Abstract
A new series of twenty two 2-exo-aryl(heteroaryl)-1,4-epoxytetrahydronaphtho[1,2-b]azepines 8–10 and eighteen cis-2-aryl(heteroaryl)-4-hydroxytetrahydronaphtho[1,2-b]azepines 11–13 were synthesized, and most of them were tested for their ability to inhibit the in vitro growth of the extracellular forms of Trypanosoma cruzi and Leishmania infantum parasites. Cell toxicity was also determined on Vero and THP-1 mammalian cells. Seventeen compounds exhibited potent activity against the epimastigotes (IC50 lower than 20 µM), without cytotoxicity on Vero cells. Ten compounds also showed remarkable anti-leishmanial properties against the promastigote form of the parasite (IC50 lower than 20 µM), but most of them were found cytotoxic for HTP-1 cells. We have also performed a quantitative structure activity relationship analysis by means of the multivariate lineal regression (MLR) technique with a family of ninety-four tetrahydro-1-benzazepine and tetrahydronaphtho[1,2-b]azepine derivatives with anti-parasitic activity. The aim of this study is to develop a tool that permits us to elucidate the structural features, which influence in the bioactivity of these compounds. The QSAR prediction models for Trypanosoma cruzi and Leishmania infantum were acceptable with a correlation coefficient values (R) of 0.668 and 0.852, respectively, in the prediction of those activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chagas disease and leishmaniasis remain a major public health problem due to the inadequate therapy, and because there are no vaccines available (Hotez et al. 2011; Diniz et al. 2013). Chagas disease (American trypanosomiasis) is caused by the flagellate protozoan Trypanosoma cruzi, mainly transmitted by the infected feces of triatomine bugs. Nevertheless, it could also be transmitted by blood transfusions and organs transplant, as well as congenitally and orally from contaminated food. Chagas disease is a devastating and neglected disease, which affects about 6 million to 7 million people worldwide, mostly in Latin America, and causes approximately 10,000 deaths annually, and it is responsible for 89% of death from tropical-clustered diseases in the region. In addition, Chagas disease causes over US$ 1.2 billion/year of productivity loss in seven of the countries where it is endemic (Rodrigues-Coura and Albajar-Viñas 2010; Crompton et al. 2010; Ayo et al. 2013; Jackson et al. 2013; World Health Organization 2016). Chagas disease presents an initial acute phase with a high number of parasites circulating the blood followed by a chronic phase which, in approximately 30% of the infected people, leads after several years to cardiac, digestive, neurological, or mixed clinical alterations (Rodrigues–Coura and Albajar–Viñas 2010). Nifurtimox (recently discontinued) and benznidazole are the two drugs available for treatment during the acute and asymptomatic chronic phases of disease. Unfortunately, these drugs have poor efficacy in the chronic stage of the disease and are related to several side effects (Rodriques–Coura and de Castro 2002; Urbina 2010; Olivieri et al. 2010; Pinazo et al. 2010), and drug resistant strains have been observed (Kessler et al. 2013, Wiggers et al. 2013, Serafim et al. 2014).
Leishmaniasis is a poverty-related group of diseases caused by several species of protozoan parasites belonging to the family Trypanosomatidae and genus Leishmania, and transmitted by the bite of more than 30 different species of sand flies (Mishra et al. 2007, World Health Organization 2012). They are digenetic parasites that proliferate as motile promastigotes in the gut of sand flies and as amastigotes inside vertebrate-host macrophages. There are two main forms of leishmaniasis, cutaneous (the most common), and visceral leishmaniasis, also known as Kala-azar (the most severe form, fatal in 85–90% of untreated cases). Leishmaniasis is currently prevalent in four continents, being endemic in 98 countries, leading to about 5,00,000 new cases and 50,000 annual deaths, mostly due to the visceral form (Alvar et al. 2012). In most of the developing countries the therapy is still based on pentavalent antimonials, sodium stibogluconate (Pentostam), and meglumine antimoniate (Glucantime), developed more than 50 years ago as first-line drugs, whereas amphotericin B (both the free and liposomal amphotericin B), pentamidine isothionate, paromomycin, and miltefosine are used as second-line chemotherapy. Unfortunately, all these drugs present several side-effects and are toxic and expensive, and have low availability, variable effectiveness, and high risk of resistance (Croft and Yardley 2002; Sundar and Chakravarty 2013; Perez-Victoria et al. 2003).
Although some progress has been made in the development of alternative drugs to treat these two diseases, efforts directed toward the discovery of non-toxic, safer, cheaper, and more effective chemotherapeutic agents are urgently needed (Barrett and Croft 2012; Molina et al. 2014; Espuelas et al. 2012; Marín et al. 2013; Chauhan et al. 2013; Galiana-Roselló et al. 2013).
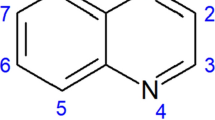
The tetrahydro-1-benzazepine nucleus is frequently encountered as scaffold in medicinal chemistry (Bankir et al. 2001; Abraham 1994; Morita et al. 2003; Barberis et al. 1999; Decaux 2001), as this heterocyclic unit is present in an extensively studied class of potent and orally active non-peptide arginine-vasopressin antagonists for both V1A and V2 receptors, (Shimada et al. 2000; Kakefuda et al. 2002; Tsunoda et al. 2003 2005; Matthews et al. 2003; Yea et al. 2008) as potent inhibitors of cyclin-dependent kinases Schultz et al. 1999; Sielecki et al. 2000; Kunick et al. 2000, 2006), as potent and orally bioavailable growth hormone secretagogue (Schoen et al. 1994; De Vita and Wyvratt 1996; De Vita et al. 1998; Ankersen 2002), and as agents against HIV-1 infection (Seto et al. 2005). Furthermore, some molecules containing this frame have been described as promising anti-leishmanicidal and anti-chagasic agents (Knockaert et al. 2002; Zuccotto et al. 2001).
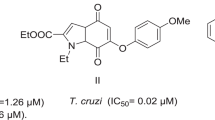
In preceding studies, we have established that compounds bearing a substituted 2-aryltetrahydronaphtho[1,2-b]azepine scaffold, like in compounds I and II (Fig. 1), possess remarkable anti-parasitic activity against both the extracellular and intracellular forms of T. cruzi and L. infantum parasites (Palma et al. 2009a, 2009b). Moreover, we have also synthetized series of compounds of the types III and IV (Fig. 1), in which the naphthalene ring of the tetrahydronaphtho[1,2-b]azepine system was replaced by benzene, and have analyzed the consequences of such structural modification, as well as the role of the substituents attached at C–2 position for their anti-chagasic and anti-leishmanicidal activities (Gómez–Ayala et al. 2010; Blanco et al. 2014).
In order to determine the anti-parasitic activity of new compounds, containing the structural scaffolds I and II, we describe the syntheses of 22 new 2-exo-aryl(heteroaryl)-1,4-epoxytetrahydronaphtho[1,2-b]azepines 8–10 and 18 new cis-2-aryl(heteroaryl)-4-hydroxy-tetrahydronaphtho[1,2-b]azepines 11–13. We have also carried out the corresponding bio-assays to check their ability to inhibit the in vitro growth of the extracellular forms of T. cruzi and L. infantum parasites along with an in vitro cytotoxicity evaluation on Vero and THP-1 mammalian cells. The choice of substituents presented in compounds 8–10 and 11–13 at C-2 position was considered in the previously described structure-activity relationships on compounds of the series III and IV (Gómez–Ayala et al. 2010; Blanco et al. 2014). On the other hand, taking the advantage to obtain a relatively extended library, formed by more than 90 compounds, we have developed a quantitative structure activity relationship (QSAR) analysis by means of the Multivariate lineal regression (MLR) technique, with the aim to elucidate the structural features, which influence in the bioactivity of these compounds to attempt predictive models able to orientate the synthesis of new derivatives with even greater anti-parasitic activity.
Results and discussion
Chemistry
The diastereoselective synthesis of the target compounds 8–10 and 11–13 was achieved employing the synthetic routes, previously described by our group (Palma et al. 2009a, 2009b; Gómez-Ayala et al. 2010; Blanco et al. 2014) and are illustrated in Scheme 1. Synthetically available 2-allyl-α-naphthylamines (Palma et al. 2009a, 2009b) 1a,b were condensed with substituted benzaldehydes 2a–i, 2-thiophenecarboxaldehydes 3a–d and 2-furancarboxaldehydes 4a–c in refluxing methanol to afford the corresponding imine intermediates, which were further reduced with sodium borohydride to give key intermediates 5a–j, 6a–g, and 7a–c. These key intermediates were oxidized with an excess of hydrogen peroxide (30% H2O2) in the presence of sodium tungstate (Na2WO4·2H2O) to afford the corresponding nitrones, which then undergo an internal 1,3-dipolar cycloaddition to generate the targeted 2-exo-aryl(heteroaryl)-1,4-epoxycycloadducts 8a–j, 9a–g, and 10a–e. Finally, reductive cleavage of the N–O bond of 8, 9, and 10 by treatment with an excess of zinc in a mixture of glacial acetic acid and concentrated hydrochloric acid at temperature ranging from 0–25 oC afforded the target cis-2-aryl(heteroaryl)-4-hydroxytetrahydronaphtho[1,2-b]azepines 11a–h, 12a–f, and 13a–d. As it was previously observed with other structural analogs (Blanco et al. 2014), the reductive cleavage of bromo-substituted cycloadduct 9g also afforded the debrominated tetrahydronaphtho[1,2-b]azepin-4-ol 12a instead of the expected 5-bromo-substituted tetrahydronaphtho[1,2-b]azepin-4-ol. Additionally, all attempts to furnish the reductive cleavage of nitro-substituted 1,4-epoxy-cycloadducts 8i, 8j, and 10e resulted in the formation of a very complex mixture of non-identifiable degradation products. The chemical structures of all the synthesized compounds were then fully characterized on the basis of their physicochemical and spectral data (see Experimental section), and the relative configurations of tertiary methynic C–2 and C–4 carbons and the conformation of the tetrahydroazepine ring in compounds 8–12 were unambiguously determined on the basis of single-crystal X-ray analysis, which confirmed what we had deduced by means of 1H NMR experiments (Palma et al. 2009a, 2009b; Yépes et al. 2013a, 2013b).
Anti-parasitic activity and cytotoxicity for the newly synthetized compounds
Anti-parasitic activity in vitro assays with T. cruzi epimastigotes and L. infantum promastigotes, as well as cytotoxicity with Vero epithelial cells (the host cells of T. cruzi) and THP-1 transformed human macrophages (the host cells of L. infantum) were conducted, as previously reported (Gómez-Ayala et al. 2010; Blanco et al. 2014). Nifurtimox and amphotericin B were used as control under the same assay conditions. Considering the low antiparasitic activity that revealed compounds of the type IV bearing a substituted furan ring at C-2 position (Blanco et al. 2014), in this work compounds 13 were not evaluated. The obtained results are listed in Tables 1 and 2. From the results observed in both tables, it is clear that some of these new compounds displayed a significant anti-parasitic activity. Unfortunately, we do not know the mechanism of action (at molecular level) for compounds reported here. Therefore, indirect approaches are the only option we have in order to better understand the structural requirements to produce the biological response; thus, in the next sections we present the results obtained by using both techniques SAR and QSAR analysis.
Qualitative structure-activity relationship analysis (SAR)
As observed from Table 1, the anti-parasitic activity and cytotoxicity of the tested 1,4-epoxycycloadducts depend on the presence or absence of bromine atom at C–6 position and upon the nature and the substitution pattern of the aromatic rings at C–2 position. These results indicated that most of the 6-bromo substituted derivatives of the series 8 did not display any activity against epimastigotes of T. cruzi and promastigotes of L. infantum; only compounds 8c and 8d showed moderate and good anti-epimastigote activity with IC50 values of 29.7 µM and 11.9 µM, respectively. Non-brominated compounds 8i and 8j, bearing meta- and para-nitrophenyl groups at C–2, also displayed good anti-parasitic activity. The nitro derivative 8i was inactive against promastigotes (IC50 = 98.6 µM), but it is active against epimastigotes (IC50 = 17.5 µM). While, the compounds 8j has the best activity of the series 8 against the epimastigotes (IC50 = 8.6 µM) and promastigotes (IC50 = 5.0 µM).
From the series 9, bearing a substituted thiophene ring at C–2 position, derivatives 9a, 9b, 9e, and 9g showed low to high anti-parasitic activity with IC50 values ranging from 54.1 to 8.3 µM against epimastigotes, and 29.3 to 10.9 µM against promastigotes. The compound 9g is the most active against epimastigotes (IC50 = 8.3 µM) and 9e the most active against promastigotes (IC50 = 10.9 µM).
Out of four compounds of the series 10, bearing a substituted furan ring at C–2 position, only derivatives 10d and 10e displayed a good anti-parasitic activity with IC50 values of 9.6 and 8.6 µM against epimastigotes, and 15.4 µM and 13.9 µM against promastigotes, respectively.
With respect to the tested 2-aryl(thiophen-2-yl)-substituted 4-hydroxytetrahydronaphtho[1,2-b]azepines 11 and 12 (Table 2), only compounds 11a and 12f were inactive against promastigotes. The rest of the analogs of these two series showed low to remarkable activity against epimastigotes (IC50 values ranging from 42.2 to 2.47 µM), and promastigotes (IC50 values ranging from 48.2 to 7.7 µM). Among the active compounds, derivatives 11b (IC50 = 7.1 µM), 11f (IC50 = 6.3 µM), 11g (IC50 = 3.3 µM) and 11h (IC50 = 2.47 µM) exhibited the highest anti-epimastigote activity, whereas derivatives 11b (IC50 = 7.7 µM), 11d (IC50 = 9.6 µM), and 11e (IC50 = 8.9 µM) were the most active against promastigotes.
Cytotoxic assays showed that most of the tested compounds of the series 8–10 have CC50 values higher than 100 µM, demonstrating that all of the active compounds were far less cytotoxic (excepting 8c and 10d, which displayed a limited cytotoxicity on HTP-1 cells) than the reference drugs, nifurtimox and amphotericin B. In majority of the cases the tested compounds of the series 11 and 12 were more cytotoxic for Vero and HTP-1 cells than their precursors 8–10.
The structure-activity relationships (SARs) analysis showed that meta substitution of benzene ring with a methoxyl group render a compound with better anti-epimastigote activity than substitutions with methyl and nitro groups, as was observed in compounds 8d, 8c, and 8i, respectively. However, the para substitution of benzene ring with a nitro group (8j) resulted in the most active compound of the series 8 for both the epimastigote and promastigote forms of the parasites. The IC50 values 8i and 8j indicated that the para position is more favorable than the meta position for providing anti-parasitic activity on nitro-derivatives. Other substitution patterns led to total loss of anti-parasitic activity, as was observed in compounds 8a, 8b, 8e, 8f, 8g, and 8h.
The introduction of methyl (9e) and bromo (9g) substituents on the position 5 of thiophene ring in compounds of the series 9, resulted in increased anti-epimastigote and anti-promastigote activities. In addition, there is an emerging pattern of decreasing anti-parasitic activity when the methyl group is changed from position 5 to 3 on the thiophene ring (9f), or when the bromine atom is incorporated at C–7 position of the base nucleus (9b).
Within the series 10, the substitution of furan ring on position 5 with methyl (10d) and nitro (10e) groups also rendered compounds with improved anti-epimastigote and anti-promastigote activities as compared to the non-substituted analogs 10a and 10c.
In the compounds of the series 11, the most potent compounds against epimastigotes (even more active than nifurtimox) were the 7-bromo-substituted derivatives 11f and 11g, bearing 4-chloro- and 2,4-dichlorophenyl moieties at C–2, respectively. Whereas the derivatives 11b, 11d, and 11e, bearing 2-methyl-, 3-methoxy- and 2-chlorophenyl moieties at C–2, respectively, emerged as the most active compounds against L. infantum promastigotes. Finally, the non-bromo-substituted derivative 11h in which the benzene ring at C–2 is 2-methylphenyl is the most active compounds against T. cruzi epimastigotes.
Unfortunately, the above molecules have significant cytotoxicity on both the Vero and THP-1 cells. As shown by the comparison of the activities of derivatives 11h and 11b, the incorporation of a bromine atom at C–7 position slightly reduced the anti-epimastigote activity (from 2.47 to 7.1 µM), but substantially enhanced the anti-promastigote activity (from 36.19 to 7.1 µM); similar effect on the activities was observed when the chlorine atom is changed from position 4 (11f) to position 2 (11e) on the phenyl moiety. Moreover, the 7-bromo-substituted derivatives 11d and 11e, bearing 3-methoxy- and 2-chlorophenyl moieties at C–2, demonstrated a strong affinity for the extracellular parasite forms with IC50 values of 11.7 and 11.1 µM against epimastigotes, and IC50 values of 9.6 µM and 8.9 µM against promastigotes, respectively. Although being inactive against promastigotes (IC50 = 617.1 µM), compound 11a was active against epimastigotes (IC50 = 12.2 µM). Finally, the IC50 values of compounds of the series 12 show that the anti-parasitic activity did not improve by the replacement of the substituted benzene ring by a substituted thiophene ring. In this series of compounds, the presence of a 5-methyl-2-thiophenyl moiety (12e) is more favorable than the presence of a 3-methyl-2-thiophenyl moiety (12f) for enhancing the anti-epimastigote and anti-promastigote activities.
Quantitative structure-activity relationship analysis (QSAR)
At this step of our study, we were interested in obtaining a model that allowed us to predict the potential anti-parasitic activity of these compounds. Thus, a QSAR analysis was carried out by searching the optimal linear regression equations by means of the Replacement Method (RM) approach (Duchowicz et al. 2006; Mercader et al. 2010), which minimizes their standard deviation (S). A total of 94 compounds, tables 5S-10S, previously described (Palma et al. 2009a, 2009b; Gómez-Ayala et al. 2010; Blanco et al. 2014), were included in the dataset. In these tables the compounds are identified as I, II, III, and IV, which corresponds to each type of compound in Fig. 1.
We analyzed the regression that includes the most “representative” d = 1–10 molecular descriptors. To define the maximum number of molecular descriptors in the lineal regression we consider the “rule of thumb”, which states that at least six data points should be present per fitting parameter, i.e., (Ntrain/d ≥ 6), where Ntrain is the number of compounds of training set and d is the number of molecular descriptors.
QSAR for the growth inhibition of Trypanosoma cruzi epimastigotes
Table 1S of the supplementary material summarized the main statistical parameters of the QSAR model for the inhibition of Trypanosoma cruzi epimastigotes. We considered that a model with five molecular descriptors shows an acceptable quality in accordance with the number of independent variables. The equation of selected QSAR model is:
The results of the validation reveal that model have an acceptable predictive capacity, according to the known criterions (Rval ≥ 0.500 and Srand > Strain). Figure 2a shows the linear trend of the relationship of the predicted and experimental values. The residuals values are plotted in the Fig. 2b. From this figure it can be appreciated that compounds IId and IIId present a residual value close to the 2.5 S limit (dotted line). However, these compounds were not considered outliers. Within the validation set, only compounds Ii and IVaa exhibit a residual value higher than the limit of deviation standard.
The molecular descriptors involved in the QSAR model are listed in Table 2S. The table presents the maximum correlation, the standardized regression coefficients of the molecular descriptors and a brief description of them. The maximum correlation between the descriptors of the model is 0.825, given by nBondsM and SpMax3_Bhv. The standardized coefficient specifies the importance of the descriptor on the biological activity, and the sign indicates whether the relationship is direct or indirect. The standardized regression coefficients values plot is presented in the Fig. 3.
The most important descriptor for the activity is SpMax3_Bhv (0.910), whole positive value expresses a direct relationship of this descriptor with the –log(IC50). This descriptor is defined as the average centered Broto-Moreau autocorrelation–lag 1/weighted by first ionization potential, and to have strong empirical relationship to electron distribution of the molecule as a whole (Todeschini and Consonni 2009). The standardized regression coefficients magnitudes for the rest of the molecular descriptors (0.516, −0.428, 0.375, 0.313) suggest that they complement each other in QSAR equation and the negative values in nBondsM indicate its indirect relationship with the –log(IC50).
QSAR for the growth inhibition of Leishmania infantum promastigotes
The statistical parameters of QSAR models (from 1 to 10 descriptors) for the inhibition of Leishmania infantum promastigotes are listed in the Table 3S. In this case, the model containing six molecular descriptors was considered appropriate, with an acceptable quality for the training and validation sets. The mathematical equation of selected QSAR model is:
The statistical parameters indicate that model has a suitable predictive power for both, the training set (Rtrain = 0.757, Strain = 0.218) and the validation set (Rval = 0.589, Sval = 0.301). Figure 3 shows the predicted and experimental –log(IC50) values plot and the residual values graphic. Figure 4a suggests the existence of a linear trend between the predicted and experimental values, while Fig. 4b indicates that there are no compounds outside the 2.5 S limit (dotted line).
Table 4S presents the maximum correlation, the standardized regression coefficients of the molecular descriptors and a brief description of molecular descriptors involved in the QSAR model. The maximum correlation value (0.684) indicates that there is a low relationship between the descriptors of model and the information is not overlapping. Figure 5 shows the standardized regression coefficients values plot.
The two most important descriptors for the activity present positive values of the standardized regression coefficients and a direct relationship with the –log(IC50); Vm (0.816) and RDF60s (0.679). The Vm descriptor is one of the most used in the QSAR model and it is relate to the molecular volume.58 While RDF60s is a radial distribution function at 6.0 Å inter-atomic distances weighted by relative I-state.57 The rest of the molecular descriptors have negative standardized regression coefficients values (−0.299, −0.301, −0.315, −0.532), indicating an indirect relationship of these descriptors with the –log(IC50).
