Abstract
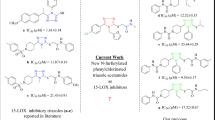
It is well established that cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) play a vital role in the initiation and progression of inflammatory reactions. Hence, thiazole and thiazolidene-based pharmacophore molecules were synthesized to obtain dual COX-2 and 5-LOX inhibitory activity. The synthesis of target compounds has been achieved by a novel green strategy. In vitro COX-1, COX-2, and 5-LOX evaluation of these molecules have shown the potential for an improved anti-inflammatory profile. Most promising compound among the series (2-(diphenylamino)-4-(4-nitrophenyl)thiazol-5-yl)(naphthalen-1-yl)methanone 7h (IC50 = 0.07 ± 0.02 μM) showed equivalent COX-2 inhibitory potency as that of positive control etoricoxib (IC50 = 0.07 ± 0.01 μM) and an enhanced selectivity index of 115.14. Compound 7h exhibited 5-LOX IC50 of 0.29 ± 0.09 μM and reference drug zileuton showed IC50 of 0.15 ± 0.05 μM. In vivo studies of 7h including carrageenan-induced paw edema assay (63% inhibition of paw edema), antiulcer studies, biochemical assays, qRT-PCR analysis, and anticancer studies indicated that the present study has identified a good lead compound for the development of a potential anti-inflammatory drug having improved gastric safety profile.
















Similar content being viewed by others
References
Haeggström JZ, Rinaldo-Matthis A, Wheelock CE, Wetterholm A. Advances in eicosanoid research, novel therapeutic implications. Biochem Biophys Res Commun. 2010;396:135–139. https://doi.org/10.1016/j.bbrc.2010.03.140.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;54:436. https://doi.org/10.1038/nature07205.
Manju SL, Ethiraj KR, Elias G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: a structure-based approach. Eur J Pharm Sci. 2018;121:356–381. https://doi.org/10.1016/j.ejps.2018.06.003.
Singh P, Kaur J, Singh G, Bhatti R. Triblock conjugates: identification of a highly potent antiinflammatory agent. J Med Chem. 2015;58:5989–6001. https://doi.org/10.1021/acs.jmedchem.5b00952.
Ding X, Zhu C, Qiang H, Zhou X, Zhou G. Enhancing antitumor effects in pancreatic cancer cells by combined use of COX-2 and 5-LOX inhibitors. Biomed Pharmacother. 2011;65:486–490. https://doi.org/10.1016/j.biopha.2011.06.009.
Chang J, Tang N, Fang Q, Zhu K, Liu L, Xiong X, et al. Inhibition of COX-2 and 5-LOX regulates the progression of colorectal cancer by promoting PTEN and suppressing PI3K/AKT pathway. Biochem Biophys Res Commun. 2019;517:1–7. https://doi.org/10.1016/j.bbrc.2018.01.061.
Harris RE, Beebe-Donk J, Alshafie GA. Cancer chemoprevention by cyclooxygenase 2 (COX-2) blockade. In: Inflammation in the pathogenesis of chronic diseases, Subcellular Biochemistry. vol 42. Springer, Dordrecht. 2007. pp. 193–212. https://doi.org/10.1007/1-4020-5688-5_9.
Omar YM, Abdel-Moty SG, Abdu-Allah HH. Further insight into the dual COX-2 and 15-LOX anti-inflammatory activity of 1, 3, 4-thiadiazole-thiazolidinone hybrids: the contribution of the substituents at 5th positions is size dependent. Bioorg Chem. 2020;97:103657. https://doi.org/10.1016/j.bioorg.2020.103657.
de Souza MVN. Synthesis and biological activity of natural thiazoles: an important class of heterocyclic compounds. J Sulphur Chem. 2005;26:429–449. https://doi.org/10.1080/17415990500322792.
Kouatly O, Geronikaki A, Kamoutsis C, Hadjipavlou-Litina D, Eleftheriou P. Adamantane derivatives of thiazolyl-N-substituted amide, as possible non-steroidal anti-inflammatory agents. Eur J Med Chem. 2009;44:1198–1204. https://doi.org/10.1016/j.ejmech.2008.05.029.
