Abstract
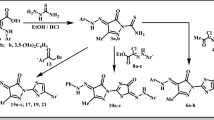
A series of 2-thioxothiazolidin-4-one derivatives were efficiently synthesized and evaluated for their cytotoxic activity against four tumor cell lines. The synthesized compounds were characterized by elemental analysis, IR, 1H NMR, 13C NMR, and mass spectral data. Compound 5c exhibited notable in vitro anticancer activity against HeLa, HT29, A549, and MCF-7 cell lines with IC50 values of 28.3, 24.5, 26.6, and 28.6 µM, respectively. Common pharmacophore model with H bond donor (2), H bond acceptor (3), Hydrophobic (1), and ring aromatic (2) was developed using phase module, and molecular docking of target compounds into the epidermal growth factor receptor kinase revealed important structural information on the plausible binding interactions.





Similar content being viewed by others
References
Alegaon SG, Alagawadi KR, Sonkusare PV, Chaudhary SM, Dadwe DH, Shah AS (2012) Novel imidazo[2,1-b][1,3,4]thiadiazole carrying rhodanine-3-acetic acid as potential antitubercular agents. Bioorg Med Chem Lett 22:1917–1921
Azizmohammadi M, Khoobi M, Ramazani A, Emami S, Zarrin A, Firuzi O, Miri R, Shafiee A (2013) 2H-Chromene derivatives bearing thiazolidine-2,4-dione, rhodanine or hydantoin moieties as potential anticancer agents. Eur J Med Chem 59:15–22
Bois F, Boumendjel A, Mariotte A, Conseil G, Di Petro A (1999) Synthesis and biological activity of 4-alkoxy chalcones: potential hydrophobic modulators of p-glycoprotein-mediated multidrug resistance. Bioorg Med Chem 7:2691–2695
Chan H, Fan YH, Natarajan A, Guo Y, Iyasere J, Harbinski F, Luus L, Christ W, Aktas H, Halperin JA (2004) Synthesis and biological evaluation of thiazolidine-2,4-dione and 2,4-thione derivatives as inhibitors of translation initiation. Bioorg Med Chem Lett 15:5401–5405
Chandrappa S, Kavitha CV, Shahabuddin MS, Vinaya K, Anand Kumar CS, Ranganath SR, Raghavan SC, Rangappa KS (2009) Synthesis of 2-(5-((5-(4-chlorophenyl)furan-2-yl)methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetic acid derivatives and evaluation of their cytotoxicity and induction of apoptosis in human leukemia cells. Bioorg Med Chem 17:2576–2584
Cutshall NS, O’Day C, Prezhdo M (2005) Rhodanine derivatives as inhibitors of JSP-1. Bioorg Med Chem Lett 15:3374–3379
Dixon SL, Smondyrev AM, Knoll EH, Rao SN, Shaw DE, Friesner RA (2006) PHASE: a new enzyme for pharmacophore perception, 3D QSAR model development, and 3D database screening. 1. Methodology and preliminary results. J Comput Aided Mol Des 20:647–671
Fadeyi OO, Adamson ST, Myles EL, Okoro CO (2008) Novel fluorinated acridone derivatives. Part 1: synthesis and evaluation as potential anticancer agents. Bioorg Med Chem Lett 18:4172–4176
Go ML (2003) Novel antiplasmodial agents. Med Res Rev 23:456–487
Go ML, Wu X, Liu XL (2005) Chalcones: an update on cytotoxic and chemoprotective properties. Curr Med Chem 12:481–499
Hardej D, Ashby CR Jr, Khadtare NS, Kulkarni SS, Singh S, Talele T (2010) The synthesis of phenylalanine-derived C5-substuted rhodanines and their activity against selected methicillin-resistant staphylococcus aureus (MRSA) strains. Eur J Med Chem 45:5827–5832
Herencia F, Ferrandiz ML, Ubeda A, Dominguez JN, Charris JE, Lobo GM, Alcaraz MJ (1998) Synthesis and anti-inflammatory activity of chalcone derivatives. Bioorg Med Chem Lett 8:1169–1174