Anti-parasitic activity prediction for the newly synthetized compounds
We apply the established QSAR models to predict the anti-parasitic activity of the newly synthesized compounds. These results are presented in Table 3. The Trypanosoma cruzi model presents a correlation coefficient (R) of 0.668, a standard deviation of 0.852 and the residue average is −0.430. Both models show acceptable values for QSAR prediction. However, same compounds accuse a poor prediction. The compounds 8c, 9f, 11a, 11c, 11e, and 11f show an error in the prediction higher than 60% for Trypanosoma cruzi. While for Leishmania infantum this percentage is achieve only for the compound 8j. It is clear that Trypanosoma cruzi model has problem to predict correctly the activity of compounds of the series 11. This may be due to they have a molecular structure different to the ones investigated in the training set.
Conclusions
We report here a new library of twenty two 2-exo-aryl(heteroaryl)-1,4-epoxytetrahydronaphtho[1,2-b]azepines and eighteen cis-2-aryl(heteroaryl)-4-hydroxytetrahydronaphtho[1,2-b]azepines. Seventeen compounds exhibited potent activity against the T. cruzi epimastigotes (IC50 lower than 20 µM), without cytotoxicity or with just limited effect on Vero cells. In addition, ten compounds also showed remarkable anti-leishmanial properties against the L. infantum promastigotes (IC50 lower than 20 µM), however in some of them were found cytotoxic effects for HTP-1 cells.
Both SAR and QSAR analyses were carried out for the compounds reported here. Thus, we performed a quantitative structure activity relationship analysis by means of the multivariate lineal regression (MLR) technique for a family of ninety four tetrahydro-1-benzazepine and tetrahydronaphtho[1,2-b]azepine derivatives. The QSAR prediction models obtained for the extracellular forms of Trypanosoma cruzi and Leishmania infantum parasites were acceptable with a correlation coefficient values (R) of 0.668 and 0.852, respectively. From our results, it is clear that the QSAR study reported here might predict the potential anti-parasitic effect of non-synthesized compounds with an acceptable degree of accuracy. Such information could be useful in order to know a priori the putative activity of new anti-parasitic compounds structurally related to these series.
Materials and methods
General
All the reagents and solvents were purchased from commercial suppliers (Aldrich, Merck), and used without further purification. Monitoring of the reactions was performed using silica gel TLC plates (silica Merck 60 F254). Column chromatography purification was performed on Merck Kieselgel 230–400 mesh (ASTM). Melting points were determined with a MEL–TEMP 1201D capillary apparatus and are uncorrected. IR spectra were recorded on a Bruker Tensor 27 spectrometer (equipped with a platinum ATR cell). 1H and 13C NMR spectra were measured on a Bruker AM–400 spectrometer, using CDCl3 as the solvent. Chemical shifts (δ) and coupling constants (J) values are reported in ppm and hertz, respectively. Chemical shifts are relative to the solvent peaks used as reference [CDCl3: δH = 7.26, and δC = 77.0]. A Hewlett–Packard (HP) 5890 A series II Gas Chromatograph interfaced to a HP 5972 Mass Selective Detector with a HP MS ChemStation Data system, and a High Resolution Mass Spectrometer Waters Micromass AutoSpect NT (equipped with a direct inlet probe) operating at 70 eV were used for MS identification. All the compounds described here were synthetized according to the experimental conditions reported in previous related works (Gómez-Ayala et al. 2010; Blanco et al. 2014).
Physicochemical and spectral data for 2–allyl–N–aryl(heteroaryl)methylnaphthylamines (5a–j), (6a–g), and (7a–e)
2–Allyl–N–benzyl–4–bromo–1–naphthylamine (5a)
Colorless viscous oil. Yield: 93%. IR (liquid film, cm−1): ṽ 3361 (N–H), 1636 (C=Callyl), 916 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.28 (dd, J = 7.5, 2.2 Hz, 1H, H–8), 8.26 (dd, J = 7.5, 2.3 Hz, 1H, H–5), 7.54–7.62 (m, 2H, H–6, H–7), 7.62 (s, 1H, H–3), 7.32–7.42 (m, 5H, H–2’, H–3’, H–4’, H–5’, H–6’), 5.94 (ddt, J = 17.1, 10.1, 6.0 Hz, 1 H, =CH), 5.14 (dq, J = 10.1, 1.5 Hz, 1H, =CH2(H–cis)), 5.03 (dq, J = 17.1, 1.5 Hz, 1H, =CH2(H–trans)), 4.35 (s, 2H, N–CH2), 3.34 (dt, J = 6.0, 1.5 Hz, 2H, –CH2–). 13C NMR (100 MHz, CDCl3): δ 142.6 (C1), 139.8 (C1’), 136.0 (=CH), 132.2 (C3), 132.1 (C4a), 130.4 (C8a), 128.9 (C2), 128.0 (C3’, C5’), 127.9 (C8), 127.7 (C4’), 127.6 (C2’, C6’), 126.7 (C6), 126.4 (C7), 123.9 (C5), 117.0 (C4), 116.7 (=CH2), 55.1 (N–CH2), 36.1 (–CH2–). GC–MS (EI, 70 eV): m/z (%) 351 (M+•, 79Br, 31), 274 (2), 260 (10), 246 (1), 180 (100), 91 (55).
2–Allyl–4–bromo–N–(2–methylbenzyl)–1–naphthylamine (5b)
Colorless viscous oil. Yield: 92%. IR (liquid film, cm−1): ṽ 3363 (N–H), 1637 (C=Callyl), 916 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.29 (dd, J = 8.0, 1.7 Hz, 1H, H–8), 8.27 (dd, J = 8.0, 1.8 Hz, 1H, H–5), 8.00 (td, J = 8.0, 1.7 Hz, 1H, H–6), 7.65 (s, 1H, H–3), 7.61 (td, J = 8.0, 1.8 Hz, 1H, H–7), 7.50–7.54 (m, 1H, H–6’), 7.21–7.34 (m, 3H, H–3’, H–4’, H–5’), 5.97 (ddt, J = 17.1, 10.1, 6.0 Hz, 1H, = CH), 5.17 (dq, J = 10.1, 1.6 Hz, 1H, =CH2(H–cis)), 5.05 (dq, J = 17.1, 1.6 Hz, 1H, =CH2(H–trans)), 4.35 (s, 2H, N–CH2), 3.42 (dt, J = 6.0, 1.6 Hz, 2H, –CH2–), 2.35 (s, 3H, 2’–CH3). 13C NMR (100 MHz, CDCl3): δ 142.9 (C1), 137.9 (C1’), 136.0 (=CH), 132.2 (C3, C4a), 132.1 (C2’), 130.6 (C3’), 130.5 (C8a), 130.4 (C5’), 128.9 (C2), 128.4 (C6’), 127.7 (C8), 127.6 (C4’), 126.8 (C6), 126.4 (C7), 124.0 (C5), 117.0 (C4), 116.7 (=CH2), 52.5 (N–CH2), 35.7 (–CH2–), 19.4 (2’–CH3). GC–MS (EI, 70 eV): m/z (%) 365 (M+•, 79Br, 18), 274 (2), 260 (10), 246 (4), 180 (70), 105 (100).
2–Allyl–4–bromo–N–(3–methylbenzyl)–1–naphthylamine (5c)
Colorless viscous oil. Yield: 88%. IR (liquid film, cm−1): ṽ 3361 (N–H), 1637 (C=Callyl), 916 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.27 (dd, J = 7.1, 2.1 Hz, 1H, H–8), 8.24 (dd, J = 8.0, 1.6 Hz, 1H, H–5), 7.62 (s, 1H, H–3), 7.54–7.60 (m, 2H, H–6, H–7), 7.28 (t, J = 7.5 Hz, 1H, H–5’), 7.24 (s, 1H, H–2’), 7.18–7.22 (m, 1H, H–4’), 7.14–7.18 (m, 1H, H–6’), 5.94 (ddt, J = 17.1, 10.1, 6.0 Hz, 1H, =CH), 5.14 (dq, J = 10.1, 1.6 Hz, 1H, =CH2(H–cis)), 5.03 (dq, J = 17.1, 1.6 Hz, 1H, =CH2(H–trans)), 4.28 (s, 2H, N–CH2), 3.40 (dt, J = 6.0, 1.6 Hz, 2H, –CH2–), 2.40 (s, 3H, 3´–CH3). 13C NMR (100 MHz, CDCl3): δ 142.5 (C1), 139.6 (C1’), 138.4 (C3’), 136.0 (=CH), 132.2 (C3), 132.1 (C4a), 130.4 (C8a), 129.0 (C2), 128.7 (C5’), 128.4 (C2’), 127.8 (C4’), 126.8 (C6), 126.4 (C7), 125.1 (C6’), 124.0 (C5, C8), 117.6 (C4), 116.7 (=CH2), 55.2 (N–CH2), 36.1 (–CH2–), 21.5 (3’–CH3). GC–MS (EI, 70 eV): m/z (%) 365 (M+•, 79Br, 7), 274 (2), 260 (5), 246 (2), 180 (100), 105 (92).
2–Allyl–4–bromo–N–(3–methoxybenzyl)–1–naphthylamine (5d)
Colorless viscous oil. Yield: 90%. IR (liquid film, cm−1): ṽ 3361 (N–H), 1635 (C=Callyl), 915 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.24 (dd, J = 8.0, 1.6 Hz, 1H, H–8), 8.22 (dd, J = 8.0, 1.6 Hz, 1H, H–5), 7.60 (s, 1H, H–3), 7.58 (td, J = 8.0, 1.6 Hz, 1H, H–7), 7.55 (td, J = 8.0, 1.6 Hz, 1H, H–6), 7.29 (t, J = 7.8 Hz, 1H, H–5’), 6.96 (d, J = 7.8 Hz, 1H, H–6’), 6.94 (d, J = 2.2 Hz, 1H, H–2’), 6.87 (dd, J = 7.8, 2.2 Hz, 1H, H–4’), 5.93 (ddt, J = 17.2, 10.1, 6.0 Hz, 1H, =CH), 5.13 (dq, J = 10.1, 1.7 Hz, 1H, =CH2(H–cis)), 5.02 (dq, J = 17.2, 1.7 Hz, 1H, =CH2(H–trans)), 4.29 (s, 2H, N–CH2), 3.38 (dt, J = 6.0, 1.7 Hz, 2H, –CH2–), 3.30 (s, 3H, 3’–OCH3). 13C NMR (100 MHz, CDCl3): δ 160.1 (C3’), 142.3 (C1), 141.3 (C1’), 136.1 (=CH), 132.3 (C3), 132.1 (C4a), 130.4 (C8a), 129.8 (C5’), 129.2 (C2), 127.8 (C8), 126.8 (C6), 126.5 (C7), 123.9 (C5), 120.3 (C2’), 120.2 (C4’), 117.1 (C4), 116.8 (=CH2), 113.3 (C6’), 55.3 (OCH3), 55.0 (N–CH2), 36.1 (–CH2–).GC–MS (EI, 70 eV): m/z (%) 381 (M+•, 79Br, 20), 274 (2), 260 (14), 246 (1), 180 (100), 121 (74).
2–Allyl–4–bromo–N–(2–chlorobenzyl)–1–naphthylamine (5e)
Colorless viscous oil. Yield: 90%. IR (liquid film, cm−1): ṽ 3367 (N–H), 1636 (C=Callyl), 916 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.26 (dd, J = 7.5, 2.6 Hz, 1H, H–8), 8.24 (dd, J = 7.5, 2.6 Hz, 1H, H–5), 7.55–7.62 (m, 2H, H–6, H–7), 7.59 (s, 1H, H–3), 7.45 (dd, J = 7.0, 1.2 Hz, 1H, H–3’), 7.30 (td, J = 7.0, 1.2 Hz, 1H, H–5’), 7.27 (td, J = 7.0, 1.8 Hz, 1H, H–6’), 7.23 (td, J = 7.0, 1.8 Hz, 1H, H–4’), 5.92 (ddt, J = 17.1, 10.1, 6.0 Hz, 1H, =CH), 5.13 (dq, J = 10.1, 1.6 Hz, 1H, =CH2(H–cis)), 5.06 (dq, J = 17.1, 1.6 Hz, 1H, =CH2(H–trans)), 4.43 (s, 2H, N–CH2), 3.37 (dt, J = 6.0, 1.6 Hz, 2H, –CH2–). 13C NMR (100 MHz, CDCl3): δ 142.2 (C1), 137.4 (C1’), 136.0 (=CH), 133.7 (C2’), 132.1 (C3, C4a), 130.2 (C8a, C6’), 129.7 (C3’), 129.0 (C2, C5’), 127.8 (C8), 127.1 (C4’), 126.8 (C6), 126.5 (C7), 123.9 (C5), 117.2 (C4), 116.7 (=CH2), 52.5 (N–CH2), 36.0 (–CH2–). GC–MS (EI, 70 eV): m/z (%) 385 (M+•, 79Br, 35Cl, 15), 274 (3), 260 (10), 246 (2), 180 (100), 125 (20).
2–Allyl–4–bromo–N–(4–chlorobenzyl)–1–naphthylamine (5f)
Colorless viscous oil. Yield: 90%. IR (liquid film, cm−1): ṽ 3361 (N–H), 1635 (C=Callyl), 916 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.22 (dd, J = 8.4, 1.6 Hz, 1H, H–8), 8.20 (dd, J = 7.6, 1.6 Hz, 1H, H–5), 7.59 (s, 1H, H–3), 7.57 (td, J = 7.6, 1.6 Hz, 1H, H–7), 7.55 (td, J = 7.6, 1.6 Hz, 1H, H–6), 7.33 (d, J = 8.5 Hz, 2H, H–3’, H–5’), 7.28 (d, J = 8.5 Hz, 2H, H–2’, H–6’), 5.91 (ddt, J = 17.1, 10.1, 6.0 Hz, 1H, =CH), 5.12 (dq, J = 10.1, 1.5 Hz, 1H, =CH2(H–cis)), 5.00 (dq, J = 17.1, 1.5 Hz, 1H, =CH2(H–trans)), 4.27 (s, 2H, N–CH2), 3.35 (dt, J = 6.0, 1.5 Hz, 2H, –CH2–).13C NMR (100 MHz, CDCl3): δ 141.8 (C1), 138.1 (C1’), 136.0 (=CH), 133.5 (C4’), 132.2 (C3), 132.1 (C4a), 130.2 (C8a), 129.1 (C2), 129.4 (C3’, C5’), 128.9 (C2’, C6’), 127.8 (C8), 126.8 (C6), 126.6 (C7), 123.8 (C5), 117.3 (C4), 116.5 (=CH2), 55.1 (N–CH2), 36.3 (–CH2–). GC–MS (EI, 70 eV): m/z (%) 385 (M+•, 79Br, 35Cl, 14), 274 (1), 260 (7), 246 (3), 180 (100), 125 (43).
2–Allyl–4–bromo–N–(2,4–dichlorobenzyl)–1–naphthylamine (5g)
Yellow viscous oil. Yield: 88%. IR (liquid film, cm−1): ṽ 3363 (N–H), 1637 (C=Callyl), 914 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.21 (dd, J = 7.5, 1.5 Hz, 1H, H–8), 8.17 (dd, J = 7.5, 1.5 Hz, 1H, H–5), 7.57 (td, J = 7.5, 1.5 Hz, 1H, H–7), 7.54 (td, J = 7.5, 1.5 Hz, 1H, H–6), 7.53 (s, 1H, H–3), 7.43 (d, J = 2.0 Hz, 1H, H–3’), 7.18 (dd, J = 8.1, 2.0 Hz, 1H, H–5’), 7.25 (d, J = 8.1 Hz, 1H, H–6’), 5.90 (ddt, J = 17.1, 10.1, 6.0 Hz, 1H, =CH), 5.12 (dq, J = 10.1, 1.6 Hz, 1H, =CH2(H–cis)), 5.03 (dq, J = 17.1, 1.6 Hz, 1H, =CH2(H–trans)), 4.37 (s, 2H, N–CH2), 3.35 (dt, J = 6.0, 1.6 Hz, 2H, –CH2–). 13C NMR (100 MHz, CDCl3): δ 141.0 (C1), 135.8 (=CH), 135.6 (C1’), 134.4 (C2’), 134.1 (C4’), 132.2 (C3, C6’), 132.0 (C4a), 130.4 (C8a), 129.5 (C3’), 129.4 (C2), 127.8 (C5’), 127.4 (C8), 126.8 (C6), 126.6 (C7), 123.8 (C5), 117.5 (C4), 116.7 (=CH2), 51.5 (N–CH2), 36.2 (–CH2–). GC–MS (EI, 70 eV): m/z (%) 421 (M+•, 79Br, 35Cl, 10), 274 (1), 260 (7), 246 (2), 180 (100), 159 (20).
4.2.8. 2–Allyl–N–(2–methylbenzyl)–1–naphthylamine (5h)
Pale Yellow viscous oil. Yield: 78%. IR (liquid film, cm−1): ṽ 3363 (N–H), 1635 (C=Callyl), 915 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.25 (dd, J = 9.0, 1.0 Hz, 1H, H–8), 7.87 (dd, J = 7.6, 2.0 Hz, 1H, H–5), 7.59 (d, J = 8.4 Hz, 1H, H–4), 7.58 (dd, J = 7.6, 1.6 Hz, 1H, H–6’), 7.33 (d, J = 8.4 Hz, 1H, H–3), 7.43–7.49 (m, 2H, H–6, H–7), 7.27–7. 30 (m, 3H, H–3’, H–4’, H–5’), 5.98–6.05 (m, 1H, =CH), 5.01–5.15 (m, 2H, =CH2), 4.36 (s, 2H, N–CH2), 3.59 (dt, J = 6.4, 1.6 Hz, 2H, –CH2–), 2.36 (s, 3H, 2’–CH3). 13C NMR (100 MHz, CDCl3): δ 143.0 (C1), 138.4 (C1’), 136.9 (C2’, =CH), 134.0 (C4a), 130.5 (C5’), 129.3 (C8a), 128.7 (C5), 128.5 (C6’), 128.3 (C3), 127.9 (C2), 127.5 (C4’), 126.4 (C3’), 125.7 (C7), 125.4 (C6), 123.4 (C4, C8), 116.1 (=CH2), 52.7 (N–CH2), 36.5 (–CH2–), 19.2 (2’–CH3). GC–MS (EI, 70 eV): m/z (%) 318 (M+•, 31), 277 (1), 274 (2), 196 (1), 182 (100), 136 (3).
2–Allyl–N–(3–nitrobenzyl)–1–naphthylamine (5i)
Yellow viscous oil. Yield: 78%. IR (liquid film, cm−1): ṽ 3360 (N–H), 1641(C=Callyl), 1528 (–NO2), 1348 (–NO2), 919 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.34 (s, 1H, H–2’), 8.18 (dd, J = 8.3, 1.1 Hz, 1H, H–8), 8.13 (dd, J = 8.2, 0.4 Hz, 1H, H–5), 7.73 (d, J = 8.4 Hz, 1H, H–6’), 7.65 (d, J = 7.0 Hz, 1H, H–4), 7.57 (t, J = 8.4 Hz, 1H, H–5’), 7.43–7.52 (m, 2H, H–6, H–7), 7.47 (d, J = 7.0 Hz, 1H, H–3), 7.30 (d, J = 8.4 Hz, 1H, H–4’), 6.00 (ddt, J = 17.1, 10.1, 6.0 Hz, 1H, =CH), 5.12 (dq, J = 10.1, 1.6 Hz, 1H, =CH2(H–cis)), 5.01 (dq, J = 17.1, 1.6 Hz, 1H, =CH2(H–trans)), 4.41 (s, 2H, N–CH2), 3.47 (dt, J = 6.0, 1.6 Hz, 2H, –CH2–). 13C NMR (100 MHz, CDCl3): δ 148.6 (C1’), 142.0 (C3’), 141.7 (C1), 136.7 (=CH2), 134.0 (C4a), 133.9 (C6’), 129.3 (C8a), 128.6 (C4’), 128.1 (C2, C4), 125.9 (C3, C6), 125.5 (C7), 123.9 (C5’), 123.0 (C5), 122.7 (C8), 122.4 (C2’), 116.3 (=CH2), 53.8 (N–CH2), 36.5 (–CH2–). GC–MS (EI, 70 eV): m/z (%) 318 (M+•, 36), 196 (6), 182 (100), 168 (1), 136 (11).
2–Allyl–N–(4–nitrobenzyl)–1–naphthylamine (5j)
Yellow viscous oil. Yield: 79%. IR (liquid film, cm−1): ṽ 3354 (N–H), 1522 (–NO2), 1350 (–NO2), 1637 (C=Callyl), 917 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.22 (d, J = 8.4 Hz, 2H, H–3’, H–5’), 8.10 (dd, J = 7.1, 1.9 Hz, 1H, H–8), 7.84 (dd, J = 7.8, 2.3 Hz, 1H, H–5), 7.58 (d, J = 8.4 Hz, 2H, H–2’, H–6’), 7.57 (d, J = 8.3 Hz, 1H, H–4), 7.42–7.51 (m, 2H, H–6, H–7), 7.29 (d, J = 8.3 Hz, 1H, H–3), 5.99 (ddt, J = 17.1, 10.2, 6.0 Hz, 1H, =CH), 5.10 (dq, J = 10.1, 1.6 Hz, 1H, =CH2(H–cis)), 4.99 (dq, J = 17.1, 1.5 Hz, 1H, =CH2(H–trans)), 4.41 (s, 2H, N–CH2), 3.44 (dt, J = 6.0, 1.6 Hz, 2H, –CH2–). 13C NMR (100 MHz, CDCl3): δ 147.8 (C1’), 147.4 (C4’), 141.9 (C1), 136.6 (=CH), 134.0 (C4a), 129.0 (C8a), 128.7 (C4), 128.6 (C3), 128.5 (C5), 128.0 (C2), 125.9 (C6), 125.5 (C7), 124.0 (C3’, C5’), 123.9 (C2’, C6’), 122.9 (C8), 116.2 (=CH2), 54.2 (N–CH2), 36.8 (–CH2–). GC–MS (EI, 70 eV): m/z (%) 318 (M+•, 29), 196 (5), 182 (100), 168 (1), 136 (7).