T Chhabria M, Patel S, Modi P, S Brahmkshatriya P. Thiazole: a review on chemistry, synthesis and therapeutic importance of its derivatives. Curr Top Med Chem. 2016;16:2841–2862.
Hsu A, Granneman GR, Bertz RJ. Ritonavir. Clin Pharmacokinet. 1998;35:275–291. https://doi.org/10.2165/00003088-199835040-00002.
Gündüz MG, Tahir MN, Armaković S, Koçak CÖ, Armaković SJ. Design, synthesis and computational analysis of novel acridine-(sulfadiazine/sulfathiazole) hybrids as antibacterial agents. J Mol Struct. 2019;1186:39–49. https://doi.org/10.1016/j.molstruc.2019.03.010.
Borelli C, Schaller M, Niewerth M, Nocker K, Baasner B, Berg D, et al. Modes of action of the new arylguanidine abafungin beyond interference with ergosterol biosynthesis and in vitro activity against medically important fungi. Chemotherapy. 2008;54:245–259. https://doi.org/10.1159/000142334.
Noble S, Balfour J. Meloxicam. Drugs. 1996;51:424–430.
Hubble JP, Koller WC, Cutler NR, Sramek JJ, Friedman J, Goetz C, et al. Pramipexole in patients with early Parkinson’s disease. Clin Neuropharmacol. 1995;18:338–347. https://doi.org/10.1097/00002826-199508000-00006.
O’Dwyer PJ, Shoemaker DD, Jayaram HN, Johns DG, Cooney DA, Marsoni S, et al. Tiazofurin: a new antitumor agent. Invest New Drugs. 1984;2:79–84. https://doi.org/10.1007/BF00173791.
Stubbe J, Kozarich JW. Mechanisms of bleomycin-induced DNA degradation. Chem Rev. 1987;87:1107–1136. https://doi.org/10.1021/cr00081a011.
Liaras K, Fesatidou M, Geronikaki A. Thiazoles and thiazolidinones as COX/LOX inhibitors. Molecules. 2018;23:685. https://doi.org/10.3390/molecules23030685.
Woods KW, McCroskey RW, Michaelides MR, Wada CK, Hulkower KI, Bell RL. Thiazole analogues of the NSAID indomethacin as selective COX-2 inhibitors. Bioorg Med Chem Lett. 2001;11:1325–1328. https://doi.org/10.1016/S0960-894X(01)00212-8.
Abdelall EK, Kamel GM. Synthesis of new thiazolo-celecoxib analogues as dual cyclooxygenase-2/15-lipoxygenase inhibitors: determination of regio-specific different pyrazole cyclization by 2D NMR. Eur J Med Chem. 2016;118:250–258. https://doi.org/10.1016/j.ejmech.2016.04.049.
Afifi OS, Shaaban OG, El Razik HAA, El SE-DAS, Ashour FA, El-Tombary AA, Abu-Serie MM. Synthesis and biological evaluation of purine-pyrazole hybrids incorporating thiazole, thiazolidinone or rhodanine moiety as 15-LOX inhibitors endowed with anticancer and antioxidant potential. Bioorg Chem. 2019;87:821–837. https://doi.org/10.1016/j.bioorg.2019.03.076.
Sinha S, Sravanthi T, Yuvaraj S, Manju S, Doble M. 2-Amino-4-aryl thiazole: a promising scaffold identified as a potent 5-LOX inhibitor. RSC Adv. 2016;6:19271–19279. https://doi.org/10.1039/C5RA28187C.
Sinha S, Doble M, Manju S. Design, synthesis and identification of novel substituted 2-amino thiazole analogues as potential anti-inflammatory agents targeting 5-lipoxygenase. Eur J Med Chem. 2018;158:34–50. https://doi.org/10.1016/j.ejmech.2018.08.098.
Sinha S, Manju S, Doble M. Chalcone-thiazole hybrids: rational design, synthesis and lead identification against 5-lipoxygenase. ACS Med Chem Lett. 2019;10:1415–1422. https://doi.org/10.1021/acsmedchemlett.9b00193.
Jacob PJ, Manju SL. Identification and development of thiazole leads as COX-2/5-LOX inhibitors through in-vitro and in-vivo biological evaluation for anti-inflammatory activity. Bioorg Chem. 2020;100:103882. https://doi.org/10.1016/j.bioorg.2020.103882.
