aaf8993
Groll M, Kim KB, Kairies N, Huber R, Crews CM. Crystal structure of epoxomicin: 20S proteasome reveals a molecular basis for selectivity of alpha ‘,beta ‘-epoxyketone proteasome inhibitors. J Am Chem Soc. 2000;122(6):1237–8. https://doi.org/10.1021/ja993588m
Wasserman HH, Ho WB. (Cyanomethylene)Phosphoranes as Novel Carbonyl 1,1-Dipole Synthons - an Efficient Synthesis of Alpha-Keto Acids, Esters, and Amides. J Org Chem. 1994;59(16):4364–6. https://doi.org/10.1021/jo00095a005
Groll M, Schellenberg B, Bachmann AS, Archer CR, Huber R, Powell TK, et al. A plant pathogen virulence factor inhibits the eukaryotic proteasome by a novel mechanism. Nature. 2008;452(7188):755–8. https://doi.org/10.1038/nature06782
Krahn D, Ottmann C, Kaiser M. The chemistry and biology of syringolins, glidobactins and cepafungins (syrbactins). Nat Prod Rep. 2011;28(11):1854–67. https://doi.org/10.1039/C1NP00048A
Clerc J, Groll M, Illich DJ, Bachmann AS, Huber R, Schellenberg B, et al. Synthetic and structural studies on syringolin A and B reveal critical determinants of selectivity and potency of proteasome inhibition. Proc Natl Acad Sci USA. 2009;106(16):6507–12. https://doi.org/10.1073/pnas.0901982106
Pirrung MC, Biswas G, Ibarra-Rivera TR. Total synthesis of syringolin A and B. Org Lett. 2010;12(10):2402–5. https://doi.org/10.1021/ol100761z
Dai C, Stephenson CR. Total synthesis of syringolin A. Org Lett. 2010;12(15):3453–5. https://doi.org/10.1021/ol101252y
Chiba T, Hosono H, Nakagawa K, Asaka M, Takeda H, Matsuda A, et al. Total synthesis of syringolin A and improvement of its biological activity. Angew Chem Int Ed Engl. 2014;53(19):4836–9. https://doi.org/10.1002/anie.201402428
Bachmann AS, Opoku-Ansah J, Ibarra-Rivera TR, Yco LP, Ambadi S, Roberts CC, et al. Syrbactin Structural Analog TIR-199 Blocks Proteasome Activity and Induces Tumor Cell Death. J Biol Chem. 2016;291(16):8350–62. https://doi.org/10.1074/jbc.M115.710053
Chiba T, Matsuda A, Ichikawa S. Structure-activity relationship study of syringolin A as a potential anticancer agent. Bioorg Med Chem Lett. 2015;25(21):4872–7. https://doi.org/10.1016/j.bmcl.2015.06.015
Totaro KA, Barthelme D, Simpson PT, Sauer RT, Sello JK. Substrate-guided optimization of the syringolins yields potent proteasome inhibitors with activity against leukemia cell lines. Bioorg Med Chem. 2015;23(18):6218–22. https://doi.org/10.1016/j.bmc.2015.07.041
Archer CR, Groll M, Stein ML, Schellenberg B, Clerc J, Kaiser M, et al. Activity enhancement of the synthetic syrbactin proteasome inhibitor hybrid and biological evaluation in tumor cells. Biochemistry (Mosc). 2012;51(34):6880–8. https://doi.org/10.1021/bi300841r
Beck P, Dubiella C, Groll M. Covalent and non-covalent reversible proteasome inhibition. Biol Chem. 2012;393(10):1101–20. https://doi.org/10.1515/hsz-2012-0212
Stein ML, Cui H, Beck P, Dubiella C, Voss C, Kruger A, et al. Systematic comparison of peptidic proteasome inhibitors highlights the alpha-ketoamide electrophile as an auspicious reversible lead motif. Angew Chem Int Ed Engl. 2014;53(6):1679–83. https://doi.org/10.1002/anie.201308984
Voss C, Scholz C, Knorr S, Beck P, Stein ML, Zall A, et al. alpha-Keto phenylamides as P1’-extended proteasome inhibitors. ChemMedChem. 2014;9(11):2557–64. https://doi.org/10.1002/cmdc.201402244
Braun HA, Umbreen S, Groll M, Kuckelkorn U, Mlynarczuk I, Wigand ME, et al. Tripeptide mimetics inhibit the 20 S proteasome by covalent bonding to the active threonines. J Biol Chem. 2005;280(31):28394–401. https://doi.org/10.1074/jbc.M502453200
Naggie S, Patel K, McHutchison J. Hepatitis C virus directly acting antivirals: current developments with NS3/4A HCV serine protease inhibitors. J Antimicrob Chemother. 2010;65(10):2063–9. https://doi.org/10.1093/jac/dkq284
De Risi C, Pollini GP, Zanirato V. Recent Developments in General Methodologies for the Synthesis of alpha-Ketoamides. Chem Rev. 2016;116(5):3241–305. https://doi.org/10.1021/acs.chemrev.5b00443
Juhl K, Jorgensen KA. Catalytic asymmetric direct alpha-amination reactions of 2-keto esters: a simple synthetic approach to optically active syn-beta-amino-alpha-hydroxy esters. J Am Chem Soc. 2002;124(11):2420–1
Shu C, Zeng X, Hao MH, Wei X, Yee NK, Busacca CA, et al. RCM macrocyclization made practical: an efficient synthesis of HCV protease inhibitor BILN 2061. Org Lett. 2008;10(6):1303–6. https://doi.org/10.1021/ol800183x
Copeland RA. Evaluation of Enzyme Inhibitors in Drug Discovery: a Guide for Medicinal Chemists and Pharmacologists. 2nd ed. Hoboken, New Jersey: John Wiley & Sons; 2013. Chapter 5
Acknowledgements
This work was supported by the Center for Drug Design at the University of Minnesota. We thank Prof Rodney Johnson on the use of the ozone generator in his laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This paper is dedicated to Professor Robert Vince on the occasion of his 80th birthday.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ding, R., Wilson, D.J. & Chen, L. Synthesis of macrocyclic α-ketoamide as a selective and reversible immunoproteasome inhibitor. Med Chem Res 30, 410–420 (2021). https://doi.org/10.1007/s00044-020-02678-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02678-2