2–Allyl–4–bromo–N–(thiophen–2–ylmethyl)–1–naphthylamine (6a)
Colorless viscous oil. Yield: 75%. IR (liquid film, cm−1): ṽ 3360 (N–H), 1635 (C=Callyl), 916 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.20 (dd, J = 8.7, 1.2 Hz, 1H, H–8), 7.57 (dd, J = 8.4, 1.5 Hz, 1H, H–5), 7.53 (ddd, J = 8.7, 7.2, 1.5 Hz, 1H, H–7), 7.51 (ddd, J = 8.4, 7.2, 1.2 Hz, 1H, H–6), 7.23 (s, 1H, H–3), 7.19 (dd, J = 3.2, 0.7 Hz, 1H, H–5’), 6.95 (dd, J = 3.5, 3.5 Hz, 1H, H–4’), 6.91 (dd, J = 3.5, 0.7 Hz, 1H, H–3’), 5.88 (ddt, J = 17.1, 10.2, 6.1 Hz, 1H, =CH), 5.07 (dq, J = 10.2, 1.8 Hz, 1H, =CH2(H–cis)), 4.97 (dq, J = 17.1, 1.8 Hz, 1H, =CH2(H–trans)), 4.40 (s, 2H, N–CH2), 3.39 (dt, J = 6.1, 1.8 Hz, 2H, –CH2–). 13C NMR (100 MHz, CDCl3): δ 142.9 (C1), 141.3 (C2’), 136.0 (=CH), 131.7 (C4a), 128.7 (C8a), 128.4 (C3), 127.8 (C2), 127.8 (C5), 126.8 (C7), 126.6 (C5’), 126.5 (C6), 125.2 (C4’), 124.9 (C3’), 123.8 (C8), 117.2 (C4), 116.6 (=CH2), 49.4 (N–CH2), 36.3 (–CH2–). GC–MS (EI, 70 eV): m/z (%) 357 (M+•, 79Br, 6), 274 (1), 260 (3), 246 (2), 97 (100).
2–Allyl–4–bromo–N–(5–methylthiophen–2–ylmethyl)–1–naphthylamine (6b)
Colorless viscous oil. Yield: 78%. IR (liquid film, cm−1): ṽ 3362 (N–H), 1635 (C=Callyl), 915 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.22 (dd, J = 7.1, 2.2 Hz, 1H, H–8), 8.20 (dd, J = 7.1, 2.3 Hz, 1H, H–5), 7.60 (s, 1H, H–3), 7.56 (td, J = 7.1, 2.3 Hz, 1H, H–7), 7.55 (td, J = 7.1, 2.2 Hz, 1H, H–6), 6.61 (dd, J = 3.3, 1.0 Hz, 1H, H–4’), 6.68 (d, J = 3.3 Hz, 1H, H–3’), 5.94 (ddt, J = 17.1, 10.1, 6.0 Hz, 1H, =CH), 5.13 (dq, J = 10.1, 1.6 Hz, 1H, =CH2(H–cis)), 5.03 (dq, J = 17.1, 1.6 Hz, 1H, =CH2(H–trans)), 4.37 (s, 2H, N–CH2), 3.40 (dt, J = 6.0, 1.6 Hz, 2H, –CH2–), 2.48 (s, 3H, 5’–CH3). 13C NMR (100 MHz, CDCl3): δ 142.3 (C1), 140.6 (C2’), 139.4 (C5’), 136.3 (=CH), 132.2 (C3, C4a), 130.4 (C8a), 129.2 (C2), 127.7 (C5), 126.8 (C7), 126.4 (C6), 125.3 (C3’), 125.0 (C4’), 124.0 (C8), 117.3 (C4), 115.2 (=CH2), 49.5 (N–CH2), 35.8 (–CH2–), 15.5 (5’–CH3). GC–MS (EI, 70 eV): m/z (%) 371 (M+•, 79Br, 3), 274 (2), 182 (4), 260 (4), 111 (100).
2–Allyl–4–bromo–N–(3–methylthiophen–2–ylmethyl)–1–naphthylamine (6c)
Colorless viscous oil. Yield: 70%. IR (liquid film, cm−1): ṽ 3360 (N–H), 1636 (C=Callyl), 916 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.24 (dd, J = 7.0, 2.1 Hz, 1H, H–8), 8.20 (dd, J = 7.0, 2.0 Hz, 1H, H–5), 7.58 (s, 1H, H–3), 7.56 (td, J = 7.0, 2.1 Hz, 1H, H–7), 7.55 (td, J = 7.0, 2.1 Hz, 1H, H–6), 7.16 (d, J = 5.1 Hz, 1H, H–5’), 6.82 (d, J = 5.1 Hz, 1H, H–4’), 5.91 (ddt, J = 17.0, 10.2, 6.0 Hz, 1H, =CH), 5.12 (dq, J = 10.2, 1.5 Hz, 1H, =CH2(H–cis)), 5.02 (dq, J = 17.0, 1.5 Hz, 1H, =CH2(H–trans)), 4.36 (s, 2H, N–CH2), 3.35 (dt, J = 6.0, 1.5 Hz, 2H, –CH2–), 2.01 (s, 3H, 3’–CH3).13C NMR (100 MHz, CDCl3): δ 142.4 (C1), 136.1 (C2’), 136.0 (=CH), 134.2 (C3’), 132.2 (C3), 132.1 (C4a), 130.5 (C8a), 130.3 (C5’), 129.2 (C2), 127.7 (C5), 126.8 (C7), 126.5 (C6), 123.2 (C8, C4’), 117.3 (C4), 116.9 (=CH2), 47.3 (N–CH2), 36.1 (–CH2–), 13.6 (3’–CH3). GC–MS (EI, 70 eV): m/z (%) 371 (M+•, 79Br, 2), 274 (3), 260 (3), 246 (2), 180 (14), 111 (100).
2–Allyl–N–(thiophen–2–ylmethyl)–1–naphthylamine (6d)
Colorless viscous oil. Yield: 76%. IR (liquid film, cm−1): ṽ 3361 (N–H), 1629 (C=Callyl), 916 (=C–Hallyl).1H NMR (400 MHz, CDCl3): δ 8.24 (dd, J = 8.4, 1.2 Hz, 1H, H–8), 7.87 (dd, J = 8.4, 1.2 Hz, 1H, H–5), 7.55 (ddd, J = 8.4, 7.0, 1.2 Hz, 1H, H–7), 7.50 (ddd, J = 8.4, 7.0, 1.2 Hz, 1H, H–6), 7.33 (d, J = 8.4 Hz, 1H, H–3), 7.29 (dd, J = 5.2, 1.2 Hz, 1H, H–5’), 7.02 (d, J = 5.2, 3.2 Hz, 1H, H–4’), 6.99 (dd, J = 3.2, 0.8 Hz, 1H, H–3’), 6.02 (ddt, J = 17.2, 10.2, 6.0 Hz, 1H, =CH), 5.14 (dq, J = 10.2, 1.6 Hz, 1H, =CH2(H–cis)), 5.07 (dq, J = 17.2, 1.6 Hz, 1H, =CH2(H–trans)), 4.55 (s, 2H, N–CH2), 3.38 (dt, J = 6.0, 1.6 Hz, 2H, –CH2–). 13C NMR (100 MHz, CDCl3): δ 142.3 (C1), 141.9 (C2’), 137.1 (=CH), 134.3 (C4a), 129.5 (C8a), 128.9 (C3), 128.8 (C2), 127.3 (C7, C4’), 126.2 (C3’), 125.8 (C6), 125.7 (C5), 124.2 (C4), 123.6 (C8), 116.5 (=CH2), 49.7 (N–CH2), 36.8 (–CH2–). GC–MS (EI, 70 eV): m/z (%) 279 (M+•, 40), 196 (1), 182 (36), 168 (6), 97 (100).
2–Allyl–N–(5–methylthiophen–2–ylmethyl)–1–naphthylamine (6e)
Colorless viscous oil. Yield: 80%. IR (liquid film, cm−1): ṽ 3361 (N–H), 1636 (C=Callyl), 915 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 30 (br d, J = 8.8 Hz, 1H, H–8), 7.90 (br d, J = 8.4 Hz, 1H, H–5), 7.62 (d, J = 8.4 Hz, 1H, H–4), 7.58 (ddd, J = 8.4, 7.0, 1.2 Hz, 1H, H–7), 7.52 (ddd, J = 8.0, 7.0, 1.2 Hz, 1H, H–6), 7.36 (d, J = 8.4 Hz, 1H, H–3), 6.80 (d, J = 3.2 Hz, 1H, H–4’), 6.70 (dd, J = 3.2, 1.0 Hz, 1H, H–3’), 6.07 (ddt, J = 17.1, 10.1, 6.0 Hz, 1H, =CH), 5.18 (dq, J = 10.1, 1.6 Hz, 1H, =CH2(H–cis)), 5.12 (dq, J = 17.1, 1.6 Hz, 1H, =CH2(H–trans)), 4.48 (s, 2H, N–CH2), 3.55 (dt, J = 6.0, 1.6 Hz, 2H, –CH2–), 2.57 (s, 3H, 5’–CH3). 13C NMR (100 MHz, CDCl3): δ 142.6 (C1), 141.4 (C2’), 139.7 (C5’), 137.2 (=CH), 134.3 (C4a), 129.6 (C8a), 129.0 (C5), 128.8 (C3), 128.5 (C2), 126.1 (C7), 125.7 (C6), 125.4 (C4’), 125.3 (C3’), 123.9 (C4), 123.7 (C8), 116.5 (=CH2), 50.1 (N–CH2), 36.9 (–CH2–), 15.9 (5’–CH3). GC–MS (EI, 70 eV): m/z (%) 293 (M+•, 10), 196 (1), 182 (4), 168 (4), 111 (100).
2–Allyl–N–(3–methylthiophen–2–ylmethyl)–1–naphthylamine (6f)
Colorless viscous oil. Yield: 81%. IR (liquid film, cm−1): ṽ 3360 (N–H), 1635 (C=Callyl), 915 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.27 (dd, J = 8.4, 1.2 Hz, 1H, H–8), 7.86 (br d, J = 7.2 Hz, 1H, H–5), 7.58 (br d, J = 8.4 Hz, 1H, H–4), 7.54 (ddd, J = 8.4, 7.0, 1.4 Hz, 1H, H–7), 7.48 (ddd, J = 7.2, 7.0, 1.2 Hz, 1H, H–6), 7.31 (br d, J = 8.4 Hz, 1H, H–3), 7.19 (d, J = 5.2 Hz, 1H, H–5’), 6.86 (d, J = 5.2 Hz, 1H, H–4’), 6.00 (ddt, J = 17.2, 10.0, 6.4 Hz, 1H, =CH), 5.13 (dq, J = 10.2, 1.5 Hz, 1H, =CH2(H–cis)), 5.06 (dq, J = 17.2, 1.6 Hz, 1H, =CH2(H–trans)), 4.43 (s, 2H, N–CH2), 3.46 (dt, J = 6.4, 1.6 Hz, 2H, –CH2–), 2.08 (s, 3H, 3’–CH3). 13C NMR (100 MHz, CDCl3): δ 142.6 (C1), 137.1 (=CH), 136.9 (C2’), 134.3 (C4a, C3’), 130.6 (C5’), (129.7 (C8a), 128.9 (C5), 128.8 (C3), 128.6 (C2), 126.1 (C7), 125.7 (C6), 124.0 (C4), 123.7 (C8), 123.4 (C4’), 116.4 (=CH2), 47.8 (N–CH2), 36.8 (–CH2–), 14.0 (3’–CH3). GC–MS (EI, 70 eV): m/z (%) 357 (M+•, 3), 274 (2), 260 (4), 246 (1), 180 (12), 111 (100).
2–Allyl–N–(5–bromothiophen–2–ylmethyl)–1–naphthylamine (6g)
Colorless viscous oil. Yield: 78%. IR (liquid film, cm−1): ṽ 3360 (N–H), 1628 (C=Callyl), 915 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.19 (br d, J = 8.4 Hz, 1H, H–8), 7.86 (br d, J = 8.4 Hz, 1H, H–5), 7.59 (d, J = 8.4 Hz, 1H, H–4), 7.53 (ddd, J = 8.4, 7.0, 1.2 Hz, 1H, H–7), 7.49 (ddd, J = 8.4, 7.0, 1.2 Hz, 1H, H–6), 7.32 (d, J = 8.4 Hz, 1H, H–3), 6.96 (d, J = 3.8 Hz, 1H, H–4’), 6.71 (dd, J = 3.8, 1.0 Hz, 1H, H–3’), 6.02 (ddt, J = 16.8, 10.2, 6.0 Hz, 1H, =CH), 5.14 (dq, J = 10.2, 1.6 Hz, 1H, =CH2(H–cis)), 5.05 (dq, J = 16.8, 1.6 Hz, 1H, =CH2(H–trans)), 4.43 (s, 2H, N–CH2), 3.50 (dt, J = 6.0, 1.6 Hz, 2H, –CH2–). 13C NMR (100 MHz, CDCl3): δ 142.0 (C1), 145.6 (C2’), 137.0 (=CH), 134.3 (C4a), 130.0 (C4’), 129.5 (C8a), 129.0 (C3), 128.8 (C5), 128.7 (C2), 126.2 (C7), 125.8 (C6), 125.4 (C3’), 124.3 (C4), 123.4 (C8), 116.5 (=CH2), 111.5, (C5’), 50.0 (N–CH2), 36.9 (–CH2–). GC–MS (EI, 70 eV): m/z (%) 357 (M+•, 79Br, 8), 196 (1), 182 (39), 175 (100).
2–Allyl–4–bromo–N–(furan–2–ylmethyl)–1–naphthylamine (7a)
Colorless viscous oil. Yield: 74%. IR (liquid film, cm−1): ṽ 3370 (N–H), 1636 (C=Callyl), 916 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.22 (dd, J = 7.0, 1.6 Hz, 1H, H–8), 8.20 (dd, J = 7.0, 1.5 Hz, 1H, H–5), 7.58 (s, 1H, H–3), 7.57 (td, J = 7.0, 1.5 Hz, 1H, H–7), 7.55 (td, J = 7.0, 1.6 Hz, 1H, H–6), 7.41 (dd, J = 1.9, 0.8 Hz, 1H, H–5’), 6.31 (dd, J = 3.1, 1.9 Hz, 1H, H–4’), 6.06 (dd, J = 3.1, 0.8 Hz, 1H, H–3’), 5.93 (ddt, J = 17.1, 10.0, 6.1 Hz, 1H, =CH), 5.15 (dq, J = 10.0, 1.5 Hz, 1H, =CH2(H–cis)), 5.05 (dq, J = 17.1, 1.5 Hz, 1H, =CH2(H–trans)), 4.28 (s, 2H, N–CH2), 3.33 (dt, J = 6.1, 1.5 Hz, 2H, –CH2–). 13C NMR (100 MHz, CDCl3): δ 153.6 (C2’), 142.2 (C5’), 142.1 (C1), 136.3 (=CH), 132.2 (C3), 132.1 (C4a), 129.2 (C2), 129.1 (C8a), 127.4 (C5), 126.7 (C7), 126.4 (C6), 124.0 (C8), 117.0 (C4), 116.4 (=CH2), 110.6 (C4’), 107.2 (C3’), 47.4 (N–CH2), 35.8 (–CH2–). GC–MS (EI, 70 eV): m/z (%) 341 (M+•, 79Br, 8), 274 (1), 260 (6), 246 (1), 81 (100).
2–Allyl–4–bromo–N–(5–methylfuran–2–ylmethyl)–1–naphthylamine (7b)
Colorless viscous oil. Yield: 82%. IR (liquid film, cm−1): ṽ 3369 (N–H), 1635 (C=Callyl), 917 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.21 (dd, J = 8.5, 2.0 Hz, 1H, H–8), 8.20 (dd, J = 8.6, 1.0 Hz, 1H, H–5), 7.57 (s, 1H, H–3), 7.56 (td, J = 8.6, 1.0 Hz, 1H, H–7), 7.54 (td, J = 8.6, 2.0 Hz, 1H, H–6), 5.93 (ddt, J = 17.1, 10.0, 6.1 Hz, 1H, =CH), 5.92 (d, J = 3.2 Hz, 1H, H–3’), 5.87 (d, J = 3.2 Hz, 1H, H–4’), 5.13 (dq, J = 10.0, 1.6 Hz, 1H, =CH2(H–cis)), 5.06 (dq, J = 17.1, 1.6 Hz, 1H, =CH2(H–trans)), 4.20 (s, 2H, N–CH2), 3.35 (dt, J = 6.1, 1.6 Hz, 2H, –CH2–), 2.31 (s, 3H, 5’–CH3). 13C NMR (100 MHz, CDCl3): δ 151.8 (C2’), 151.6 (C5’),142.4 (C1), 136.2 (=CH), 132.1 (C3), 130.4 (C8a), 132.0 (C4a), 129.0 (C2), 127.7 (C5), 126.7 (C7), 126.4 (C6), 124.0 (C8), 117.0 (C4), 116.6 (=CH2), 108.1 (C3’), 106.3 (C4’), 47.6 (N–CH2), 35.9 (–CH2–). GC–MS (EI, 70 eV): m/z (%) 355 (M+•, 79Br, 4), 274 (1), 260 (2), 246 (2), 95 (100).
2–Allyl–N–(furan–2–ylmethyl)–1–naphthylamine (7c)
Colorless viscous oil. Yield: 77%. IR (liquid film, cm−1): ṽ 3369 (N–H), 1634 (C=Callyl), 915 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.23 (br d, J = 8.4 Hz, 1H, H–8), 7.84 (br d, J = 8.1 Hz, 1H, H–5), 7.55 (d, J = 8.4 Hz, 1H, H–4), 7.51 (ddd, J = 8.4, 8.1, 1.5 Hz, 1H, H–7), 7.47 (td, J = 8.1, 1.2 Hz, 1H, H–6), 7.43 (dd, J = 3.0, 0.7 Hz, 1H, H–5’), 7.28 (d, J = 8.4 Hz, 1H, H–3), 6.33 (dd, J = 3.0, 1.8 Hz, 1H, H–4’), 6.11 (d, J = 3.0 Hz, 1H, H–3’), 5.99 (ddt, J = 17.1, 10.1, 6.0 Hz, 1H, =CH), 5.12 (dq, J = 10.1, 1.5 Hz, 1H, =CH2(H–cis)), 5.06 (dq, J = 17.1, 1.5 Hz, 1H, =CH2(H–trans)), 4.32 (s, 2H, N–CH2), 3.41 (dt, J = 6.0, 1.5Hz, 2H, –CH2–). 13C NMR (100 MHz, CDCl3): δ 153.7 (C2’), 142.2 (C5’), 142.0 (C1), 136.9 (=CH), 133.9 (C4a), 128.6 (C8a), 128.4 (C3, C5), 128.1 (C2), 125.7 (C7), 125.4 (C6), 123.6 (C8), 123.4 (C4), 116.1 (=CH2), 107.2 (C3’), 47.0 (N–CH2), 36.6 (–CH2–). GC–MS (EI, 70 eV): m/z (%) 263 (M+•, 28), 196 (1), 182 (34), 168 (60), 81 (100).
2–Allyl–N–(5–methylfuran–2–ylmethyl)–1–naphthylamine (7d)
Colorless viscous oil. Yield: 76%. IR (liquid film, cm−1): ṽ 3369 (N–H), 1635 (C=Callyl), 917 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.24 (br d, J = 8.4 Hz, 1H, H–8), 7.84 (br d, J = 8.4 Hz, 1H, H–5), 7.55 (d, J = 8.2 Hz, 1H, H–4), 7.51 (td, J = 8.4, 1.4 Hz, 1H, H–7), 7.45 (td, J = 8.4, 1.1 Hz, 1H, H–6), 7.29 (d, J = 8.2 Hz, 1H, H–3), 6.01 (ddt, J = 17.1, 10.1, 6.0 Hz, 1H, =CH), 5.99 (d, J = 3.0 Hz, 1H, H–3’), 5.91 (dd, J = 3.0, 1.0 Hz, 1H, H–4’), 5.14 (dq, J = 10.1, 1.2 Hz, 1H, =CH2(H–cis)), 5.09 (dq, J = 17.1, 1.2 Hz, 1H, =CH2(H–trans)), 4.26 (s, 2H, N–CH2), 3.46 (dt, J = 6.0, 1.2 Hz, 2H, –CH2–), 2.35 (s, 3H, 5’-CH3). 13C NMR (100 MHz, CDCl3): δ 151.9 (C2’), 151.7 (C5’), 142.3 (C1), 136.9 (=CH), 133.9 (C4a), 129.3 (C8a), 128.5 (C3), 128.4 (C5), 128.0 (C2), 125.6 (C7), 125.3 (C6), 123.5 (C4, C8), 115.9 (=CH2), 108.1 (C3’), 106.2 (C4’), 47.0 (N–CH2), 36.2 (–CH2–), 13.9 (5’–CH3). GC–MS (EI, 70 eV): m/z (%) 277 (M+•, 8), 196 (1), 182 (3), 168 (6), 95 (100).