Zhang P, Ye D, Chu Y. An efficient one-pot procedure for the synthesis of 1, 5-benzothiazepinones catalyzed by tetrabutylammonium fluoride (TBAF). Tetrahedron Lett. 2016;57:3743–3745. https://doi.org/10.1016/j.tetlet.2016.07.012.
Bramley SE, Dupplin V, Goberdhan DG, Meakins GD. The Hantzsch thiazole synthesis under acidic conditions: change of regioselectivity. J Chem Soc [Perkin Trans 1]. 1987;1:639–643. https://doi.org/10.1039/P19870000639.
Brindley JC, Caldwell JM, Meakins GD, Plackett SJ, Price SJ. N′-substituted N-acyl-and N-imidoyl-thioureas: preparation and conversion of N′, N′-disubstituted compounds into 2-(N, N-disubstituted amino) thiazol-5-yl ketones. J Chem Soc [Perkin Trans 1]. 1987;1:1153–1158. https://doi.org/10.1039/P19870001153.
Pan Y, Li H, Zheng S, Zhang B, Deng Z-y. Implication of the significance of dietary compatibility: based on the antioxidant and anti-inflammatory interactions with different ratios of hydrophilic and lipophilic antioxidants among four daily agricultural crops. J Agr Food Chem. 2018;66:7461–7474. https://doi.org/10.1021/acs.jafc.8b01690.
Singh P, Prasher P, Dhillon P, Bhatti R. Indole based peptidomimetics as anti-inflammatory and anti-hyperalgesic agents: dual inhibition of 5-LOX and COX-2 enzymes. Eur J Med Chem. 2015;97:104–123. https://doi.org/10.1016/j.ejmech.2015.04.044.
Xu G-L, Liu F, Ao G-Z, He S-Y, Ju M, Zhao Y, Xue T. Anti-inflammatory effects and gastrointestinal safety of NNU-hdpa, a novel dual COX/5-LOX inhibitor. Eur J Pharmacol. 2009;611:100–106. https://doi.org/10.1016/j.ejphar.2009.03.062.
Abdelall EK. Synthesis and biological evaluations of novel isoxazoles and furoxan derivative as anti-inflammatory agents. Bioorg Chem. 2020;94:103441. https://doi.org/10.1016/j.bioorg.2019.103441.
Abdelrahman MH, Youssif BG, Abdelazeem AH, Ibrahim HM, Abd El Ghany AM, Treamblu L, Bukhari SNA. Synthesis, Biological evaluation, docking study and ulcerogenicity profiling of some novel quinoline-2-carboxamides as dual COXs/LOX inhibitors endowed with anti-inflammatory activity. Eur J Med Chem. 2017;127:972–985. https://doi.org/10.1016/j.ejmech.2016.11.006.
Ganguly A, Bhatnagar O. Effect of bilateral adrenalectomy on production of restraint ulcers in the stomach of albino rats. Can J Physiol Pharm. 1973;51:748–750. https://doi.org/10.1139/y73-113.
Ganguly A. A method for quantitative assessment of experimentally produced ulcers in the stomach of albino rats. Experientia. 1969;25:1224.
Sabt A, Eldehna WM, Al-Warhi T, Alotaibi OJ, Elaasser MM, Suliman H, et al. Discovery of 3, 6-disubstituted pyridazines as a novel class of anticancer agents targeting cyclin-dependent kinase 2: synthesis, biological evaluation and in silico insights. J Enzyme Inhib Med Chem. 2020;35:1616–1630. https://doi.org/10.1080/14756366.2020.1806259.
Shrivastava SK, Srivastava P, Bandresh R, Tripathi PN, Tripathi A. Design, synthesis, and biological evaluation of some novel indolizine derivatives as dual cyclooxygenase and lipoxygenase inhibitor for anti-inflammatory activity. Bioorg Med Chem. 2017;25:4424–4432. https://doi.org/10.1016/j.bmc.2017.06.027.
Lee ES, Park BC, Paek SH, Lee YS, Basnet A, Jin DQ, et al. Potent analgesic and anti-inflammatory activities of 1-furan-2-yl-3-pyridin-2-yl-propenone with gastric ulcer sparing effect. Biol Pharm Bull. 2006;29:361–364. https://doi.org/10.1248/bpb.29.361.
