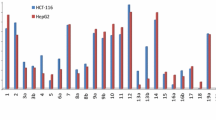
Abstract
The multi-component reaction of 2-acetylthiophene with aromatic aldehydes and either malononitrile or ethyl cyanoacetate gave the pyran derivatives 4a–4f and pyridine derivatives 5a–5f. On the other hand, the reaction of the 2-acetylthiophene with elemental sulfur and either malononitrile or ethyl cyanoacetate gave the thiophene derivatives 6a and 6b; respectively. Compounds 6a and 6b underwent a series of heterocyclic reactions to give thiazole and thiophene derivatives. All the products were assessed for antitumor activity towards human cancer human gastric cancer (NUGC and HR), human colon cancer (DLD1), human liver cancer (HA22T and HEPG2), human breast cancer (MCF), nasopharyngeal carcinoma (HONE1) cell lines. Compounds 4e, 4f, 5e, 5f, 7b, 8b, 10e, 10f, 11e, 11f, 14d-f, 15d-f, 16a,b and 18b exhibited optimal cytotoxic effect against cancer cell lines, with IC50’s in the nM range. Moreover, 7b, 10e, 14d, 15e and 16b showed no toxicity against shrimp larvae. Anti-proliferative cell activity against cancer cell lines of the most potent compounds showed that compounds 5f and 10e achieved the highest activities among the tested compounds.



Similar content being viewed by others
References
Brayn B, Timothy M, Tore S (2009) General and applied toxicology, 3rd edn., vol. I, p 52
Calleja MC, Persoone G (1992) The potential of ecotoxicological tests for the prediction of acute toxicityin man as evaluated on the 1st 10 chemicals on the MEIC program. Atla 20:396–405
Carballo JL, Inda ZLH, Pérez P, Grávalos MDG (2002) A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol 2:17
Chatterjee PN, Roy S (2011) Alkylation of 1,3-dicarbonyl compounds with benzylic and propargylic alcohols using Ir-bimetallic catalyst: synthesis of fully decorated furans and pyrroles. Tetrahedron 67:4569–4577
Choudhary MI, Thomsen WJ, Bioassay techniques for drug development, Harwood Academic Publishers,New York, 2001, pp. 9–10.
Delorenzo ME, Ghung K, Key PB, Wison WA, Black MC (2007) Toxicity of fipronil and its enantiomers to marine and freshwater non-targets. J Environ Sci Health 42:471–480
Elghandour AHH, Mohamed KA, Ibrahim MKA, Elshikh SMM, Fawzy MM, Ali FMM (1992) Studies with polyfunctionally substituted heteroaromatics: synthesis of several new .thiazoles, pyrazolo[5,1-c]triazines and of polyfunctionally substituted pyridines and pyrimidines. Tetrahedron 48:9295–9304
Fakhr IMI, Radwan MAA, El-Batran S, Abd El-Salam OME, El-Shenawy SM (2009) Synthesis and pharmacological evaluation of 2-substituted benzo[b]thiophenes as anti-inflammatory and analgesic agents. Eur J Med Chem 44:1718–1725
Giri RS, Thaker HM, Giordano T, Williams J, Rogers D, Vasu KK, Sudarsanam V (2010) Design, synthesis and evaluation of novel 2-thiophen-5-yl-3H-quinazolin-4-one analogues as inhibitors of transcription factors NF-кB and AP-1 mediated transcriptional activation: Their possible utilization as anti-inflammatory and anti-cancer. Bioorg Med Chem 18:2796–2808
Harinath Y, Reddy HK, Kumar BN, Apparao C, Seshaiah K (2013) Synthesis, spectral characterization and antioxidant activity studies of a bidentate Schiff base, 5-methyl thiophene-2-carboxaldehyde-carbohydrazone and its Cd(II), Cu(II), Ni(II) and Zn(II) complexes. Spectrochim Acta A Mol Biomol Spectrosc 01:264–272