2–Allyl–N–(5–nitrofuran–2–ylmethyl)–1–naphthylamine (7e)
Colorless viscous oil. Yield: 70%. IR (liquid film, cm−1): ṽ 3373 (N–H), 1636 (C=Callyl), 1497 (–NO2), 1356 (–NO2), 918 (=C–Hallyl). 1H NMR (400 MHz, CDCl3): δ 8.11 (dd, J = 8.3, 1.1 Hz, 1H, H–8), 7.85 (dd, J = 7.4, 1.2 Hz, 1H, H–5), 7.59 (d, J = 8.5 Hz, 1H, H–4), 7.52 (td, J = 8.3, 1.2 Hz, 1H, H–7), 7.47 (td, J = 7.4, 1.1 Hz, 1H, H–6), 7.29 (d, J = 8.5 Hz, 1H, H–3), 7.24 (d, J = 3.6 Hz, 1H, H–4’), 6.33 (d, J = 3.6 Hz, 1H, H–3’), 6.01 (ddt, J = 17.1, 10.1, 5.9 Hz, 1H, =CH), 5.15 (dq, J = 10.1, 1.7 Hz, 1H, =CH2(H–cis)), 5.06 (dq, J = 17.1, 1.7 Hz, 1H, =CH2(H–trans)), 4.41 (s, 2H, N–CH2), 3.48 (dt, J = 5.9, 1.7 Hz, 2H, –CH2–). 13C NMR (100 MHz, CDCl3): δ 157.6 (C5’), 151.9 (C2’), 141.0 (C1), 136.5 (=CH), 134.0 (C4a), 129.0 (C8a), 128.7 (C5), 128.6 (C3), 128.5 (C2), 126.1 (C7), 125.6 (C6), 124.3 (C4), 122.7 (C8), 116.2 (=CH2), 112.8 (C4’), 110.7 (C3’), 47.0 (N–CH2), 36.6 (–CH2–). GC–MS (EI, 70 eV): m/z (%) 308 (M+•, 34), 196 (14), 182 (74), 167 (100), 126 (20).
Physicochemical and spectral data for (2SR,4RS)–2–aryl(heteroaryl)–1,4–epoxy–2,3,4,5–tetrahydro–naphtho[1,2–b]azepines (8a–j), (9a–g), and (10a–e)
(2SR,4SR)–7–Bromo–2–phenyl–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (8a)
Reaction time: 35 h. Yield: 49%. White solid, mp 123 °C (heptane). IR (KBr, cm−1): ṽ 1044 (C–O), 996, (N–O). 1H NMR (400 MHz, CDCl3): δ 8.26 (br d, J = 8.2 Hz, 1H, H–11), 8.19 (br d, J = 8.2 Hz, 1H, H–8), 7.58 (td, J = 8.2, 1.0 Hz, 1H, H–9), 7.57 (d, J = 7.6 Hz, 2H, H–2’, H–6’), 7.56 (s, 1H, H–6), 7.51 (td, J = 8.2, 1.1 Hz, 1H, H–10), 7.44 (t, J = 7.6 Hz, 2H, H–3’, H–5’), 7.34 (t, J = 7.6 Hz, 1H, H–4’), 5.07 (ddd, J = 7.2, 5.3, 1.9 Hz, 1H, H–4), 4.73 (dd, J = 8.3, 2.8 Hz, 1H, H–2), 3.55 (dd, J = 16.8, 5.3 Hz, 1H, HB–5), 2.69 (dddd, J = 12.6, 7.2, 2.8, 0.8 Hz, 1H, HB–3), 2.63 (ddd, J = 12.6, 8.3, 1.9 Hz, 1H, HA–3), 2.61 (br d, J = 16.8 Hz, 1H, HA–5). 13C NMR (100 MHz, CDCl3): δ 145.6 (C11b), 143.6 (C1’), 131.1 (C6), 131.0 (C7a), 128.9 (C11a), 128.8 (C3’, C5’), 127.3 (C4’), 127.2 (C8), 127.1 (C9, C2’, C6’), 126.4 (C10), 122.6 (C11), 122.1 (C5a), 119.6 (C7), 75.4 (C4), 74.5 (C2), 43.2 (C3), 35.0 (C5). GC–MS (EI, 70 eV): m/z (%) 365 (M+•, 79Br, 35Cl, 10), 335 (7), 322 (5), 257 (9), 231 (2), 220 (100), 193 (2). HR–MS (EI–MS) m/z calcd. for C20H16BrNO, 365.0415; found, 365.0421.
(2SR,4SR)–7–Bromo–2–(o–tolyl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (8b)
Reaction time: 60 h. Yield: 50%. Pale yellow solid, mp 177 °C (heptane). IR (KBr, cm−1): ṽ 1048 (C–O), 1000 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.21 (br d, J = 7.3 Hz, 1H, H–11), 8.19 (br d, J = 8.0 Hz, 1H, H–8), 8.09 (br d, J = 7.7 Hz, 1H, H–6’), 7.58 (s, 1H, H–6), 7.56 (td, J = 8.2, 1.1 Hz, 1H, H–9), 7.48 (td, J = 8.2, 0.9 Hz, 1H, H–10), 7.37 (t, J = 7.7 Hz, 1H, H–5’), 7.25 (td, J = 7.6, 1.1 Hz, 1H, H–4’), 7.19 (br d, J = 7.6 Hz, 1H, H–3’), 5.02 (ddd, J = 7.9, 5.4, 1.5 Hz, 1H, H–4), 4.80 (dd, J = 8.6, 2.9 Hz, 1H, H–2), 3.55 (dd, J = 16.9, 5.4 Hz, 1H, HB–5), 2.70 (ddd, J = 12.2, 8.6, 1.5 Hz, 1H, HA–3), 2.63 (br d, J = 16.9 Hz, 1H, HA–5), 2.47 (dddd, J = 12.2, 7.9, 2.9, 1.2 Hz, 1H, HB–3), 2.17 (s, 3H, 2’–CH3). 13C NMR (100 MHz, CDCl3): δ 145.9 (C11b), 141.8 (C1’), 133.9 (C2’), 131.0 (C6), 130.3 (C7a, C3’), 128.9 (C11a), 127.4 (C9), 127.3 (C4’), 127.2 (C10), 127.0 (C8), 126.5 (C5’), 125.7 (C6’), 122.6 (C11), 122.3 (C5a), 119.6 (C7), 75.4 (C4), 72.0 (C2), 43.4 (C3), 35.1 (C5), 19.7 (2’–CH3). GC–MS (EI, 70 eV): m/z (%) 379 (M+•, 79Br, 8), 349 (3), 336 (6), 220 (100), 193 (4). HR–MS (EI–MS) m/z calcd. for C21H18BrNO, 379.0566; found, 379.0572.
(2SR,4SR)–7–Bromo–2–(m–tolyl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (8c)
Reaction time: 58 h. Yield: 51%. Yellow solid, mp 80 °C (heptane). IR (KBr, cm−1): ṽ 1046 (C–O), 992 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.27 (br d, J = 8.3 Hz, 1H, H–11), 8.19 (br d, J = 8.3 Hz, 1H, H–8), 7.59 (d, J = 7.7 Hz, 1H, H–6’), 7.58 (td, J = 8.3, 0.8 Hz, 1H, H–9), 7.55 (s, 1 H, H–6), 7.50 (td, J = 8.2, 0.8 Hz, 1H, H–10), 7.41 (s, 1H, H–2’), 7.33 (t, J = 7.7 Hz, 1H, H–5’), 7.16 (br d, J = 7.7 Hz, 1H, H–4’), 5.07 (ddd, J = 7.0, 5.4, 1.6 Hz, 1H, H–4), 4.70 (dd, J = 8.3, 2.8 Hz, 1H, H–2), 3.55 (dd, J = 16.8, 5.3 Hz, 1H, HB–5), 2.68 (dddd, J = 12.5, 7.0, 2.8, 0.9 Hz, 1H, HB–3), 2.63 (ddd, J = 12.5, 8.3, 1.6 Hz, 1H, HA–3), 2.60 (br d, J = 16.8 Hz, 1H, HA–5), 2.44 (s, 3H, 3’–CH3). 13C NMR (100 MHz, CDCl3): δ 145.7 (C11b), 143.3 (C1’), 138.4 (C3’), 131.0 (C6), 130.1 (C7a), 128.8 (C11a), 128.0 (C4’), 127.3 (C10, C6’), 127.2 (C8), 127.1 (C9), 127.0 (C2’), 123.6 (C5’), 122.5 (C11), 122.1 (C5a), 119.5 (C7), 75.3 (C4), 74.6 (C2), 43.0 (C3), 34.7 (C5), 21.6 (3’–CH3). GC–MS (EI, 70 eV): m/z (%) 379 (M+•, 79Br, 11), 349 (4), 336 (4), 220 (100), 193 (3). HR–MS (EI–MS) m/z calcd. for C21H18BrNO, 379.0563; found, 379.0572.
(2SR,4SR)–7–Bromo–2–(3–methoxyphenyl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (8d)
Reaction time: 57 h. Yield: 48%. Viscous yellow oil. IR (liquid film, cm−1): ṽ 1048 (C–O), 991 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.26 (br d, J = 8.3 Hz, 1H, H–11), 8.18 (br d, J = 8.3 Hz, 1H, H–8), 7.87 (dd, J = 8.0, 2.3 Hz, 1H, H–4’), 7.58 (td, J = 8.1, 1.0 Hz, 1H, H–9), 7.55 (s, 1H, H–6), 7.50 (td, J = 8.2, 1.1 Hz, 1H, H–10), 7.34 (t, J = 7.9 Hz, 1H, H–5’), 7.18 (br d, J = 2.3 Hz, 1H, H–2’), 7.10 (d, J = 7.9 Hz, 1H, H–6’), 5.06 (ddd, J = 7.0, 5.4, 1.5 Hz, 1H, H–4), 4.89 (dd, J = 8.3, 2.8 Hz, 1H, H–2), 3.87 (s, 3H, 3’–OCH3), 3.54 (dd, J = 16.8, 5.4 Hz, 1H, HB–5), 2.66 (dddd, J = 12.5, 7.0, 2.8, 1.0 Hz, 1H, HB–3), 2.61 (ddd, J = 12.5, 8.3, 1.5 Hz, 1H, HA–3), 2.60 (br d, J = 16.8 Hz, 1H, HA–5). 13C NMR (100 MHz, CDCl3): δ 159.9 (C3’), 145.6 (C11b), 145.2 (C1’), 131.1 (C6), 131.0 (C7a), 129.8 (C5’), 128.8 (C11a), 127.3 (C10), 127.2 (C9, C8), 122.5 (C11), 122.2 (C5a), 119.6 (C7), 118.8 (C6’), 112.5 (C4’), 112.1 (C2’), 75.4 (C4), 74.2 (C2), 43.4 (C3), 34.7 (C5), 55.5 (3’–OCH3). GC–MS (EI, 70 eV): m/z (%) 395 (M+•, 79Br, 3), 365 (1), 352 (1), 220 (100), 193 (1). HR–MS (EI–MS) m/z calcd. for C21H18BrNO2, 395.0509; found, 395.0521.
(2SR,4SR)–7–Bromo–2–(2–chlorophenyl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (8e)
Reaction time: 61 h. Yield: 48%. Pale yellow solid, mp 17 °C (heptane). IR (KBr, cm−1): ṽ 1054 (C–O), 955 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.21 (br d, J = 7.2 Hz, 1H, H–11), 8.17 (dd, J = 7.3, 1.1 Hz, 1H, H–8), 8.16 (dd, J = 7.8, 1.3 Hz, 1H, H–6’), 7.60 (s, 1H, H–6), 7.58 (td, J = 7.2, 1.3 Hz, 1H, H–9), 7.49 (td, J = 7.2, 1.3 Hz, 1H, H–10), 7.43 (td, J = 7.8, 1.3 Hz, 1H, H–5’), 7.39 (td, J = 8.0, 1.3 Hz, 1H, H–4’), 7.29 (dd, J = 7.9, 1.6 Hz, 1H, H–3’), 5.03 (ddd, J = 7.7, 5.4, 1.5 Hz, 1H, H–4), 4.96 (dd, J = 8.6, 2.7 Hz, 1H, H–2), 3.57 (dd, J = 16.9, 5.4 Hz, 1H, HB–5), 2.81 (ddd, J = 12.8, 8.6, 1.5 Hz, 1H, HA–3), 2.54 (dddd, J = 12.8, 7.7, 2.7, 0.9 Hz, 1H, HB–3), 2.65 (br d, J = 16.9 Hz, 1H, HA–5). 13C NMR (100 MHz, CDCl3): δ 145.3 (C11b), 140.9 (C1’), 132.0 (C2’), 131.1 (C7a), 131.0 (C6), 129.4 (C4’), 128.8 (C11a), 128.3 (C3’), 127.8 (C9), 127.4 (C10), 127.3 (C5’), 127.2 (C8), 122.5 (C11), 122.4 (C5a, C6’), 119.8 (C7), 75.3 (C4), 71.8 (C2), 43.4 (C3), 34.8 (C5). GC–MS (EI, 70 eV): m/z (%) 399 (M+•, 79Br, 35Cl, 1), 365 (100), 348 (6), 335 (4), 322 (2). HR–MS (EI–MS) m/z calcd. for C20H15BrClNO, 399.0022; found, 399.0026.
(2SR,4SR)–7–Bromo–2–(4–chlorophenyl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (8f)
Reaction time: 61 h. Yield: 48%. Yellow solid, mp 181 °C (heptane). IR (KBr, cm−1): ṽ 1047 (C–O), 958 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.28 (br d, J = 8.5 Hz, 1H, H–11), 8.18 (br d, J = 8.5 Hz, 1H, H–8), 7.52–7.58 (m, 2H, H–9, H–10), 7.55 (s, 1H, H–6), 7.48 (d, J = 7.6 Hz, 2H, H–2’, H–6’), 7.38 (d, J = 7.6 Hz, 2H, H–3’, H–5’), 5.06 (ddd, J = 8.0, 5.1, 1.4 Hz, 1H, H–4), 4.66 (dd, J = 8.4, 2.0 Hz, 1H, H–2), 3.53 (dd, J = 16.7, 5.1 Hz, 1H, HB–5), 2.69 (m, 2H, HA–3, HB–3), 2.70 (br d, J = 16.7 Hz, 1H, HA–5). 13C NMR (100 MHz, CDCl3): δ 145.2 (C11b), 142.0 (C1’), 133.0 (C4’), 131.0 (C6, C7a), 128.9 (C3’, C5’), 128.8 (C11a), 127.7 (C2’, C6’), 127.3 (C8), 127.2 (C9, C10), 122.3 (C11), 122.1 (C5a), 119.8 (C7), 75.4 (C4), 73.8 (C2), 43.1 (C3), 34.8 (C5). GC–MS (EI, 70 eV): m/z (%) 399 (M+•, 79Br, 35Cl, 5), 369 (3), 356 (2), 220 (100), 193 (3). HR–MS (EI–MS) m/z calcd. for C20H15BrClNO, 399.0008; found, 399.0021.
(2SR,4SR)–7–Bromo–2–(2,4–dichlorophenyl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (8g)
Reaction time: 60 h. Yield: 49%. White solid, mp 192 °C (heptane). IR (KBr, cm−1): ṽ 1045 (C–O), 993 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.19 (br d, J = 8.3 Hz, 1H, H–11), 8.11 (br d, J = 8.7 Hz, 1H, H–8), 8.09 (d, J = 8.9 Hz, 1H, H–6’), 7.57 (s, 1H, H–6), 7.56 (td, J = 8.7, 1.2 Hz, 1H, H–9), 7.47 (td, J = 8.3, 1.2 Hz, 1H, H–10), 7.40 (dd, J = 8.9, 2.0 Hz, 1H, H–5’), 7.39 (d, J = 2.0 Hz, 1H, H–3’), 5.00 (ddd, J = 7.9, 5.4, 1.5 Hz, 1H, H–4), 4.87 (dd, J = 8.6, 2.8 Hz, 1H, H–2), 3.54 (dd, J = 16.9, 5.4 Hz, 1H, HB–5), 2.76 (ddd, J = 12.8, 8.6, 1.5 Hz, 1H, HA–3), 2.62 (br d, J = 16.9 Hz, 1H, HA–5), 2.50 (dddd, J = 12.8, 7.9, 2.8, 1.3 Hz, 1H, HB–3). 13C NMR (100 MHz, CDCl3): δ 145.3 (C11b), 139.9 (C1’), 133.7 (C4’), 133.0 (C2’), 131.3 (C7a), 131.2 (C6), 129.2 (C6’), 129.0 (C11a), 127.8 (C9, C5’), 127.7 (C10), 127.6 (C8, C3’), 122.6 (C5a), 122.5 (C11), 120.4 (C7), 75.7 (C4), 71.9 (C2), 43.9 (C3), 35.3 (C5). GC–MS (EI, 70 eV): m/z (%) 432 (M+•, 79Br, 35Cl, 1), 399 (100), 382 (5), 369 (3), 356 (1). HR–MS (EI–MS) m/z calcd. for C20H14BrCl2NO, 432.9639; found, 432.9636.
(2SR,4SR)–2–(o–Tolyl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (8h)
Reaction time: 58 h. Yield: 47%. Pale yellow solid, mp 198 °C (heptane). IR (KBr, cm−1): ṽ 1046 (C–O), 995 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.15 (dd, J = 8.0, 1.0 Hz, 1H, H–11), 8.10 (dd, J = 8.0, 1.3 Hz, 1H, H–8), 7.79 (dd, J = 7.2, 1.5 Hz, 1H, H–6’), 7.64 (d, J = 8.5 Hz, 1H, H–7), 7.44 (td, J = 8.0, 1.3 Hz, 1H, H–10), 7.40 (td, J = 8.0, 1.0 Hz, 1H, H–9), 7.35 (t, J = 7.2 Hz, 1H, H–5’), 7.24 (d, J = 7.2 Hz, 1H, H–3’), 7.22 (td, J = 7.2, 1.0 Hz, 1H, H–4’), 7.16 (d, J = 8.5 Hz, 1H, H–6), 5.03 (ddd, J = 8.2, 5.2, 1.8 Hz, 1H, H–4), 4.81 (dd, J = 8.8, 2.8 Hz, 1H, H–2), 3.59 (dd, J = 16.8, 5.2 Hz, 1H, HB–5), 2.73 (ddd, J = 12.0, 8.8, 1.8 Hz, 1H, HA–3), 2.64 (d, J = 16.8, 1H, HA–5), 2.47 (dddd, J = 12.0, 7.2, 2.8, 0.8 Hz, 1H, HB–3), 2.16 (s, 3H, 2’–CH3). 13C NMR (100 MHz, CDCl3): δ 145.9 (C11b), 142.2 (C1’), 134.0 (C2’), 132.7 (C7a), 130.2 (C3’), 127.8 (C8), 127.7 (C11a), 127.4 (C6), 126.9 (C4’), 126.4 (C10, C6’), 126.0 (C5’), 125.8 (C9), 125.6 (C7), 122.3 (C11), 121.1 (C5a), 75.7 (C4), 72.0 (C2), 43.7 (C3), 35.3 (C5), 19.6 (2’–CH3). GC–MS (EI, 70 eV): m/z (%) 301 (M+•, 41), 272 (7), 258 (21), 142 (80), 115 (100). HR–MS (EI–MS) m/z calcd. for C21H19NO, 301.1473; found, 301.1467.
(2SR,4SR)–2–(3–Nitrophenyl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (8i)
Reaction time: 58 h.Yield: 48%. Pale yellow solid. mp 137 °C (heptane). IR (KBr, cm−1): ṽ 1525 (–NO2), 1325 (–NO2), 1046 (C–O), 1004 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.39 (s, 1H, H–2’), 8.16 (ddd, J = 8.0, 2.0, 0.8 Hz, 1H, H–4’), 7.95 (ddd, J = 8.0, 2.0, 0.8, Hz, 1H, H–6’), 7.81 (dd, J = 7.0, 2.7 Hz, 1H, H–11), 7.79 (dd, J = 7.0, 2.5 Hz, 1H, H–8), 7.64 (d, J = 8.4 Hz, 1H, H–7), 7.57 (t, J = 7.6 Hz, 1H, H–5’), 7.41–7.47 (m, 2H, H–9, H–10), 7.21 (d, J = 8.4 Hz, 1H, H–6), 5.09 (ddd, J = 7.3, 5.5, 1.8 Hz, 1H, H–4), 4.80 (dd, J = 8.6, 2.8 Hz, 1H, H–2), 3.57 (dd, J = 16.7, 5.5 Hz, 1H, HB–5), 2.70 (ddd, J = 12.5, 8.6, 1.8 Hz, 1H, HA–3), 2.63 (br d, J = 16.7 Hz, 1H, HA–5), 2.60 (dddd, J = 12.5, 7.3, 2.8, 1.0 Hz, 1H, HB–3). 13C NMR (100 MHz, CDCl3): δ 148.6 (C3’), 145.9 (C1’), 144.7 (C11b), 132.9 (C6’), 132.7 (C7a), 129.7 (C5’), 128.0 (C8), 127.5 (C11a), 127.4 (C6), 126.6 (C9), 126.0 (C10, C7), 122.2 (C11), 121.7 (C4’), 121.1 (C2’), 120.9 (C5a), 75.8 (C4), 73.6 (C2), 43.3 (C3), 35.2 (C5). GC–MS (EI, 70 eV): m/z (%) 332 (M+•, 10), 302 (1), 289 (7), 142 (100), 115 (31). HR–MS (EI–MS) m/z calcd. for C20H16N2O3, 332.1160; found, 332.1161.
