Abstract
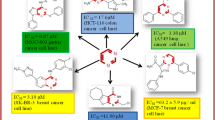
To obtain more active and selective anti-platelet candidate drugs, we tried to introduce a methyl group at the 5-position and 6-position of the parent benzene ring first time, respectively or simultaneously. The idea could inspect compound with tetra-substituted or penta-substituted characteristics rather than retained classical 1,3,4-position triple substitutions characteristic whether it continues to have anti-platelet activity in vitro. The biological evaluation revealed that most of compounds with this novel structure were more potent than the positive control drug Picotamide. At the concentration of 1.3 μmol/L, using Arachidonic acid as an inducer, it was found that the anti-platelet activity in vitro of five compounds 1a, 1b, 1c, 2f, and 3d was higher than that of Picotamide and the series 1 compounds were generally higher than that of the series 2 and 3. And with ADP as an inducer, the activity in vitro of nine compounds 2a, 2b, 2d, 2f, 2g, 2h, 3a, 3b, and 3c was more elevated than that of Picotamide and the compounds of series 2 and 3 were all evidently even more active than that of series 1. The proportion of newly designed target compounds with active is higher than that of previously developed series of compounds. Based on the in vitro activity results, a preliminary analysis of the structure–activity relationship was deduced. Meanwhile, cytotoxic effects in vitro of 11 target compounds 1b, 1c, 2f, 2a, 2b, 3a, 3b, 3c, 2d, 2g, and 2h on L-929 cells were analyzed, but the data analysis shows that at two concentrations, target compounds have higher effect on L-929 cells than that of control drug Picotamide. The reason or mechanism for obtaining higher in vitro activity and higher cytotoxicity of the target compound under tetra- or penta- substitutions requires further relevant research work before conclusion can be drawn.



Similar content being viewed by others
Abbreviations
- AA:
-
arachidonic acid
- ADP:
-
adenosine triphosphate
- CCK-8:
-
cell counting kit-8
- DMSO:
-
dimethyl sulfoxide
- PGI2 :
-
prostaglandin I2
- SARs:
-
structure–activity relationships
- TLC:
-
thin layer chromatography
- TXA2 :
-
thromboxane A2
References
Born GVR (1962) Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 194(4832):927–929
Deng QS, Zhang QX, Wang CQ, Liu XJ (2017) Synthesis and in vitro activity on anti-platelet aggregation of N 1 , N 3-benzhydryl-4-methoxy-1,3-benzenedisulfonam-idederivatives. Chin J Med Chem 22(5):355–359+366
Fontenele JB, Leal LK, Silveira ER, Felix FH, Bezerra Felipe CF, Viana GS (2009) Anti-platelet effects of piplartine, analkamide isolated from Piper tuberculatum: possible involvement of cyclooxygenase blockade and antioxidant activity. J Pharm Pharmacol 61(4):511–515
Giridhar R, Tamboli RS, Ramajayam R, Prajapati DG, Yadav MR (2012) Assessment of anti-platelet activity of 2-aminopyrimidines. Cheminform 50(33):428–432
Li GA, Wang X, Meng X, Lin YB, Li X, Liu XJ (2015) Design, synthesis of novel N, N'- bis-(halogenophenyl)-4-methoxybenzene-1,3-disulfonamides and evaluation of their anti- platelet aggregation activity. Acta Pharm Sin 50(2):185–190
Liu XJ, Wang CQ, Meng J, Shi XX, Yan YN, Liu XG (2018) Design, synthesis and biological evaluation of 4-methoxy diarylisophthalates as anti-platelet agents. Med Chem Res 27(2):488–496
Liu XJ, Wang SQ, Zhang J, Zhang FX, Li GZ, Wang BJ, Shao YL, Zhang LG, Fang L, Cheng MS (2006) Design, synthesis, and activities of novel derivatives of isophthalamide and benzene-1,3-disulfonamide. Chem Res Chin Univ 22(3):356–359
Liu XJ, Wang Y, Liu LL, Chen GL (2018) Synthesis and in vitro activities on anti-platelet aggregation of 4-methoxyisophthalamides. Med Chem Res 27(8):1971–1983
Liu XJ, Shi TE, Wang X, Wei TT, Meng X (2015) Synthesis and the evaluations in vitro anti-platelet aggregation activities of 4-ethoxyisophthalamides. Cardiovasc Hematol Agents Med Chem 13(2):124–128
Liu XJ, Shi XX, Zhong YL, Liu N, Liu K (2012) Design, synthesis and in vitro activities on anti-platelet aggregation of 4-methoxybenzene-1,3-isophthalamides. Bioorg Med Chem Lett 22(21):6591–6595
Lu N, Li L, Zheng X, Zhang S, Li Y, Yuan J, Wei Q, Xu Y, Meng F (2018) Synthesis of a novel series of amino acid prodrugs based on thienopyridine scaffolds and evaluation of their anti-platelet activity. Molecules 23(5):1041
Moura LA, de Almeida AC, da Silva AV, de Souza VR, Ferreira VF, Menezes MV, Kaiser CR, Ferreira SB, Fuly AL (2016) Synthesis, anticlotting and anti-platelet effects of 1,2,3-triazoles derivatives. Med Chem 12(8):733–741
Tong ZE, Chen WH, Peng SX (1992) Synthesis of a platelet antiaggregant—pieotamide and its analogues. J China Pharm Univ 23(1):1–4
Vijaya Bhaskar Reddy M, Tsai WJ, Qian K, Lee KH, Wu TS (2011) Structure–activity relationships of chalcone analogs as potential inhibitors of ADP- and collagen-induced platelet aggregation. Bioorg Med Chem 19(24):7711–7719
Violi F, Ghiselli A, Iuliano L, Praticò D, Alessandri C, Balsano F (1988) Inhibition bypicotamide of thromboxane production in vitro and ex vivo. Eur J Clin Pharmacol 33(6):599–602
Mirfazli SS, Kobarfard F, Firoozpour L, Asadipour A, Esfahanizadeh M, Tabib K, Shafiee A, Foroumadi A (2014) N-substituted indolecarbohydrazide derivatives: synthesis and evaluation of their anti-platelet aggregation activity. Daru 22(1):65
Youssef KM, Al-Omar MA, El-Subbagh HI, Abou-Zeid LA, Abdel-Gader AGM, Haress NG, Al-Tuwaijri AS (2011) Synthesis, anti-platelet aggregation activity, and molecular modeling study of novel substituted-piperazine analogues. Med Chem Res 20(7):898–911
Acknowledgements
The authors are grateful to the National Science Foundation of China (11341014) and the Committee of Science and Technology of Tianjin (15JCZDJC33100) & the College Students’ Platform for Innovation and Shenyang Pharmaceutical University of China for running platelet aggregation assays.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiu-jie, L., Zhi-hao, Z., Qing-song, D. et al. Synthesis, in vitro cytotoxicity and anti-platelet activity of new 1,3-bentzenedisulfonamides. Med Chem Res 28, 1864–1872 (2019). https://doi.org/10.1007/s00044-019-02419-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02419-0