Larré S, Tran N, Fan C, Hamadeh H, Champigneulles J, Azzouzi R, et al. PGE2 and LTB4 tissue levels in benign and cancerous prostates. Prostaglandins Other Lipid Mediat. 2008;87:14–19. https://doi.org/10.1016/j.prostaglandins.2008.05.001.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. https://doi.org/10.1006/meth.2001.1262.
Dhanamjayulu P, Boga RB, Mehta A. Inhibition of aflatoxin B1 biosynthesis and down regulation of aflR and aflB genes in presence of benzimidazole derivatives without impairing the growth of Aspergillus flavus. Toxicon. 2019;170:60–67. https://doi.org/10.1016/j.toxicon.2019.09.018.
Karthikeyan K, Sudhakaran R. Experimental horizontal transmission of Enterocytozoon hepatopenaei in post‐larvae of whiteleg shrimp, Litopenaeus vannamei. J Fish Dis. 2019;42:397–404. https://doi.org/10.1111/jfd.12945.
Sasidharan R, Sreedharannair Leelabaiamma M, Mohanan R, Jose SP, Mathew B, Sukumaran S. Anti-inflammatory effect of synthesized indole-based chalcone (2E)-3-(4-bromophenyl)-1-(1 H-indol-3-yl) prop-2-en-1-one: an in vitro and in vivo studies. Immunopharmacol Immunotoxicol. 2019;2019:1–9. https://doi.org/10.1080/08923973.2019.1672177.
Adjimani JP, Asare P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol Rep. 2015;2:721–728. https://doi.org/10.1016/j.toxrep.2015.04.005.
Chew Y-L, Goh J-K, Lim Y-Y. Assessment of in vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food Chem. 2009;116:13–18. https://doi.org/10.1016/j.foodchem.2009.01.091.
Chai T-T, Wong F-C. Whole-plant profiling of total phenolic and flavonoid contents, antioxidant capacity and nitric oxide scavenging capacity of Turnera subulata. J Med Plant Res. 2012;6:1730–1735. https://doi.org/10.5897/JMPR11.1541.
Jan MS, Ahmad S, Hussain F, Ahmad A, Mahmood F, Rashid U, et al. Design, synthesis, in-vitro, in-vivo and in-silico studies of pyrrolidine-2, 5-dione derivatives as multitarget anti-inflammatory agents. Eur J Med Chem. 2020;186:111863. https://doi.org/10.1016/j.ejmech.2019.111863.
Wu SY, Zhao XY, Li HP, Yang Y, Roesky HW. Synthesis and Characterization of N, N‐Di‐substituted Acylthiourea Copper (II) Complexes. Z Anorg Allg Chem. 2015;641(5):883–889.https://doi.org/10.1002/zaac.201400605.
Bai L, Li S, Wang JX, Chen M. Synthesis of benzoyl-N-phenylthioureas under microwave irradiation and phase transfer catalysis conditions. Synth. Commun. 2002;32(1):127–132. https://doi.org/10.1081/SCC-120001519.
Dzurilla M, Kutschy P, Imrich J, Brtoš S. Hugershoff Reaction of N-1-or N-2-Naphthoyl-N′-monosubstituted and N′, N′-Disubstituted Thiourea Derivatives. Collect. Czech. Chem. Commun. 1994;59(12):2663–2676. https://doi.org/10.1135/cccc19942663.
Hemdan MM, Fahmy AF, El-Sayed AA. Synthesis and antimicrobial study of 1, 2, 4-triazole, quinazoline and benzothiazole derivatives from 1-naphthoylisothiocyanate. J. Chem. Res. 2010;34(4):219–221. https://doi.org/10.3184/030823410X12707543946812.
Acknowledgements
The authors greatly acknowledge VIT, Vellore for providing “VIT Seed Grant” for research. Jaismy Jacob P recognizes CSIR, Govt. of India, for the financial support through Senior Research Fellowship for research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
P, J.J., S L, M. Novel approach of multi-targeted thiazoles and thiazolidenes toward anti-inflammatory and anticancer therapy—dual inhibition of COX-2 and 5-LOX enzymes. Med Chem Res 30, 236–257 (2021). https://doi.org/10.1007/s00044-020-02655-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02655-9