(2SR,4SR)–2–(4–Nitrophenyl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (8j)
Reaction time: 58 h. Yield: 50%. Yellow solid, mp 64 °C (heptane). IR (KBr, cm−1): ṽ 1519 (–NO2), 1342 (–NO2), 1048 (C–O), 960 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.27 (d, J = 8.5 Hz, 2H, H–3’, H–5’), 8.11 (dd, J = 7.6, 1.8 Hz, 1H, H–11), 7.81 (dd, J = 7.6, 1.7 Hz, 1H, H–8), 7.75 (d, J = 8.5 Hz, 2H, H–2’, H–6’), 7.66 (d, J = 8.3 Hz, 1H, H–7), 7.47 (td, J = 7.6, 1.8 Hz, 1H, H–10), 7.44 (td, J = 7.6, 1.8 Hz, 1H, H–9), 7.23 (d, J = 8.3 Hz, 1H, H–6), 5.09 (ddd, J = 6.9, 5.2, 1.4 Hz, 1H, H–4), 4.81 (dd, J = 8.7, 2.5 Hz, 1H, H–2), 3.59 (dd, J = 16.9, 5.2 Hz, 1H, HB–5), 2.72 (ddd, J = 12.4, 8.7, 1.4 Hz, 1H, HA–3), 2.65 (br d, J = 16.9 Hz, 1H, HA–5), 2.60 (dddd, J = 12.4, 6.9, 2.5, 1.0 Hz, 1H, HB–3). 13C NMR (100 MHz, CDCl3): δ 151.0 (C4’), 147.2 (C1’), 144.7 (C11b), 132.7 (C7a), 128.0 (C8), 127.4 (C6), 127.3 (C11a, C2’, C6’), 126.7 (C9), 126.3 (C10), 126.1 (C7), 124.1 (C3’, C5’), 121.6 (C11), 121.0 (C5a), 75.9 (C4), 73.7 (C2), 43.2 (C3), 35.1 (C5). GC–MS (EI, 70 eV): m/z (%) 332 (M+•, 12), 302 (1), 289 (7), 142 (100), 115 (31). HR–MS (EI–MS) m/z calcd. for C20H16N2O3, 332.1158; found, 332.1161.
(2SR,4SR)–7–Bromo–2–(thiophen–2–yl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (9a)
Reaction time: 39 h. Yield: 52%. Pale Yellow solid, mp 139 °C (heptane). IR (KBr, cm−1): ṽ 1044 (C–O), 996 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.38 (dd, J = 7.3, 2.4 Hz, 1H, H–11), 8.18 (dd, J = 7.3, 2.1 Hz, 1H, H–8), 7.59 (td, J = 7.3, 2.4 Hz, 1H, H–9), 7.57 (td, J = 7.3, 2.1 Hz, 1H, H–10), 7.54 (s, 1H, H–6), 7.30 (dd, J = 4.2, 2.0 Hz, 1H, H–5’), 7.02 (dd, J = 4.3, 2.0 Hz, 1H, H–3’), 7.00 (dd, J = 4.2, 4.3 Hz, 1H, H–4’), 5.12 (ddd, J = 7.7, 5.3, 1.8 Hz, 1H, H–4), 4.94 (dd, J = 8.3, 2.0 Hz, 1H, H–2), 3.56 (dd, J = 16.9, 5.3 Hz, 1H, HB–5), 2.81 (dddd, J = 12.6, 7.7, 2.0, 1.0 Hz, 1H, HB–3), 2.60 (br d, J = 16.9 Hz, 1H, HA–5), 2.56 (ddd, J = 12.6, 8.3, 1.8 Hz, 1H, HA–3). 13C NMR (100 MHz, CDCl3): δ 147.3 (C2’), 144.7 (C11b), 133.1 (C7a), 131.0 (C6), 128.0 (C11a), 127.4 (C8, C10), 127.3 (C9), 126.9 (C5’), 125.0 (C4’), 124.1 (C3’), 122.6 (C11), 121.0 (C5a), 120.0 (C7), 75.6 (C4), 70.9 (C2), 42.7 (C3), 35.1 (C5). GC–MS (EI, 70 eV): m/z (%) 371 (M+•, 79Br, 18), 341 (5), 328 (7), 257 (4), 231 (2), 220 (100), 193 (1). HR–MS (EI–MS) m/z calcd. for C18H14BrNOS, 370.9976; found, 370.9979.
(2SR,4SR)–7–Bromo–2–(5–methylthiophen–2–yl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (9b)
Reaction time: 40 h. Yield: 47%. White solid, mp 122 °C (heptane). IR (KBr, cm−1): ṽ 1050 (C–O), 989 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.37–8.41 (m, 1H, H–11), 8.15–8.22 (m, 1H, H–8), 7.54–7.63 (m, 2H, H–9, H–10), 7.53 (s, 1H, H–6), 6.79 (d, J = 3.4 Hz, 1H, H–3’), 6.65 (dd, J = 3.2, 0.8 Hz, 1H, H–4’), 5.06–5.12 (m, 1H, H–4), 4.97 (br d, J = 7.9 Hz, 1H, H–2), 3.54 (dd, J = 16.8, 5.3 Hz, 1H, HB–5), 2.77 (dddd, J = 12.0, 7.5, 2.0, 1.0 Hz, 1H, HB–3), 2.62 (br d, J = 16.8 Hz, 1H, HA–5), 2.51 (s, 3H, 5’–CH3), 2.43–2.53 (m, 1H, HA–3). 13C NMR (100 MHz, CDCl3): δ 144.8 (C11b), 144.2 (C2’), 139.5 (C5’), 132.0 (C7a), 131.0 (C6), 128.9 (C11a), 127.4 (C9, C10), 127.3 (C8), 124.8 (C4’), 124.0 (C3’), 122.7 (C11), 122.0 (C5a), 119.9 (C7), 75.2 (C4), 70.9 (C2), 42.4 (C3), 34.9 (C5), 15.4 (5’–CH3). GC–MS (EI, 70 eV): m/z (%) 385 (M+•, 79Br, 8), 355 (3), 342 (6), 257 (4), 231 (3), 220 (100), 193 (3). HR–MS (EI–MS) m/z calcd. for C19H16BrNOS, 385.0134; found, 385.0136.
(2SR,4SR)–7–Bromo–2–(3–methylthiophen–2–yl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (9c)
Reaction time: 34 h. Yield: 44%. Pale yellow solid, mp 145 °C (heptane). IR (KBr, cm−1): ṽ 1041 (C–O), 988 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.30 (dd, J = 8.4, 0.7 Hz, 1H, H–11), 8.18 (dd, J = 8.3, 1.0 Hz, 1H, H–8), 7.59 (td, J = 8.3, 0.7 Hz, 1H, H–9), 7.55 (s, 1H, H–6), 7.51 (td, J = 8.4, 1.0 Hz, 1H, H–10), 7.20 (d, J = 5.1 Hz, 1H, H–5’), 6.82 (dd, J = 5.1, 0.8 Hz, 1H, H–4’), 5.12 (ddd, J = 7.5, 5.5, 2.1 Hz, 1H, H–4), 4.95 (dd, J = 8.0, 2.6 Hz, 1H, H–2), 3.56 (dd, J = 16.9, 5.5 Hz, 1H, HB–5), 2.67 (dddd, J = 12.5, 7.5, 2.6, 1.2 Hz, 1H, HB–3), 2.62 (ddd, J = 12.5, 8.0, 2.1 Hz, 1H, HA–3), 2.60 (br d, J = 16.9 Hz, 1H, HA–5), 2.14 (s, 3H, 3’–CH3). 13C NMR (100 MHz, CDCl3): δ 144.9 (C11b), 140.7 (C2’), 132.3 (C3’), 131.0 (C6, C7a), 129.8 (C5’), 128.7 (C11a), 127.4 (C8), 127.3 (C10), 127.2 (C9), 122.6 (C4’), 122.0 (C5a), 121.9 (C11), 119.9 (C7), 75.3 (C4), 69.8 (C2), 43.2 (C3), 34.9 (C5), 14.2 (3’–CH3). GC–MS (EI, 70 eV): m/z (%) 385 (M+•, 79Br, 14), 355 (5), 342 (7), 257 (1), 231 (1), 220 (100), 193 (1). HR–MS (EI–MS) m/z calcd. for C19H16BrNOS, 385.0137; found, 385.0136.
(2SR,4SR)–2–(Thiophen–2–yl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (9d)
Reaction time: 34 h. Yield: 48%. Pale yellow solid, mp 134 °C (heptane). IR (KBr, cm−1): ṽ 1041 (C–O), 986 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.39 (br d, J = 8.2 Hz, 1H, H–11), 7.81 (br d, J = 8.2 Hz, 1H, H–8), 7.65 (d, J = 8.4 Hz, 1H, H–7), 7.53 (ddd, J = 8.2, 6.8, 1.4 Hz, 1H, H–10), 7.49 (ddd, J = 8.2, 6.8, 1.4 Hz, 1H, H–9), 7.29 (dd, J = 4.5, 1.7 Hz, 1H, H–5’), 7.21 (d, J = 8.4 Hz, 1H, H–6), 7.04 (dd, J = 4.6, 1.7 Hz, 1H, H–3’), 7.02 (td, J = 4.6, 4.5 Hz, 1H, H–4’), 5.12 (ddd, J = 7.8, 5.2, 2.0 Hz, 1H, H–4), 4.97 (dd, J = 8.2, 1.8 Hz, 1H, H–2), 3.58 (dd, J = 16.8, 5.2 Hz, 1H, HB–5), 2.77–2.83 (m, 1H, HB–3), 2.62 (br d, J = 16.8 Hz, 1H, HA–5), 2.56–2.61 (m, 1H, HA–3). 13C NMR (100 MHz, CDCl3): δ 147.8 (C2’), 144.7 (C11b), 132.7 (C7a), 127.9 (C8), 127.6 (C11a), 127.3 (C6), 126.8 (C5’), 126.5 (C10), 126.0 (C9), 125.9 (C7), 124.8 (C4’), 124.0 (C3’), 122.2 (C11), 120.9 (C5a), 75.5 (C4), 70.9 (C2), 42.9 (C3), 35.3 (C5). GC–MS (EI, 70 eV): m/z (%) 293 (M+•, 18), 263 (3), 250 (24), 180 (6), 142 (100), 115 (26). HR–MS (EI–MS) m/z calcd. for C18H15NOS, 293.0868; found, 293.0874.
(2SR,4SR)–2–(5–Methylthiophen–2–yl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (9e)
Reaction time: 36 h. Yield: 50%. Pale yellow solid, mp 132 °C (heptane). IR (KBr, cm−1): ṽ 1048 (C–O), 991 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.38 (br d, J = 8.4 Hz, 1H, H–11), 7.80 (br d, J = 8.4 Hz, 1H, H–8), 7.63 (d, J = 8.4 Hz, 1H, H–7), 7.53 (ddd, J = 8.4, 6.8, 1.4 Hz, 1H, H–10), 7.47 (ddd, J = 8.4, 6.8, 1.4 Hz, 1H, H–9), 7.20 (d, J = 8.4 Hz, 1H, H–6), 6.81 (dd, J = 3.2, 0.4 Hz, 1H, H–4’), 6.66 (dd, J = 3.2, 1.0 Hz, 1H, H–3’), 5.10 (ddd, J = 7.6, 5.2, 2.0 Hz, 1H, H–4), 4.89 (br d, J = 8.4 Hz, 1H, H–2), 3.57 (dd, J = 16.8, 5.2 Hz, 1H, HB–5), 2.78 (dddd, J = 12.8, 7.6, 2.0, 1.2 Hz, 1H, HB–3), 2.60 (br d, J = 16.8 Hz, 1H, HA–5), 2.54 (ddd, J = 12.8, 8.4, 1.6 Hz, 1H, HA–3), 2.52 (s, 3H, 5’–CH3). 13C NMR (100 MHz, CDCl3): δ 145.5 (C11b), 145.1 (C2’), 139.7 (C5’), 133.0 (C7a), 128.2 (C8), 127.9 (C11a), 127.6 (C6), 126.7 (C9), 126.2 (C10), 126.1 (C7), 125.0 (C4’), 124.1 (C3’), 122.5 (C11), 121.1 (C5a), 75.7 (C4), 71.4 (C2), 42.8 (C3), 35.6 (C5), 15.7 (5’–CH3). GC–MS (EI, 70 eV): m/z (%) 307 (M+•, 18), 277 (4), 264 (30), 180 (8), 142 (100), 115 (25). HR–MS (EI–MS) m/z calcd. for C20H17NOS, 307.1033; found, 307.1031.
(2SR,4SR)–2–(3–Methylthiophen–2–yl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (9f)
Reaction time: 28 h. Yield: 50%. Pale yellow solid, mp 149 °C (heptane). IR (KBr, cm−1): ṽ 1045 (C–O), 986 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.26–8.29 (m, 1H, H–11), 7.79–7.81 (m, 1H, H–8), 7.64 (d, J = 8.4 Hz, 1H, H–7), 7.44–7.49 (m, 2H, H–9, H–10), 7.21 (d, J = 8.4 Hz, 1H, H–6), 7.20 (d, J = 5.0 Hz, 1H, H–5’), 6.82 (d, J = 5.0 Hz, 1H, H–4’), 5.13 (ddd, J = 7.2, 5.2, 2.0 Hz, 1H, H–4), 4.99 (dd, J = 8.0, 2.8 Hz, 1H, H–2), 3.59 (dd, J = 16.8, 5.2 Hz, 1H, HB–5), 2.68 (dddd, J = 12.4, 7.2, 2.8, 0.8 Hz, 1H, HB–3), 2.62 (br d, J = 16.8 Hz, 1H, HA–5), 2.59–2.65 (m, 1H, HA–3), 2.15 (s, 3H, 3’–CH3). 13C NMR (100 MHz, CDCl3): δ 145.3 (C11b), 141.4 (C2’), 132.9 (C7a), 132.4 (C3’), 130.0 (C5’), 128.2 (C8), 127.9 (C11a), 127.6 (C6), 126.7 (C9), 126.3 (C10), 126.1 (C7), 123.5 (C4’), 122.6 (C11), 121.0 (C5a), 75.8 (C4), 70.0 (C2), 43.7 (C3), 35.5 (C5), 14.5 (3’–CH3). GC–MS (EI, 70 eV): m/z (%) 307 (M+•, 14), 277 (3), 264 (14), 180 (9), 142 (100), 115 (31). HR–MS (EI–MS) m/z calcd. for C19H17NOS, 307.1019; found, 307.1031.
(2SR,4SR)–2–(5–bromothiophen–2–yl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (9g)
Reaction time: 20 h. Yield: 49%. Pale yellow solid, mp 135 °C (heptane). IR (KBr, cm−1): ṽ 1044 (C–O), 981 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.33 (br d, J = 8.4 Hz, 1H, H–11), 7.82 (br d, J = 8.4 Hz, 1H, H–8), 7.66 (d, J = 8.4 Hz, 1H, H–7), 7.55 (ddd, J = 8.4, 7.0, 1.4 Hz, 1H, H–10), 7.50 (ddd, J = 8.4, 7.0, 1.4 Hz, 1H, H–9), 7.21 (d, J = 8.4 Hz, 1H, H–6), 6.97 (d, J = 3.8 Hz, 1H, H–4’), 6.76 (dd, J = 3.8, 1.0 Hz, 1H, H–3’), 5.11 (ddd, J = 7.6, 5.2, 2.0 Hz, 1H, H–4), 4.88 (dd, J = 8.4, 2.0 Hz, 1H, H–2), 3.59 (dd, J = 16.8, 5.2 Hz, 1H, HB–5), 2.75 (dddd, J = 12.4, 7.6, 2.0, 0.8 Hz, 1H, HB–3), 2.63 (br d, J = 16.8 Hz, 1H, HA–5), 2.57 (ddd, J = 12.4, 8.4, 2.0 Hz, 1H, HA–3). 13C NMR (100 MHz, CDCl3): δ 149.7 (C2’), 144.5 (C11b), 133.0 (C7a), 129.8 (C4’), 128.3 (C8), 127.8 (C11a), 127.6 (C6), 126.9 (C10), 126.4 (C9), 126.3 (C7), 124.3 (C3’), 122.2 (C11), 121.1 (C5a), 112.0 (C5’), 75.8 (C4), 71.3 (C2), 42.8 (C3), 35.6 (C5). GC–MS (EI, 70 eV): m/z (%) 371 (M+•, 79Br, 8), 341 (4), 328 (7), 180 (5), 154 (2), 142 (100), 115 (27). HR–MS (EI–MS) m/z calcd. for C18H14BrNOS, 370.9980; found, 370.9979.
(2SR,4SR)–7–Bromo–2–(furan–2–yl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (10a)
Reaction time: 39 h. Yield: 50%. Pale yellow solid, mp 187 °C (heptane). IR (KBr, cm−1): ṽ 1049 (C–O), 993 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.49 (dd, J = 7.6, 2.0 Hz, 1H, H–11), 8.20 (dd, J = 7.6, 2.1 Hz, 1H, H–8), 7.61 (td, J = 7.6, 2.1 Hz, 1H, H–10), 7.59 (td, J = 7.6, 2.0 Hz, 1H, H–9), 7.53 (s, 1H, H–6), 7.48 (dd, J = 3.2, 1.8 Hz, 1H, H–5’), 6.44 (d, J = 3.2 Hz, 1H, H–4’), 6.41 (dd, J = 3.2, 1.8 Hz, 1H, H–3’), 5.09 (ddd, J = 7.8, 5.3, 1.8 Hz, 1H, H–4), 4.73 (dd, J = 8.6, 2.5 Hz, 1H, H–2), 3.54 (dd, J = 16.8, 5.3 Hz, 1H, HB–5), 2.85 (dddd, J = 12.6, 7.8, 2.5, 1.1 Hz, 1H, HB–3), 2.60 (br d, J = 16.8 Hz, 1H, HA–5), 2.42 (ddd, J = 12.6, 8.6, 1.8 Hz, 1H, HA–3). 13C NMR (100 MHz, CDCl3): δ 155.1 (C2’), 144.9 (C11b), 142.4 (C5’), 131.1 (C7a), 130.9 (C6), 128.7 (C11a), 127.4 (C8, C9), 127.3 (C10), 122.7 (C11), 122.0 (C5a), 119.0 (C7), 110.5 (C3’), 106.8 (C4’), 74.9 (C4), 68.9 (C2), 38.7 (C3), 34.9 (C5). GC–MS (EI, 70 eV): m/z (%) 355 (M+•, 79Br, 12), 325 (6), 312 (7), 257 (2), 231 (2), 220 (100), 193 (3). HR–MS (EI–MS) m/z calcd. for C18H14BrNO2, 355.0207, found, 355.0208.
(2SR,4SR)–7–Bromo–2–(5–methylfuran–2–yl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (10b)
Reaction time: 39 h. Yield: 48%. Pale Yellow solid, mp 123 °C (heptane). IR (KBr, cm−1): ṽ 1054 (C–O), 989 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.49 (dd, J = 7.7, 2.2 Hz, 1H, H–11), 8.18 (dd, J = 7.7, 2.2 Hz, 1H, H–8), 7.60 (td, J = 7.7, 2.2 Hz, 1H, H–10), 7.59 (td, J = 7.7, 2.2 Hz, 1H, H–9), 7.53 (s, 1H, H–6), 6.29 (dd, J = 3.0 Hz, 1H, H–3’), 5.98 (dd, J = 3.0, 2.8 Hz, 1H, H–4’), 5.08 (ddd, J = 7.8, 5.3, 1.6 Hz, 1H, H–4), 4.68 (dd, J = 8.6, 2.2 Hz, 1H, H–2), 3.54 (dd, J = 16.7, 5.3 Hz, 1H, HB–5), 2.85 (dddd, J = 12.6, 7.8, 2.2, 1.0 Hz, 1H, HB–3), 2.59 (br d, J = 16.7 Hz, 1H, HA–5), 2.40 (ddd, J = 12.6, 8.6, 1.6 Hz, 1H, HA–3), 2.36 (s, 3H, 5’–CH3). 13C NMR (100 MHz, CDCl3): δ 153.2 (C2’), 152.1 (C5’), 144.9 (C11b), 130.9 (C7a), 130.8 (C6), 127.9 (C11a), 127.2 (C8, C9), 127.1 (C10), 122.6 (C11), 122.0 (C5a), 119.7 (C7), 107.6 (C3’), 106.1 (C4’), 74.7 (C4), 68.6 (C2), 38.3 (C3), 34.5 (C5), 13.7 (5’–CH3). GC–MS (EI, 70 eV): m/z (%) 369 (M+•, 79Br, 13), 339 (3), 326 (9), 231 (4), 220 (100), 193 (4). HR–MS (EI–MS) m/z calcd. for C19H16BrNO2, 369.0363; found, 369.0364.