Huang LJ, Kuo SC, Perng CY, Chao YH, Wu TS, McPhail AT, Mauger A, Cheng HH, Lee KH (1998) Synthesis and cytotoxicity of acetyl-4H,9H-naphtho[2,3-b]thiophene-4,9-diones. Bioorg Med Chem Lett 8:2763–2768
Karthikeyan SV, Perumal S, Shetty KA, Yogeeswari P, Sriram DA (2009) Microwave-assisted facile regioselective Fischer indole synthesis and antitubercular evaluation of novel 2-aryl-3,4-dihydro-2H-thieno[3,2-b]indoles. Bioorg Med Chem Lett 19:3006–3009
Martorana A, Gentile C, Perriconem U, Piccionello AP, Bartolotta R, Terenzi A, Pace A, Mingoia F, Almerico AM, Lauria A (2015) Synthesis, antiproliferative activity, and in silico insights of new 3-benzoylamino-benzo[b]thiophene derivatives. Eur J Med Chem 90:537–546
Mathew B, Suresh J, Anbazhaga S (2014) Synthesis, in silico preclinical evaluation, antidepressant potential of 5-substituted phenyl-3-(thiophen-2-yl)-4,5-dihydro-1h-pyrazole-1-carboxamides. Biomed Aging Pathol 4:327–333
Mohareb RM, Al-Omran F (2012) Reaction of pregnenolone with cyanoacetylhydrazine: Novel synthesis of hydrazide–hydrazone, pyrazole, pyridine, thiazole, thiophene derivatives and their cytotoxicity evaluations. Steroids 77:1551–1559
Revelant G, Hesse S, Kirsch G (2011) Synthesis of novel 2-aminothieno[3,2-d]thiazoles and selenolo[3,2-d]thiazoles. Tetrahedron 67:9352–9357
Rodenhuis N, Timmerman W, Wikström HV, Dijkstra D (2000) Thiophene analogs of naphthoxazines and 2-aminotetralins: bioisosteres with improved relative oral bioavailability, as compared to 5-OH-DPAT. Eur J Pharmacol 394:255––263
Romagnoli R, Baraldi PG, Cara CL, Hamel E, Basso G, Bortolozzi R, Viola G (2010) Synthesis and biological evaluation of 2-(3′,4′,5′-trimethoxybenzoyl)-3-aryl/arylaminobenzo[b]thiophene derivatives as a novel class of antiproliferative. Eur J Med Chem 45:5781–5791
Sable NP, Ganguly S, Chaudhario PD (2014) An efficient one-pot three-component synthesis and antimicrobial evaluation of tetra substituted thiophene derivatives. Chin Chem Lett 25:1099–1103
Sharma S, Athar F, Maurya MR, Azam A (2005) Copper (II) complexes with substituted thiosemicarbazones of thiophene-2-carboxaldehyde: synthesis, characterization and antiamoebic activity against E. histolytica. Eur J Med Chem 40:1414–1419
Shi W, Lowary TL (2011) Reprint of “Effect of carbohydrate amino group modifications on the cytotoxicity of glycosylated 2-phenyl-benzo[b]thiophenes and 2-phenyl-benzo[b]furans”. Bioorg Med Chem Lett 21:5107–5112
Terzidis MA, Tsiaras VG, Drosos NM, Kasapidou PM, Stephanatou JS, Tsoleridis CA, Buth G, Kostakis GE (2014) Chromeno[2,3-c]pyrroles by one-pot multicomponent domino addition–amination reaction. Tetrahedron Lett 55:5601–5604
Zhang H, Tanimoto H, Morimoto T, Nishiyama Y, Kakiuci K (2014) Acid-mediated synthesis of fully substituted 1,2,3-triazoles: multicomponent coupling reactions, mechanistic study, synthesis of serine hydrolase inhibitor and its derivatives. Tetrahedron 70:9828–9835
Acknowledgment
R. M. Mohareb would like to thank the Alexander von Humboldt Foundation in Germany for affording research fund facilitating to complete this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Mohareb, R.M., Ibrahim, R.A. & Wardakhan, W.W. Synthesis of pyridine, pyran and thiazole containing thiophene derivatives and their anti-tumor evaluations. Med Chem Res 25, 2187–2204 (2016). https://doi.org/10.1007/s00044-016-1654-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1654-3