(2SR,4SR)–2–(Furan–2–yl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (10c)
Reaction time: 36 h. Yield: 51%. White solid, mp 96 °C (heptane). IR (KBr, cm−1): ṽ 1051 (C–O), 996 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.47 (dd, J = 8.4, 0.9 Hz, 1H, H–11), 7.81 (dd, J = 8.4, 1.1 Hz, 1H, H–8), 7.64 (d, J = 8.4 Hz, 1H, H–7), 7.56 (td, J = 8.4, 1.1 Hz, 1H, H–10), 7.50 (td, J = 8.4, 0.9 Hz, 1H, H–9), 7.48 (dd, J = 3.3, 1.9 Hz, 1H, H–5’), 7.20 (d, J = 8.4 Hz, 1H, H–6), 6.45 (dd, J = 3.1, 3.3 Hz, 1H, H–4’), 6.42 (dd, J = 3.1, 1.9 Hz, 1H, H–3’), 5.10 (ddd, J = 7.9, 5.3, 1.7 Hz, 1H, H–4), 4.77 (dd, J = 8.6, 2.3 Hz, 1H, H–2), 3.57 (dd, J = 16.7, 5.3 Hz, 1H, HB–5), 2.87 (dddd, J = 12.7, 7.9, 2.3, 1.5 Hz, 1H, HB–3), 2.62 (br d, J = 16.7 Hz, 1H, HA–5), 2.44 (ddd, J = 12.7, 8.6, 1.7 Hz, 1H, HA–3). 13C NMR (100 MHz, CDCl3): δ 155.7 (C2’), 144.9 (C11b), 142.6 (C5’), 132.6 (C7a), 127.9 (C8), 127.6 (C11a), 127.3 (C6), 126.5 (C9), 125.9 (C10), 125.8 (C7), 122.9 (C11), 120.9 (C5a), 110.4 (C4’), 106.6 (C3’), 75.5 (C4), 68.7 (C2), 38.8 (C3), 35.1 (C5). GC–MS (EI, 70 eV): m/z (%) 277 (M+•, 27), 247 (4), 234 (25), 180 (4), 142 (100), 115 (22). HR–MS (EI–MS) m/z calcd. for C18H15NO2, 277.1097; found, 277.1103.
(2SR,4SR)–2–(5–Methylfuran–2–yl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (10d)
Reaction time: 31 h. Yield: 50%. White solid, mp 117 °C (heptane). IR (KBr, cm−1): ṽ 1054 (C–O), 991 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.47 (dd, J = 8.1, 1.2 Hz, 1H, H–11), 7.80 (dd, J = 8.1, 1.2 Hz, 1H, H–8), 7.63 (d, J = 8.4 Hz, 1H, H–7), 7.56 (td, J = 8.1, 1.2 Hz, 1H, H–10), 7.48 (td, J = 8.1, 1.0 Hz, 1H, H–9), 7.19 (d, J = 8.4 Hz, 1H, H–6), 6.31 (d, J = 2.9 Hz, 1H, H–3’), 5.99 (dd, J = 2.9, 0.8 Hz, 1H, H–4’), 5.11 (ddd, J = 7.8, 5.3, 1.8 Hz, 1H, H–4), 4.71 (dd, J = 8.5, 2.2 Hz, 1H, H–2), 3.57 (dd, J = 16.7, 5.3 Hz, 1H, HB–5), 2.87 (dddd, J = 12.8, 7.8, 2.2, 1.1 Hz, 1H, HB–3), 2.61 (br d, J = 16.7 Hz, 1H, HA–5), 2.41 (ddd, J = 12.8, 8.5, 1.8 Hz, 1H, HA–3), 2.37 (s, 3H, 5’–CH3). 13C NMR (100 MHz, CDCl3): δ 153.5 (C2’), 152.2 (C5’), 145.1 (C11b), 132.7 (C7a), 127.9 (C8), 127.6 (C11a), 127.3 (C6), 126.4 (C9), 125.9 (C10), 125.8 (C7), 122.3 (C11), 120.9 (C5a), 107.4 (C3’), 106.4 (C4’), 75.1 (C4), 68.7 (C2), 38.6 (C3), 35.2 (C5), 14.0 (5’–CH3). GC–MS (EI, 70 eV): m/z (%) 291 (M+•, 20), 261 (3), 248 (22), 180 (7), 142 (100), 115 (26). HR–MS (EI–MS) m/z calcd. for C19H17NO2, 291.1261; found, 291.1259.
(2SR,4SR)–2–(5–Nitrofuran–2–yl)–2,3,4,5–tetrahydro–1,4–epoxynaphtho[1,2–b]azepine (10e)
Reaction time: 34 h. Yield: 51%. Pale yellow solid, mp 147 °C (heptane/ethyl acetate 2:1). IR (KBr, cm−1): ṽ 1494 (–NO2), 1356 (–NO2), 1048 (C–O), 993 (N–O). 1H NMR (400 MHz, CDCl3): δ 8.28 (dd, J = 8.2, 1.0 Hz, 1H, H–11), 7.81 (dd, J = 8.2, 1.2 Hz, 1H, H–8), 7.66 (d, J = 8.4 Hz, 1H, H–7), 7.54 (td, J = 8.2, 1.2 Hz, 1H, H–10), 7.48 (td, J = 8.2, 1.0 Hz, 1H, H–9), 7.36 (d, J = 3.7 Hz, 1H, H–4’), 7.20 (d, J = 8.4 Hz, 1H, H–6), 6.84 (d, J = 3.7 Hz, 1H, H–3’), 5.09 (ddd, J = 7.8, 5.3, 1.6 Hz, 1H, H–4), 4.77 (dd, J = 8.4, 1.3 Hz, 1H, H–2), 3.57 (dd, J = 16.9, 5.3 Hz, 1H, HB–5), 2.87 (dddd, J = 12.9, 7.8, 1.3, 1.1 Hz, 1H, HB–3), 2.64 (br d, J = 16.9 Hz, 1H, HA–5), 2.53 (ddd, J = 12.9, 8.4, 1.6 Hz, 1H, HA–3). 13C NMR (100 MHz, CDCl3): δ 159.2 (C2’), 151.7 (C5’), 143.9 (C11b), 132.7 (C7a), 128.1 (C8), 127.3 (C11a), 127.2 (C6), 126.8 (C9), 126.5 (C10), 126.2 (C7), 121.5 (C11), 120.9 (C5a), 112.9 (C4’), 109.9 (C3’), 75.6 (C4), 68.4 (C2), 39.1 (C3), 34.9 (C5). GC–MS (EI, 70 eV): m/z (%) 322 (M+•, 10), 292 (2), 279 (4), 180 (10), 142 (100), 115 (38). HR–MS (EI–MS) m/z calcd. for C18H14N2O2, 322.0958; found, 322.0954.
Physicochemical and spectral data for cis–2–aryl(heteroaryl)–4–hydroxy–2,3,4,5–tetrahydronaphtho[1,2–b]azepines (11a–h), (12a–f), and (13a–d)
7–Bromo–cis–2–phenyl–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (11a)
Reaction time: 12 h. Yield: 70%. White solid, mp 147 °C (heptane). IR (KBr, cm−1): ṽ 3322 (N–H, O–H), 1267 (C–N), 1013 (C–O). 1H NMR (400 MHz, CDCl3): δ 8.22 (br d, J = 8.3 Hz, 1H, H–11), 7.66 (s, 1H, H–6), 7.61 (br d, J = 8.3 Hz, 1H, H–8), 7.53 (td, J = 8.3, 1.0 Hz, 1H, H–9), 7.48 (t, J = 8.3 Hz, 1H, H–4’), 7.45 (t, J = 8.3 Hz, 2H, H–3’, H–5’), 7.44 (t, J = 8.3 Hz, 1H, H–10), 7.42 (dd, J = 8.3, 1.6 Hz, 2H, H–2’, H–6’), 4.40 (br s, 1H, NH), 4.01 (dd, J = 12.0, 6.3 Hz, 1H, Hax–2), 3.94 (tdd, J = 9.8, 3.4, 1.8 Hz, 1H, Hax–4), 3.28 (dd, J = 16.6, 9.8 Hz, 1H, Hax–5), 3.12 (dt, J = 13.6, 1.8 Hz, 1H, Heq–5), 2.16–2.33 (m, 2H, Hax–3, Heq–3), 2.01 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 144.4 (C11b), 144.1 (C1’), 133.4 (C6), 131.7 (C7a), 128.3 (C4’), 128.1 (C3’, C5’), 128.0 (C8), 127.5 (C11a), 126.8 (C10), 126.7 (C2’, C6’), 126.5 (C9), 125.3 (C5a), 120.6 (C11), 114.1 (C7), 69.7 (C4), 61.3 (C2), 47.4 (C3), 44.1 (C5). GC–MS (EI, 70 eV): m/z (%) 367 (M+•, 79Br, 78), 323 (25), 290 (2), 246 (7), 234 (100), 219 (2). HR–MS (EI–MS) m/z calcd. for C20H18BrNO, 367.0559; found, 367.0572.
7–Bromo–cis–2–(o–tolyl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (11b)
Reaction time: 12 h. Yield: 79%. White solid, mp 147 °C (heptane). IR (KBr, cm−1): ṽ 3377 (N–H, O–H), 1267 (C–N), 1027 (C–O). 1H NMR (400 MHz, CDCl3): δ 8.23 (br d, J = 8.3 Hz, 1H, H–11), 7.70 (br d, J = 7.5 Hz, 2H, H–6’), 7.66 (s, 1H, H–6), 7.61 (br d, J = 8.3 Hz, 1H, H–8), 7.53 (t, J = 8.3 Hz, 1H, H–9), 7.43 (td, J = 8.3, 1.0 Hz, 1H, H–10), 7.24 (dd, J = 7.5, 0.9 Hz, 1H, H–3’), 7.33 (td, J = 7.5, 1.5 Hz, 1H, H–5’), 7.28 (td, J = 7.5, 1.5 Hz, 1H, H–4’), 4.64 (br s, 1H, NH), 4.25 (dd, J = 11.3, 1.7 Hz, 1H, Hax–2), 3.95 (tdd, J = 10.1, 4.1, 1.7 Hz, 1H, Hax–4), 3.36 (dd, J = 13.5, 10.1 Hz, 1H, Hax–5), 3.14 (br d, J = 13.5 Hz, 1H, Heq–5), 2.28 (ddd, J = 13.0, 11.3, 10.1 Hz, 1H, Hax–3), 2.22 (ddt, J = 13.0, 4.1, 1.7 Hz, 1H, Heq–3), 2.28 (s, 3H, 2’–CH3), 2.00 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 143.7 (C11b), 142.0 (C1’), 134.8 (C2’), 133.6 (C6), 131.6 (C7a), 131.0 (C4’), 128.0 (C8, C3’,C5’), 127.5 (C11a), 126.8 (C10), 126.7 (C9), 125.8 (C6’), 125.6 (C5a), 120.4 (C11), 115.3 (C7), 69.9 (C4), 57.2 (C2), 46.2 (C3), 44.1 (C5), 19.8 (2’–CH3). GC–MS (EI, 70 eV): m/z (%) 381 (M+•, 79Br, 26), 337 (8), 290 (1), 246 (6), 234 (100), 219 (1). HR–MS (EI–MS) m/z calcd. for C20H18BrNO, 381.0732; found, 381.0728.
7–Bromo–cis–2–(m–tolyl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (11c)
Reaction time: 10 h. Yield: 82%. Yellow solid, mp 80 °C (heptane). IR (KBr, cm−1): ṽ 3306 (N–H, O–H), 1242 (C–N), 1015 (C–O). 1H NMR (400 MHz, CDCl3): δ 8.23 (dd, J = 8.3, 1.2 Hz, 1H, H–11), 7.65 (s, 1H, H–6), 7.63 (dd, J = 8.3, 1.0 Hz, 1H, H–8), 7.53 (td, J = 8.3, 1.0 Hz, 1H, H–9), 7.44 (td, J = 8.3, 1.2 Hz, 1H, H–10), 7.33 (t, J = 7.6 Hz, 1H, H–5’), 7.31 (s, 1H, H–2’), 7.28 (d, J = 7.6 Hz, 1H, H–6’), 7.21 (d, J = 7.6 Hz, 1H, H–4’), 4.50 (br s, 1H, NH), 3.97 (dd, J = 10.2, 3.8 Hz, 1H, Hax–2), 3.91 (tdd, J = 9.9, 3.5, 2.2 Hz, 1H, Hax–4), 3.30 (dd, J = 13.5, 9.9 Hz, 1H, Hax–5), 3.11 (dd, J = 13.5, 2.2 Hz, 1H, Heq–5), 2.20–2.31 (m, 2H, Hax–3, Heq–3), 2.42 (s, 3H, 3’–CH3), 2.11 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 144.1 (C11b), 143.8 (C1’), 139.0 (C3’), 133.5 (C6), 131.6 (C7a), 129.2 (C6’), 129.0 (C4’), 128.1 (C8, C5’), 127.7 (C11a), 127.3 (C2’), 126.7 (C10), 126.5 (C9), 125.5 (C5a), 120.5 (C11), 114.9 (C7), 69.6 (C4), 61.7 (C2), 47.1 (C3), 44.3 (C5), 21.6 (3’–CH3). GC–MS (EI, 70 eV): m/z (%) 381 (M+•, 79Br, 50), 337 (18), 290 (1), 246 (10), 234 (100), 219 (1). HR–MS (EI–MS) m/z calcd. for C21H20BrNO, 381.0735; found, 381.0733.
7–Bromo–cis–2–(3–methoxyphenyl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (11d)
Reaction time: 12 h. Yield: 77%. Viscous yellow oil. IR (liquid film, cm−1): ṽ 3382 (N–H, O–H), 1265 (C–N), 1038 (C–O). 1H NMR (400 MHz, CDCl3): δ 8.21 (dd, J = 8.2, 1.1 Hz, 1H, H–11), 7.65 (s, 1H, H–6), 7.64 (dd, J = 8.2, 0.7 Hz, 1H, H–8), 7.53 (td, J = 8.2, 0.7 Hz, 1H, H–9), 7.45 (td, J = 8.2, 1.1 Hz, 1H, H–10), 7.35 (t, J = 8.1 Hz, 1H, H–5’), 7.06 (d, J = 2.4 Hz, 1H, H–2’), 7.05 (dd, J = 8.1, 0.7 Hz, 1H, H–6’), 6.92 (ddd, J = 8.1, 2.4, 0.7 Hz, 1H, H–4’), 4.67 (br s, 1H, NH), 4.01 (dd, J = 10.0, 4.3 Hz, 1H, Hax–2), 3.93 (tdd, J = 9.9, 3.6, 2.3 Hz, 1H, Hax–4), 3.84 (s, 3H, 3’–OCH3), 3.33 (dd, J = 13.5, 9.9 Hz, 1H, Hax–5), 3.12 (dt, J = 13.5, 2.3 Hz, 1H, Heq–5), 2.29–2.33 (m, 2H, Hax–3, Heq–3), 2.10 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 160.3 (C3’), 145.4 (C1’), 143.2 (C11b), 133.4 (C6), 131.6 (C7a), 130.4 (C5’), 128.2 (C8), 127.7 (C11a), 126.8 (C10), 126.7 (C9), 125.7 (C5a), 120.6 (C11), 119.1 (C2’), 115.3 (C7), 113.8 (C4’), 112.3 (C6’), 69.6 (C4), 61.6 (C2), 55.6 (3’–OCH3), 46.9 (C3), 44.0 (C5). GC–MS (EI, 70 eV): m/z (%) 397 (M+•, 79Br, 28), 353 (8), 290 (1), 246 (9), 234 (100), 219 (1).
7–Bromo–cis–2–(2–chlorophenyl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (11e)
Reaction time: 8 h. Yield: 80%. Pale Yellow solid, mp 84 °C (heptane). IR (KBr, cm−1): ṽ 3376 (N–H, O–H), 1268 (C–N), 1038 (C–O). 1H NMR (400 MHz, CDCl3): δ 8.22 (br d, J = 8.4 Hz, 1H, H–11), 7.75 (dd, J = 7.7, 1.2 Hz, 1H, H–6’), 7.66 (br d, J = 8.4 Hz, 1H, H–8), 7.66 (s, 1H, H–6), 7.53 (t, J = 8.4 Hz, 1H, H–9), 7.43 (t, J = 8.4 Hz, 1H, H–10), 7.41 (br d, J = 7.7 Hz, 1H, H–3’), 7.40 (t, J = 7.7 Hz, 1H, H–5’), 7.31 (td, J = 7.7, 1.2 Hz, 1H, H–4’), 4.55 (dd, J = 11.3, 1.6 Hz, 1H, Hax–2), 4.23 (br s, 1H, NH), 3.99 (tdd, J = 9.8, 3.5, 1.8 Hz, 1H, Hax–4), 3.27 (dd, J = 13.6, 9.8 Hz, 1H, Hax–5), 3.14 (br d, J = 13.6 Hz, 1H, Heq–5), 2.27 (ddt, J = 12.7, 3.5, 1.6 Hz, 1H, Heq–3), 2.18 (ddd, J = 12.7, 11.3, 9.8 Hz, 1H, Hax–3), 2.04 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 144.0 (C11b), 141.6 (C1’), 133.5 (C6), 132.8 (C2’), 131.6 (C7a), 129.2 (C4’), 128.0 (C3’, C5’), 127.9 (C8), 127.8 (C11a), 127.2 (C10), 126.8 (C6’), 126.5 (C9), 125.4 (C5a), 120.7 (C11), 114.9 (C7), 69.6 (C4), 56.5 (C2), 46.0 (C3), 44.3 (C5). GC–MS (EI, 70 eV): m/z (%) 401 (M+•, 79Br, 35Cl, 98), 357 (30), 290 (3), 246 (11), 234 (100), 219 (2). HR–MS (EI–MS) m/z calcd. for C20H17BrClNO, 401.0180; found, 401.0182.
7–Bromo–cis–2–(4–chlorophenyl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (11f)
Reaction time: 12 h. Yield: 75%. Yellow solid, mp 109 °C (heptane). IR (KBr, cm−1): ṽ 3354 (N–H, O–H), 1267 (C–N), 1013 (C–O). 1H NMR (400 MHz, CDCl3): δ 8.22 (br d, J = 8.3 Hz, 1H, H–11), 7.64 (s, 1H, H–6), 7.58 (br d, J = 8.3 Hz, 1H, H–8), 7.53 (t, J = 8.3 Hz, 1H, H–9), 7.45 (t, J = 8.3 Hz, 1H, H–10), 7.40–7.42 (m, 4H, H–2’, H–3’, H–5’, H–6’), 4.31 (br s, 1H, NH), 3.95–3.99 (m, 1H, Hax–2), 3.87–3.94 (m, 1H, Hax–4), 3.25 (dd, J = 13.5, 9.9 Hz, 1H, Hax–5), 2.61 (br d, J = 13.5 Hz, 1H, Heq–5), 2.17–2.22 (m, 2H, Hax–3, Heq–3), 2.05 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 143.8 (C11b), 142.7 (C1’), 134.0 (C4’), 133.5 (C6), 131.6 (C7a), 129.5 (C3’, C5’), 128.2 (C2’, C6’), 128.1 (C8), 127.7 (C11a), 126.7 (C10), 126.6 (C9), 125.5 (C5a), 120.3 (C11), 115.1 (C7), 69.7 (C4), 60.6 (C2), 47.3 (C3), 44.1 (C5). GC–MS (EI, 70 eV): m/z (%) 401 (M+•, 79Br, 35Cl, 20), 357 (9), 290 (1), 246 (6), 234 (100), 219 (1).
7–Bromo–cis–2–(2,4–dichlorophenyl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (11g)
Reaction time: 12 h. Yield: 72%. Pale yellow solid, mp 80 °C (heptane). IR (KBr, cm−1): ṽ 3354 (N–H, O–H), 1267 (C–N), 1040 (C–O). 1H NMR (400 MHz, CDCl3): δ 8.23 (dd, J = 8.4, 1.2 Hz, 1H, H–11), 7.72 (d, J = 8.4 Hz, 1H, H–6’), 7.68 (br d, J = 8.4 Hz, 1H, H–8), 7.65 (s, 1H, H–6), 7.55 (td, J = 8.4, 1.2 Hz, 1H, H–9), 7.47 (td, J = 8.4, 1.2 Hz, 1H, H–10), 7.47 (d, J = 2.1 Hz, 1H, H–3’), 7.36 (dd, J = 8.4, 2.1 Hz, 1H, H–5’), 4.51 (dd, J = 10.2, 3.3 Hz, 1H, Hax–2), 4.45 (br s, 1H, NH), 3.96 (tdd, J = 10.1, 4.6, 2.3 Hz, 1H, Hax–4), 3.34 (dd, J = 13.5, 10.1 Hz, 1H, Hax–5), 3.11 (br d, J = 13.5 Hz, 1H, Heq–5), 2.09–2.30 (m, 2H, Heq–3, Hax–3), 2.07 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 142.1 (C11b), 139.2 (C1’), 133.8 (C4’), 133.5 (C6), 133.0 (C2’), 131.6 (C7a), 129.9 (C3’), 128.8 (C6’), 128.3 (C5’), 128.1 (C8), 127.6 (C11a), 127.0 (C10), 126.9 (C9), 125.7 (C5a), 120.7 (C7, C11), 69.1 (C4), 56.8 (C2), 45.6 (C3), 44.0 (C5). GC–MS (EI, 70 eV): m/z (%) 437 (M+•, 79Br, 35Cl, 14), 393 (10), 290 (1), 246 (8), 234 (100), 219 (1). HR–MS (EI–MS) m/z calcd. for C20H16BrCl2NO, 434.9795; found, 434.9792.
cis–2–(o–Tolyl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (11h)
Reaction time: 12 h. Yield: 65%. Pale yellow solid, mp 116 °C (heptane). IR (KBr, cm−1): ṽ 3322 (N–H, O–H), 1278 (C–N), 1025 (C–O). 1H NMR (400 MHz, CDCl3): δ 7.83 (dd, J = 7.6, 1.1 Hz, 1H, H–11), 7.73 (d, J = 8.0 Hz, 1H, H–6’), 7.62 (dd, J = 8.0, 1.0 Hz, 1H, H–8), 7.48 (d, J = 8.4 Hz, 1H, H–7), 7.40–7.42 (m, 2H, H–9, H–10), 7.35 (d, J = 8.4 Hz, 1H, H–6), 7.28–7.36 (m, 1H, H–5’), 7.25–7.28 (m, 2H, H–3’, H–4’), 4.37 (br s, 1H, NH), 4.27 (dd, J = 11.2, 2.0 Hz, 1H, Hax–2), 3.96 (tdd, J = 10.0, 4.4, 2.6 Hz, 1H, Hax–4), 3.39 (dd, J = 13.2, 10.0 Hz, 1H, Hax–5), 3.20 (dt, J = 13.2, 1.6 Hz, 1H, Heq–5), 2.19–2.32 (m, 2H, Hax–3, Heq–3), 2.29 (s, 3H, 2’–CH3). 13C NMR (100 MHz, CDCl3): δ 143.9 (C11b), 142.5 (C1’), 134.8 (C2’), 133.6 (C7a), 131.0 (C3’), 130.1 (C6), 128.9 (C8), 127.8 (C4’), 127.1 (C11a), 126.3 (C6’), 125.9 (C5’), 125.8 (C10), 125.4 (C9), 124.5 (C5a), 122.0 (C7), 119.9 (C11), 70.1 (C4), 57.0 (C2), 46.7 (C3), 44.8 (C5), 19.6 (2’–CH3). GC–MS (EI, 70 eV): m/z (%) 303 (M+•, 60), 259 (22), 168 (29), 156 (100), 141 (8). HR–MS (EI–MS) m/z calcd. for C21H21NO, 303.1622; found, 303.1623.
7–Bromo–cis–2–(thiophen–2–yl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (12a)
Reaction time: 3 h. Yield: 82%. Pale yellow solid, mp 66 °C (heptane). IR (KBr, cm−1): ṽ 3349 (N–H, O–H), 1268 (C–N), 1023 (C–O). 1H NMR (400 MHz, CDCl3): δ 8.24 (dd, J = 8.4, 1.5 Hz, 1H, H–11), 7.75 (br d, J = 8.4 Hz, 1H, H–8), 7.66 (s, 1H, H–6), 7.56 (td, J = 8.4, 1.2 Hz, 1H, H–9), 7.51 (td, J = 8.4, 1.5 Hz, 1H, H–10), 7.34 (dd, J = 5.1, 1.1 Hz, 1H, H–5’), 7.14 (dd, J = 3.5, 1.1 Hz, 1H, H–3’), 7.07 (dd, J = 5.1, 3.5 Hz, 1H, H–4’), 4.43 (br s, 1H, NH), 4.34 (dd, J = 11.7, 1.9 Hz, 1H, Hax–2), 3.95 (tdd, J = 10.0, 4.1, 2.0 Hz, 1H, Hax–4), 3.29 (dd, J = 13.6, 10.0 Hz, 1H, Hax–5), 3.11 (dt, J = 13.6, 2.0 Hz, 1H, Heq–5), 2.46 (ddt, J = 12.2, 4.1, 1.9 Hz, 1H, Heq–3), 2.30 (ddd, J = 12.2, 11.7, 10.0 Hz, 1H, Hax–3), 1.96 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 147.2 (C2’), 143.5 (C11b), 133.4 (C6), 131.5 (C7a), 128.0 (C8), 127.9 (C11a), 127.1 (C5’), 126.8 (C10), 126.7 (C9), 125.7 (C5a), 124.8 (C4’), 124.3 (C3´), 120.7 (C11), 115.2 (C7), 69.5 (C4), 55.7 (C2), 47.8 (C3), 43.9 (C5). GC–MS (EI, 70 eV): m/z (%) 373 (M+•, 79Br, 11), 329 (2), 290 (1), 246 (4), 234 (100), 219 (5). HR–MS (EI–MS) m/z calcd. for C18H14BrNOS, 370.9976; found, 370.9979.
7–Bromo–cis–2–(5–methylthiophen–2–yl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (12b)
Reaction time: 2 h. Yield: 70%. Pale yellow solid, mp 53 °C (heptane). IR (KBr, cm−1): ṽ 3364 (N–H, O–H), 1268 (C–N), 1022 (C–O). 1H NMR (400 MHz, CDCl3): δ 8.22 (dd, J = 8.0, 1.3 Hz, 1H, H–11), 7.76 (dd, J = 8.0, 1.1 Hz, 1H, H–8), 7.63 (s, 1H, H–6), 7.54 (td, J = 8.0, 1.1 Hz, 1H, H–9), 7.51 (td, J = 8.0, 1.1 Hz, 1H, H–10), 6.89 (d, J = 3.4 Hz, 1H, H–3’), 6.68 (dd, J = 3.4, 1.0 Hz, 1H, H–4’), 4.41 (br s, 1H, NH), 4.22 (dd, J = 11.6, 2.0 Hz, 1H, Hax–2), 3.91 (tdd, J = 9.9, 4.0, 2.0 Hz, 1H, Hax–4), 3.26 (dd, J = 13.6, 9.9 Hz, 1H, Hax–5), 3.08 (dt, J = 13.6, 2.0 Hz, 1H, Heq–5), 2.42 (ddt, J = 12.6, 4.0, 2.0 Hz, 1H, Heq–3), 2.25 (ddd, J = 12.6, 11.6, 9.9 Hz, 1H, Hax–3), 2.53 (s, 3H, 5’–CH3), 2.03 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 144.8 (C2’), 143.6 (C11b), 139.4 (C5’), 133.5 (C6), 131.6 (C7a), 128.1 (C8), 127.8 (C11a), 126.8 (C10), 126.7 (C9), 125.6 (C5a), 125.0 (C4’), 124.3 (C3’), 120.7 (C11), 115.0 (C7), 69.7 (C4), 56.1 (C2), 47.7 (C3), 43.9 (C5), 15.6 (5’–CH3). GC–MS (EI, 70 eV): m/z (%) 387 (M+•, 79Br, 10), 343 (4), 290 (2), 246 (3), 234 (100), 219 (2). HR–MS (EI–MS) m/z calcd. for C19H18BrNOS, 387.0305; found, 387.0292.
7–Bromo–cis–2–(3–methylthiophen–2–yl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (12c)
Reaction time: 2 h. Yield: 85%. Yellow solid, mp 65 °C (heptane). IR (KBr, cm−1): ṽ 3352 (N–H, O–H), 1269 (C–N), 1039 (C–O). 1H NMR (400 MHz, CDCl3): δ 8.23 (dd, J = 8.4, 1.4 Hz, 1H, H–11), 7.66 (s, 1H, H–6), 7.65 (br d, J = 8.4 Hz, 1H, H–8), 7.54 (td, J = 8.4, 1.1 Hz, 1H, H–9), 7.48 (td, J = 8.4, 1.4 Hz, 1H, H–10), 7.24 (d, J = 5.1 Hz, 1H, H–5’), 6.89 (d, J = 5.1 Hz, 1H, H–4’), 4.36 (br s, 1H, NH), 4.34 (dd, J = 11.1, 2.5 Hz, 1H, Hax–2), 3.90–3.99 (m, 1H, Hax–4), 3.29 (dd, J = 13.5, 10.0 Hz, 1H, Hax–5), 3.12 (dt, J = 13.5, 2.0 Hz, 1H, Heq–5), 2.36 (ddt, J = 12.1, 3.5, 2.5 Hz, 1H, Heq–3), 2.29 (ddd, J = 12.1, 11.1, 10.0 Hz, 1H, Hax–3), 2.20 (s, 3H, 3’–CH3), 1.93 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 143.7 (C11b), 140.5 (C2’), 133.6 (C7a, C3’), 133.5 (C6), 130.3 (C5’), 128.3 (C8), 127.6 (C11a), 126.8 (C10), 126.6 (C9), 125.4 (C5a), 123.4 (C4’), 120.4 (C11), 115.0 (C7), 69.8 (C4), 54.3 (C2), 47.9 (C3), 44.1 (C5), 13.9 (3’–CH3). GC–MS (EI, 70 eV): m/z (%) 387 (M+•, 79Br, 8), 343 (1), 290 (1), 246 (4), 234 (100), 219 (1).
cis–2–(Thiophen–2–yl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (12d)
Reaction time: 2 h. Yield: 77%. Pale brown oil. IR (liquid film, cm−1): ṽ 3357 (N–H, O–H), 1273 (C–N), 1025 (C–O). 1H NMR (400 MHz, CDCl3): δ 7.84 (dd, J = 7.4, 2.0 Hz, 1H, H–11), 7.75–7.77 (m, 1H, H–8), 7.44–7.50 (m, 2H, H–9, H–10), 7.49 (br d, J = 8.0 Hz, 1H, H–7), 7.35 (br d, J = 8.0 Hz, 1H, H–6), 7.32 (dd, J = 5.1, 1.4 Hz, 1H, H–5’), 7.14 (dd, J = 3.5, 1.4 Hz, 1H, H–3’), 7.06 (dd, J = 5.1, 3.5 Hz, 1H, H–4’), 4.44 (br s, 1H, NH), 4.36 (dd, J = 11.6, 2.0 Hz, 1H, Hax–2), 3.96 (tdd, J = 10.0, 3.5, 1.8 Hz, 1H, Hax–4), 3.27 (dd, J = 13.6, 10.0 Hz, 1H, Hax–5), 3.17 (dt, J = 13.6, 1.8 Hz, 1H, Heq–5), 2.48 (ddt, J = 12.4, 4.0, 2.0 Hz, 1H, Heq–3), 2.34 (ddd, J = 12.4, 11.6, 10.0 Hz, 1H, Hax–3), 2.16 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 147.9 (C2’), 143.9 (C11b), 133.9 (C7a), 130.0 (C6), 129.1 (C8), 127.3 (C5’), 127.0 (C11a, C4’), 126.3 (C10), 125.7 (C9), 125.1 (C5a), 124.3 (C3’), 122.5 (C7), 120.6 (C11), 70.0 (C4), 56.3 (C2), 48.5 (C3), 44.7 (C5). GC–MS (EI, 70 eV): m/z (%) 295 (M+•, 22), 251 (4), 212 (1), 168 (6), 156 (100), 141 (5).
cis–2–(5–Methylthiophen–2–yl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (12e)
Reaction time: 2 h. Yield: 79%. Viscous brown oil. IR (liquid film, cm−1): ṽ 3359 (N–H, O–H), 1276 (C–N), 1022 (C–O). 1H NMR (400 MHz, CDCl3): δ 7.83 (dd, J = 7.0, 2.6 Hz, 1H, H–11), 7.78 (dd, J = 7.2, 2.0 Hz, 1H, H–8), 7.42–7.48 (m, 2H, H–9, H–10), 7.46 (br d, J = 8.4 Hz, 1H, H–7), 7.32 (br d, J = 8.4 Hz, 1H, H–6), 7.26 (d, J = 5.2 Hz, 1H, H–3’), 6.92 (d, J = 5.2 Hz, 1H, H–4’), 4.44 (br s, 1H, NH), 4.23 (dd, J = 11.6, 2.0 Hz, 1H, Hax–2), 3.93 (tdd, J = 10.0, 4.0, 2.0 Hz, 1H, Hax–4), 3.31 (dd, J = 13.2, 10.0 Hz, 1H, Hax–5), 3.14 (dt, J = 13.2, 2.0 Hz, 1H, Heq–5), 2.54 (s, 3H, 5’–CH3), 2.42 (ddt, J = 12.4, 4.0, 2.0 Hz, 1H, Heq–3), 2.27 (ddd, J = 12.4, 11.6, 10.0 Hz, 1H, Hax–3), 2.08 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 145.5 (C2’), 144.0 (C11b), 139.5 (C5’), 133.9 (C7a), 130.2 (C6), 129.1 (C8), 126.9 (C11a), 126.2 (C10), 125.7 (C9), 125.3 (C4’), 124.9 (C5a), 124.4 (C3’), 122.3 (C7), 120.5 (C11), 70.1 (C4), 56.5 (C2), 48.2 (C3), 44.7 (C5), 15.8 (5’–CH3). GC–MS (EI, 70 eV): m/z (%) 309 (M+•, 26), 265 (5), 212 (1), 168 (7), 156 (100), 141 (4).
cis–2–(3–Methylthiophen–2–yl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (12f)
Reaction time: 2 h. Yield: 80%. Yellow solid, mp 160 °C (heptane). IR (KBr, cm−1): ṽ 3300 (N–H, O–H), 1273 (C–N), 1032 (C–O). 1H NMR (400 MHz, CDCl3): δ 7.86 (dd, J = 7.0, 2.8 Hz, 1H, H–11), 7.70 (dd, J = 7.0, 2.4 Hz, 1H, H–8), 7.49 (br d, J = 8.4, 1H, H–7), 7.43–7.47 (m, 2H, H–9, H–10), 7.36 (br d, J = 8.4, 1H, H–6), 6.90 (d, J = 3.2 Hz, 1H, H–5’), 6.69 (dd, J = 3.2, 0,8 Hz, 1H, H–4’), 4.40 (br s, 1H, NH), 4.38 (dd, J = 11.2, 2.4 Hz, 1H, Hax–2), 3.96–4.00 (m, 1H, Hax–4), 3.36 (dd, J = 13.6, 10.0 Hz, 1H, Hax–5), 3.19 (dt, J = 13.6, 2.4 Hz, 1H, Heq–5), 2.29–2.41 (m, 2H, Hax–3, Heq–3), 2.23 (s, 3H, 3’–CH3), 2.24 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 144.0 (C11b), 141.1 (C2’), 133.8 (C7a), 133.7 (C3’), 130.4 (C5’), 130.3 (C6), 129.1 (C8), 126.6 (C11a), 126.2 (C10), 125.6 (C9), 124.7 (C5a), 123.5 (C4’), 122.2 (C7), 120.2 (C11), 70.1 (C4), 54.6 (C2), 48.4 (C3), 44.8 (C5), 14.4 (3’–CH3). GC–MS (EI, 70 eV): m/z (%) 309 (M+•, 14), 265 (1), 212 (1), 168 (6), 156 (100), 141 (7). HR–MS (EI–MS) m/z calcd. for C19H19NOS, 309.1194; found, 309.1187.
7–Bromo–cis–2–(furan–2–yl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (13a)
Reaction time: 0.7 h. Yield: 72%. Yellow solid, mp 58 °C (heptane). IR (KBr, cm−1): ṽ 3344 (N–H, O–H), 1271 (C–N), 1015 (C–O). 1H NMR (400 MHz, CDCl3): δ 8.21 (dd, J = 7.0, 2.8 Hz, 1H, H–11), 7.93 (dd, J = 7.5, 1.9 Hz, 1H, H–8), 7.64 (s, 1H, H–6), 7.52–7.60 (m, 2H, H–9, H–10), 7.51 (dd, J = 1.9, 0.7 Hz, 1H, H–5’), 6.42 (dd, J = 3.2, 1.9 Hz, 1H, H–4’), 6.34 (dd, J = 3.2, 0.8 Hz, 1H, H–3’), 4.73 (br s, 1H, NH), 4.13 (dd, J = 11.8, 2.0 Hz, 1H, Hax–2), 3.94 (tdd, J = 9.8, 4.1, 2.0 Hz, 1H, Hax–4), 3.26 (dd, J = 13.5, 9.8 Hz, 1H, Hax–5), 3.08 (dt, J = 13.5, 2.0 Hz, 1H, Heq–5), 2.46 (ddt, J = 12.7, 4.1, 2.0 Hz, 1H, Heq–3), 2.22 (ddd, J = 12.7, 11.8, 9.8 Hz, 1H, Hax–3), 2.01 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 155.9 (C2’), 143.4 (C11b), 142.4 (C5’), 133.5 (C6), 131.7 (C7a), 128.1 (C11a), 128.0 (C8), 126.8 (C10), 126.7 (C9), 126.0 (C5a), 120.7 (C11), 115.3 (C7), 110.8 (C4’), 106.0 (C3’), 69.3 (C4), 53.7 (C2), 44.0 (C3), 43.4 (C5). GC–MS (EI, 70 eV): m/z (%) 357 (M+•, 79Br, 20), 313 (5), 290 (1), 246 (4), 234 (100), 219 (3).
7–Bromo–cis–2–(5–methylfuran–2–yl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (13b)
Reaction time: 1.5 h. Yield: 82%. Viscous yellow oil. IR (liquid film, cm−1): ṽ 3351 (N–H, O–H), 1271 (C–N), 1023 (C–O). 1H NMR (400 MHz, CDCl3): δ 8.22 (dd, J = 7.2, 2.0 Hz, 1H, H–11), 7.90 (dd, J = 8.0, 2.2 Hz, 1H, H–8), 7.64 (s, 1H, H–6), 7.51–7.60 (m, 2H, H–9, H–10), 6.20 (d, J = 3.0 Hz, 1H, H–3’), 5.99 (dd, J = 3.0, 0.9 Hz, 1H, H–4’), 4.70 (br s, 1H, NH), 4.07 (dd, J = 11.5, 2.0 Hz, 1H, Hax–2), 3.93 (tdd, J = 9.8, 4.0, 2.0 Hz, 1H, Hax–4), 3.25 (dd, J = 13.6, 9.8 Hz, 1H, Hax–5), 3.08 (dt, J = 13.6, 2.0 Hz, 1H, Heq–5), 2.42 (ddt, J = 12.4, 4.0, 2.0 Hz, 1H, Heq–3), 2.37 (s, 3H, 5’–CH3), 2.21 (ddd, J = 12.4, 11.5, 9.8 Hz, 1H, Hax–3), 2.02 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 154.0 (C2’), 152.0 (C5’), 143.7 (C11b), 133.4 (C6), 131.6 (C7a), 128.0 (C8, C11a), 126.7 (C10), 126.6 (C9), 125.8 (C5a), 120.6 (C11), 115.0 (C7), 106.7 (C4’), 106.4 (C3’), 69.3 (C4), 53.9 (C2), 44.0 (C3), 43.4 (C5), 13.8 (5’–CH3). GC–MS (EI, 70 eV): m/z (%) 371 (M+•, 79Br, 6), 327 (1), 290 (1), 246 (20), 234 (100), 219 (2).
cis–2–(Furan–2–yl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (13c)
Reaction time: 0.5 h. Yield: 77%. Viscous yellow oil. IR (liquid film, cm−1): ṽ 3359 (N–H, O–H), 1285 (C–N), 1031 (C–O). 1H NMR (400 MHz, CDCl3): δ 7.94 (br d, J = 8.2 Hz, 1H, H–11), 7.84 (br d, J = 8.2 Hz, 1H, H–8), 7.53 (td, J = 8.2, 1.2 Hz, 1H, H–9), 7.51 (dd, J = 1.9, 0.8 Hz, 1H, H–5’), 7.47 (d, J = 8.3 Hz, 1H, H–7), 7.46 (td, J = 8.2, 1.3 Hz, 1H, H–10), 7.32 (d, J = 8.3 Hz, 1H, H–6), 6.43 (dd, J = 3.1, 1.9 Hz, 1H, H–4’), 6.35 (dd, J = 3.1, 0.8 Hz, 1H, H–3’), 4.75 (br s, 1H, NH), 4.15 (dd, J = 11.6, 2.0 Hz, 1H, Hax–2), 3.95 (tdd, J = 10.0, 4.0, 2.0 Hz, 1H, Hax–4), 3.31 (dd, J = 13.5, 10.0 Hz, 1H, Hax–5), 3.14 (dt, J = 13.5, 2.0 Hz, 1H, Heq–5), 2.47 (ddt, J = 12.5, 4.0, 2.0 Hz, 1H, Heq–3), 2.25 (ddd, J = 12.5, 11.6, 10.0 Hz, 1H, Hax–3), 1.83 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 156.4 (C2’), 143.7 (C11b), 142.1 (C5’), 133.7 (C7a), 129.8 (C6), 128.9 (C8), 126.9 (C11a), 126.2 (C10), 125.5 (C9), 125.0 (C5a), 122.2 (C7), 120.2 (C11), 110.7 (C4’), 105.8 (C3’), 69.5 (C4), 54.0 (C2), 44.6 (C3), 43.8 (C5). GC–MS (EI, 70 eV): m/z (%) 279 (M+•, 28), 235 (4), 212 (1), 168 (7), 156 (100), 141 (4). HR–MS (EI–MS) m/z calcd. for C18H17NO2, 279.1246; found, 279.1259.
cis–2–(5–Methylfuran–2–yl)–2,3,4,5–tetrahydro–1H–naphtho[1,2–b]azepin–4–ol (13d)
Reaction time: 1.5 h. Yield: 78%. Viscous yellow oil. IR (liquid film, cm−1): ṽ 3395 (N–H, O–H), 1276 (C–N), 1022 (C–O). 1H NMR (400 MHz, CDCl3): δ 7.92 (br d, J = 8.2 Hz, 1H, H–11), 7.84 (br d, J = 8.2 Hz, 1H, H–8), 7.50 (td, J = 8.2, 1.0 Hz, 1H, H–9), 7.46 (d, J = 8.3 Hz, 1H, H–7), 7.45 (td, J = 8.2, 1.3 Hz, 1H, H–10), 7.31 (d, J = 8.3 Hz, 1H, H–6), 6.20 (d, J = 3.0 Hz, 1H, H–3’), 5.99 (dd, J = 3.0, 1.0 Hz, 1H, H–4’), 4.74 (br s, 1H, NH), 4.09 (dd, J = 11.6, 2.0 Hz, 1H, Hax–2), 3.94 (tdd, J = 9.9, 4.1, 2.2 Hz, 1H, Hax–4), 3.30 (dd, J = 13.7, 9.9 Hz, 1H, Hax–5), 3.13 (dt, J = 13.7, 2.2 Hz, 1H, Heq–5), 2.43 (ddt, J = 12.1, 4.1, 2.2 Hz, 1H, Heq–3), 2.24 (ddd, J = 12.1, 11.6, 9.9 Hz, 1H, Hax–3), 2.38 (s, 3H, 5’–CH3), 1.72 (br s, 1H, 4–OH). 13C NMR (100 MHz, CDCl3): δ 151.9 (C5’), 154.4 (C2’), 143.7 (C11b), 133.5 (C7a), 129.9 (C6), 128.8 (C8), 126.7 (C11a), 125.8 (C10), 125.4 (C9), 124.9 (C5a), 122.0 (C7), 120.1 (C11), 106.4 (C3’), 105.5 (C4’), 69.7 (C4), 54.2 (C2), 44.4 (C3), 43.8 (C5), 14.1 (5’–CH3). GC–MS (EI, 70 eV): m/z (%) 293 (M+•, 14), 249 (1), 212 (1), 168 (17), 156 (100), 141 (6).
QSAR analysis
Dataset and biological activity
A total of 94 compounds, synthetized in our laboratory and previously published (Palma et al. 2009a, 2009b; Gómez-Ayala et al. 2010; Blanco et al. 2014), were included in the dataset. Their anti-parasitic activity (IC50) evaluated against the extracellular form of Trypanosoma cruzi and Leishmania infantum parasites were converted to −log(IC50) and used as depended variable in two different QSAR models. The biological activity was evaluated by a single protocol at the same laboratory in identical experimental conditions (Gómez-Ayala et al. 2010; Blanco et al. 2014). The compounds with IC50 identified in the Tables as >100 were consider outlier to QSAR models, due they do not have a defined IC50 value. The Tables 5S–10S in the Supplementary Material display a listing of the compounds used to develop the QSAR models and their experimental biological activities. The dataset was divided into training set and validation set with a percentage of 80/20. To setup the sets we used the k-means clustering technique (Mamy et al. 2015). Thus, we performed a rational sets partition avoiding the random selection which is sometimes inappropriate (Andrada et al. 2015).
Structure and molecular descriptors
The molecular structures were optimized with MOPAC 09 software (Stewart 2008) using the semiempirical PM6 (parametric method-6) method (Stewart 2007). Then, a set of 1875 molecular descriptors of different types were calculated by PaDEL software (Yap 2011). They included, acidic group count, ALOGP, APol, Aromatic atoms count, Aromatic bonds count, Atom count, Autocorrelation, Barysz matrix, Basic group count, BCUT, Bond count, BPol, Burden modified eigenvalues, Carbon types, Chi, Constitutional, Crippen logP and MR, Detour matrix, Eccentric connectivity index, Atom type electrotopological state, Extended topochemical atom, FMFDescriptor, Fragment complexity, Hbond acceptor count, Hbond donor count, Hybridization ratio, Information content, Kappa shape indices, Largest chain, Largest Pi system, Longest aliphatic chain, Mannhold LogP, McGowan volume, Molecular distance edge, Molecular linear free energy relation, Path counts, Petitjean number, Ring count, Rotatable bonds count, Rule of five, Topological, Van der Waals volume, Vertex adjacency information (magnitude), Walk counts, Weight, Weighted path, Wiener numbers, XLogP, Zagreb index, 3D autocorrelation, Charged partial surface area, Gravitational index, Length over breadth, Moment of inertia, Petitjean shape index, RDF, and WHIM. To avoid redundant information, the descriptors with a correlation higher than 0.9 were removed from the total set.
QSAR modeling
The Replacement Method (RM) (Duchowicz et al. 2006; Mercader et al. 2010), implemented in Matlab software (Matlab 2014), was used as the variable selection procedure in the search of the best Multivariate lineal regression (MLR) equations. The RM is an optimization tool, which generates multivariate linear equation for the QSAR models by searching an optimal subset of descriptors from the total set descriptors (1875) with minimum standard deviation (S) of the model. The quality of the model and the validation was quantified with the traditional statistic parameters: the regression coefficient (R), the determination coefficient (R2), Root-mean-square (RMS) and the standard deviation (S).
To validate the developed QSAR models, we used a validation set containing about a 20% of the total set of compounds. In addition, we used the internal-validation procedures; the leave-one-out (loo) and the leave-more-out (lmo). We selected about the 10% of the compounds and generated a million cases of random data removal. Finally, 100,000 cases of y-randomization validation were performed. The y-randomization consists in the interchange of the experimental property such that property value and the compound do not match. Thus, it is demonstrated that the best model was not found by chance.
References
Abraham WT (1994) New neurohormonal antagonists and atrial natriuretic peptide in the treatment of congestive heart failure. Coron Artery Dis 5:127–136
Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 7:e35671
Andrada MF, Vega-hissi EG, Estrada MR, Garro Martinez JC (2015) Application of k-means clustering, linear discriminant analysis and multivariate linear regression for the development of a predictive QSAR model on 5-lipoxygenase inhibitors. Chemom Intell Lab Syst 143:122–129
Ankersen M (2002) Growth hormone secretagogues: discovery of small orally active molecules by peptidomimetic strategies. Progr Med Chem 39:173–214
Ayo CM, De O, Dalalio MM, Visentainer JEL, Reis PG, Sippert EÂ, Jarduli LR, Alves HV, Sell AM (2013) Genetic susceptibility to Chagas disease: an overview about the infection and about the association between disease and the immune response genes. Biomed Res Int. 2013:1–13
Bankir L, Bardoux P, Ahloulay M (2001) Vasopressin and diabetes mellitus. Nephron 87:8–18
Barberis C, Morin D, Durroux T, Mouillac B, Guillon G, Seyer R, Hibert M, Tribollet E, Manning M (1999) Molecular pharmacology of AVP and OT receptors and therapeutic potential. Drug News Perspect 12:279–298
Barrett MP, Croft SL (2012) Management of trypanosomiasis and leishmaniasis. Br Med Bull 104:175–179
Blanco MC, Escobar P, Leal SM, Bahsas A, Cobo J, Nogueras M, Palma A (2014) Synthesis of novel polysubstituted (2SR,4RS)-2-heteroaryltetrahydro-1,4-epoxy-1-benzazepines and cis-2-heteroaryl-4-hydroxytetrahydro-1H-1-benzazepines as antiparasitic agents. Eur J Med Chem 86:291–309
Chauhan K, Sharma M, Shivahare R, Debnath U, Gupta S, Prabhakar YS, Chauhan PMS (2013) Discovery of triazine mimetics as potent antileishmanial agents. ACS Med Chem Lett 4:1108–1113
Croft SL, Yardley V (2002) Chemotherapy of leishmaniasis. Curr Pharm Des 8:319–342
Crompton DWT, Daumerie D, Peters P, Savioli L (2010) Working to overcome the global impact of neglected tropical diseases first WHO report on neglected tropical diseases. World Health Organization. Deptartment of Control of Neglected Tropical Diseases, Geneva, Switzerland
De Vita RJ, Wyvratt MJ (1996) Benzolactam growth hormone secretagogues. Drugs Future 21:273–281
Decaux G (2001) Long term treatment of inappropriate secretion of antidiuretic hormone by the vasopressin antagonist conivaptan, urea or furosemide. Am J Med 110:582–584
DeVita RJ, Bochis R, Frontier AJ, Kotliar A, Fisher MH, Schoen WR, Wyvratt MJ, Cheng K, Chan WWS, Butler B, Jacks TM, Hickey GJ, Schleim KD, Leung K, Chen Z, Lee Chiu SH, Feeney WP, Cunningham PK, Smith RG (1998) A potent, orally bioavailable benzazepinone growth hormone secretagogue. J Med Chem 41:1716–1728
Diniz L de F, Urbina JA, de Andrade IM, Mazzeti AL, Martins TAF, Caldas IS, Talvani A, Ribeiro I, Bahia MT (2013) Benznidazole and posaconazole in experimental Chagas disease: positive interaction in concomitant and sequential treatments. PLoS Negl Trop Dis 7:e2367
Duchowicz PR, Castro EA, Fernández FM (2006) Alternative algorithm for the search of an optimal set of descriptors in QSAR-QSPR studies. MATCH Commun Math Comput Chem 55:179–192
Espuelas S, Plano D, Nguewa P, Font M, Palop JA, Irache JM, Sanmartin C (2012) Innovative lead compounds and formulation strategies as newer kinetoplastid therapies. Curr Med Chem 19:4259–4288
Galiana-Roselló C, Bilbao-Ramos P, Dea-Ayuela MA, Rolón M, Vega C, Bolas-Fernández F, García-España E, Alfonso J, Coronel C, González-Rosende ME (2013) In vitro and in vivo antileishmanial and trypanocidal studies of new N-benzene- and N-naphthalenesulfonamide derivatives. J Med Chem 56:8984–8998
Gómez-Ayala S, Castrillón JA, Palma A, Leal SM, Escobar P, Bahsas A (2010) Synthesis, structural elucidation and in vitro antiparasitic activity against Trypanosoma cruzi and Leishmania chagasi parasites of novel tetrahydro-1-benzazepine derivatives. Bioorg Med Chem 18:4721–4739
Hotez PJ, Dumonteil E, Heffernan MJ, Bottazzi ME (2011) Innovation for the ‘bottom 100 million’: eliminating neglected tropical diseases in the Americas. Adv Exp Med Biol 764:1–12
Jackson Y, Chatelain E, Mauris A, Holst M, Miao Q, Chappuis F, Ndao M (2013) Serological and parasitological response in chronic Chagas patients 3 years after nifurtimox treatment. BMC Infect Dis 13:85
Kakefuda A, Tsukada J, Kusayama T, Tahara A, Tsukamoto S (2002) N-Methylbenzanilide derivatives as a novel class of selective V1A receptor antagonists. Bioorg Med Chem Lett 12:229–232
Kessler RL, Soares MJ, Probst CM, Krieger MA (2013) Trypanosoma cruzi response to sterol biosynthesis inhibitors: morphophysiological alterations leading to cell death. PLoS One 8:e55497
Knockaert M, Wieking K, Schmitt S, Leost M, Grant KM, Mottram JC, Kunick C, Meijer L (2002) Intracellular targets of paullones: Identification following affinity purification on immobilized inhibitor. J Biol Chem 277:25493–25501
Kunick C, Bleeker C, Prühs C, Totzke F, Schächtele C, Kubbutat MHG, Link A (2006) Matrix COMPARE analysis discriminates subtle structural differences in a family of novel antiproliferative agents, diaryl-3-hydroxy-2,3,3a,10a-tetrahydro-benzo[b]cylopenta[e]azepine-4,10(1H,5H)-diones. Bioorg Med Chem Lett 16:2148–2153
Kunick C, Schultz C, Lemcke T, Zaharevitz DW, Gussio R, Jalluri RK, Sausville EA, Leost M, Meijer L (2000) 2-Substituted paullones: CDK1/cyclin B-inhibiting property and in vitro anti- proliferative activity. Bioorg Med Chem Lett 10:567–569
Mamy L, Patureau D, Barriuso E, Bedos C, Bessac F, Louchart X, Martin-laurent F, Miege C, Benoit P (2015) Prediction of the fate of organic compounds in the environment from their molecular properties: a review. Crit Rev Environ Sci Technol 45:1277–1377
Marín C, Clares MP, Ramírez-Macías I, Blasco S, Olmo F, Soriano C, Verdejo B, Rosales MJ, Gomez-Herrera D, García-España E, Sánchez Moreno M (2013) In vitro activity of scorpiand-like azamacrocycle derivatives in promastigotes and intracellular amastigotes of Leishmania infantum and Leishmania braziliensis. Eur J Med Chem 62:466–477
Matlab 7.0. The MathWorks Inc., Natick, Massachusetts, 2014; mathworks.com software. http://www.mathworks.com. Accessed 6 November 2015
Matthews JM, Greco MN, Hecker LR, Hoekstra WJ, Andrade-Gordon P, de Garavilla L, Demarest KT, Ericson E, Gunnet JW, Hageman W, Look R, Moore JB, Maryanoff BE (2003) Synthesis and biological evaluation of novel indoloazepine derivatives as non-peptide vasopressin V2 receptor antagonists. Bioorg Med Chem Lett 13:753–756
Mercader AG, Duchowicz PR, Fernandez FM, Castro EA (2010) Replacement method and enhanced replacement method versus the genetic algorithm approach for the selection of molecular descriptors in QSPR/QSAR theories. J Chem Inf Model 50:1542–1548
Mishra J, Saxena A, Singh S (2007) Chemotherapy of leishmaniasis: past and future. Curr Med Chem 14:1153–1169
Molina I, Gómez i Prat J, Salvador F, Treviño B, Sulleiro E, Serre N, Pou D, Roure S, Cabezos J, Valerio L, Blanco-Grau A, Sánchez-Montalvá A, Vidal X, Pahissa A (2014) Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N Engl J Med 370:1899–1908
Morita M, Ohkubo-Suzuki A, Takahashi T, Nagashima A, Sawada Y, Ohkawa T, Nishimura S, Kita Y (2003) Molecular analysis of antilipemic effects of FR218944, a novel vasopressin V1a receptor antagonist, in genetically diabetic db/db mice in comparison with pioglitazone and fenofibrate. Drug Dev Res 60:241–251
Olivieri BP, Molina JT, de Castro SL, Pereira MC, Calvet CM, Urbina JA, Araújo-Jorge TC (2010) A comparative study of posaconazole and benznidazole in the prevention of heart damage and promotion of trypanocidal immune response in a murine model of Chagas disease. Int J Antimicrob Agents 36:79–83
Palma A, Bahsas A, Yépes AF, Cobo J, Hursthouse MB, Glidewell C (2009a) (2R,4S)-7-Bromo-2-phenyl-2,3,4,5-tetrahydro-1,4-epoxynaphtho[1,2-b]azepine, (2RS, 4SR)-7-bromo-2-(4-chlorophenyl-2,3,4,5-tetrahydro-1,4-epoxynaphtho[1,2-b]azepine and (2RS, 4SR)-2-(4-fluorophenyl)-2,3,4,5-tetrahydro-1,4-epoxynaphtho[1,2-b]azepine: hydrogen-bonded structures in two and three dimensions. Acta Cryst 65:140–145
Palma A, Yépes AF, Leal SM, Coronado CA, Escobar P (2009b) Synthesis and in vitro activity of new tetrahydronaphtho[1,2-b]azepine derivatives against Trypanosoma cruzi and Leishmania chagasi parasites. Bioorg Med Chem 19:2360–2363
Perez-Victoria FJ, Castanys S, Gamarro F (2003) Leishmania donovani resistance to miltefosine involves a defective inward translocation of the drug. Antimicrob Agents Chemother 47:2397–2403
Pinazo MJ, Muñoz J, Posada E, López-Chejade P, Gállego M, Ayala E, del Cacho E, Soy D, Gascon J (2010) Tolerance of benznidazole in treatment of Chagas’ disease in adults. Antimicrob Agents Chemother 54:4896–4899
Rodrigues-Coura J, Albajar-Viñas P (2010) Chagas disease: a new worldwide challenge. Nature 465:S6–S7
Rodriques-Coura J, de Castro SL (2002) A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz 97:3–24
Schoen WR, Pisano JM, Prendergast K, Wyvratt Jr MJ, Fisher MH, Cheng K, Chan WWS, Butler B, Smith RG, Ball RG (1994) A novel 3-substituted benzazepinone growth hormone secretagogue (L-692, 429). J Med Chem 37:897–906
Schultz C, Link A, Leost M, Zaharevitz DW, Gussio R, Sausville EA, Meijer L, Kunick C (1999) Paullones, a series of cyclin-dependent kinase inhibitors: synthesis, evaluation of CDK1/cyclin B inhibition, and in vitro antitumor activity. J Med Chem 42:2909–2919
Serafim RAM, Gonçalves JE, de Souza FP, de Melo-Loureiro AP, Storpirtis S, Krogh R, Andricopulo AD, Dias LC, Ferreira EI (2014) Design, synthesis and biological evaluation of hybrid bioisoster derivatives of N-acylhydrazone and furoxan groups with potential and selective anti-Trypanosoma cruzi activity. Eur J Med Chem 82:418–425
Seto M, Miyamoto N, Aikawa K, Aramaki Y, Kanzaki N, Iizawa Y, Baba M, Shiraishi M (2005) Bioorg Med Chem 13:363–386
Shimada Y, Taniguchi N, Matsuhisa A, Sakamoto K, Yatsu T, Tanaka A (2000) Highly Potent and Orally Active Non-Peptide Arginine Vasopressin Antagonists for Both V1A and V2 Receptors: Synthesis and Pharmacological Properties of 4’-[(4, 4-Difluoro-5-methylidene-2, 3, 4, 5-tetrahydro-1H-1-benzoazepin-1-yl)carbonyl]-2-phenylbenzanilide Derivatives. Chem Pharm Bull 48:1644–1651
Sielecki TM, Boylan JF, Benfield PA, Trainor GL (2000) Cyclin-dependent kinase inhibitors: useful targets in cell cycle regulation. J Med Chem 43:1–18
Stewart JJP (2007) Optimization of parameters for semiempirical methods V: modification of NDDO approximations and application to 70 elements. J Mol Model 13:1173–1213
Stewart JJP (2008) In: Stewart Computational Chemistry. Colorado Springs: MOPAC2009, CO, USA. http://OpenMOPAC.net
Sundar S, Chakravarty J (2013) Leishmaniasis: an update of current pharmacotherapy. Expert Opin Pharmacother 14:53–63
Todeschini R, Consonni V (2009) Molecular descriptors for chemoinformatics. Mannhold R, Kubinyi H, Folkers G (Series Editors), Weinheim, Wiley–VCH, pp. 714–726
Tsunoda T, Yamazaki A, Iwamoto H, Sakamoto S (2003) Practical synthesis of N-{4-[(2-Methyl-4,5-dihydroimidazo[4,5-d][1]benzazepin-6(1H)-yl)carbonyl]phenyl}biphenyl-2-carboxamide monohydrochloride: an arginine vasopressin antagonist. Org Process Res Dev 7:883–887
Tsunoda T, Yamazaki A, Mase T, Sakamoto S (2005) A scalable process for the synthesis of 2-Methyl-1,4,5,6-tetrahydroimidazo[4,5-d][1]benzazepine monohydrate and 4-[(Biphenyl-2-ylcarbonyl)amino]benzoic acid: two new key intermediates for the synthesis of the AVP antagonist conivaptan hydrochloride. Org Process Res Dev 9:593–598
Urbina JA (2010) Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop 115:55–68
Wiggers HJ, Rocha JR, Fernandes WB, Sesti-Costa R, Carneiro ZA, Cheleski J, da Silva ABF, Juliano L, Cezari MHS, Silva JS, McKerrow JH, Montanari CA (2013) Non-peptidic cruzain inhibitors with trypanocidal activitydiscovered by virtual screening and in vitro assay. PLoS Negl Trop Dis 7:e2370
World Health Organization (2012) Research priorities for Chagas disease, human African trypanosomiasis and leihsmaniasis. Tech Rep Ser 975:1–100
World Health Organization, WHO Chagas Disease (American trypanosomiasis) (2016).
Yap CW (2011) PaDEL‐descriptor: an open source software to calculate molecular descriptors and fingerprints. J Comput Chem 32:1466–1474
Yea CM, Allan CE, Ashworth DM, Barnett J, Baxter AJ, Broadbridge JD, Franklin RJ, Hampton SL, Hudson P, Horton JA, Jenkins PD, Penson AM, Pitt GRW, Riviere P, Robson PA, Rooker DP, Semple G, Sheppard A, Haigh RM, Roe MB (2008) New benzylureas as a novel series of potent, nonpeptidic vasopressin V2 receptor agonists. J Med Chem 51:8124–8134
Yépes AF, Palma A, Cobo J, Glidewell C (2013) A hydrogen-bonded tetramer in (2RS,4SR)-7-bromo-2-(2-methylphenyl)-2,3,4,5-tetrahydro-1H-naphtho[1,2-b]azepin-4-ol and a three-dimensional hydrogen-bonded framework in (2RS,4SR)-2-(3-methylthiophen-2-yl)-2,3,4,5-tetrahydro-1H-naphtho[1,2-b]azepin-4-ol. Acta Cryst 69:448–452
Yépes AF, Palma A, Cobo J, Glidewell C (2013) Three closely related thienyl-substituted 1,4-epoxynaphtho[1,2-b]azepines: hydrogen-bonded assembly in one, two and three dimensions. Acta Cryst 69:307–312
Zuccotto F, Zvelebil M, Brun R, Chowdhury SF, Di Lucrezia R, Leal I, Maes L, Ruiz-Perez LM, Pacanowska DG, Gilbert IH (2001) Novel inhibitors of Trypanosoma cruzi dihydrofolate reductase. Eur J Med Chem 36:395–405
Acknowledgements
Authors thanks Colombian Institute for Science and Research (COLCIENCIAS, grant No 110265842651), Universidad de Jaén and Consejería de Economía, Innovación, Ciencia y Empleo (Junta de Andalucía, Spain) for financial support. JC thanks Centro de Instrumentación Científico-Técnico of Universidad de Jaén and the staff for spectrometric data acquisition. Grants from Universidad Nacional de San Luis (UNSL), partially supported this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Additional information
These authors contributed equally: Alirio Palma, Juan C. Garro Martinez.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yépes, A.F., Bahsas, A., Escobar, P. et al. Synthesis, anti-parasitic activity and QSAR study of a new library of polysubstituted tetrahydronaphtho[1,2-b]azepines. Med Chem Res 27, 2239–2264 (2018). https://doi.org/10.1007/s00044-018-2232-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-018-2232-7