Hotta N, Akanuma Y, Kawamori R, Matsuoka K, Oka Y, Shichiri M, Toyata T, Nakashima M, Yoshimura I, Sakamoto N, Shigeta Y (2006) Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3-year, multicenter, comparatives aldose reductase inhibitor-diabetes complications trial. Diabetes Care 29:1538–1544
Jin X, Zeheng CJ, Song MX, Wu Y, Sun LP, Li YJ, Yu LJ, Piao HR (2012) Synthesis and antimicrobial evaluation of l-phenylalanine-derived C5-substituted rhodanine and chalcone derivatives containing thibarbituric acid or 2-thioxo-4-thiazolidinone. Eur J Med Chem 56:203–209
Kashfi K, Enna SJ, Michael W (2009) Anti-inflammatory agents as cancer therapeutics. Advances in pharmacology. Academic Press, New York, pp 31–89
Katsori AM, Hadjipavlou-Litina D (2009) Chalcone in cancer: understanding their rolein terms of QSAR. Curr Med Chem 16:1062–1081
Kimura Y (2005) New anticancer agents: in vitro and in vivo evaluation of the antitumor and antimetastatic actions of various compounds isolated from medicinal plants. In Vivo 19:37–60
Kontogiorgis C, Mantzanidou M, Hadjipavlou-Litina D (2008) Chalcones and their potential role in inflammation. Mini Rev Med Chem 8:1224–1242
Lawrence NJ, McGown AT (2005) The chemistry and biology of antimitotic chalcones and related enone systems. Curr Pharm Des 11:1679–1693
Lin YM, Zhou Y, Flavin MT, Zhou LM, Nie W, Chen FC (2002) Chalcones and flavonoids as anti-tuberculosis agents. Bioorg Med Chem 10:2795–2802
Liu M, Wilairat P, Croft SL, Tan AL, Go ML (2003) Structure-activity relationship of antileishmanial and antimalarial chalcones. Bioorg Med Chem 11:2729–2738
Liu HL, Jiang WB, Xie MX (2010) Flavonoids: recent advances as anticancer drugs. Recent Pat Anticancer Drug Discov 5:152–164
Maestro (2013) version 9.3; Schrodinger, LLC, New York, NY
Min G, Lee S, Kim H, Han Y, Lee R, Jeong DW, Han DC, Kwon B (2013) Rhodanine-based PRL-3 inhibitors blocked the migration and invasion of metastatic cancer cells. Bioorg Med Chem Lett 23:3769–3774
Momose Y, Meguro K, Ikeda H, Hatanaka C, Oi S, Sohda T (1991) Studies on antidiabetic agents. X. Synthesis and biological activities of pioglitazone and related compounds. Chem Pharm Bull 39:1440–1445
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survrvial. Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Nowakowska Z (2007) Areview of anti-infective and anti-inflammatory chalcones. Eur J Med Chem 42:125–137
Ohishi Y, Mukai T, Nagahara M, Yajima M, Kajikawa N, Miyahara K, Takano T (1990) Preparation of 5-alkylmethylidene-3-carboxymethylrhodanine derivatives and their aldose reductase inhibitory activity. Chem Pharm Bull 38:1911–1919
Opletalova V (2000) Chalcones and there heterocyclic analogs as potential therapeutic agents in bacterial diseases. Ceska Slov Farm 49:278–284
Opletalova V, Sedivy D (1999) Chalcones and there heterocyclic analogs as potential antifungal chemotherapeutic agents. Ceska Slov Farm 48:252–255
Opletalova V, Jahodar L, Jun D, Opletal L (2003) Chalcones (1,3-diarylpropen-1-ones) and their analogs as potential therapeutic agents incardiovascular system diseases. Ceska Slov Farm 52:12–19
Padhye S, Ahmad A, Oswal N, Sarkar FH (2009) Emerging role of garcinol, the antioxidant chalcone from garcinia indica choisy and its synthetic analogs. J Hematol Oncol 2:38
Phase (2013) version 3.4, Schrödinger, LLC, New York, NY
Ravi S, Chiruvella KK, Rajesh K, Prabhu V, Raghavan SC (2010) 5-Isopropylidene-3-ethyl rhodanine induce growth inhibition followed by apoptosis in leukemia cells. Eur J Med Chem 45:2748–2752
Rostom SAF (2006) Synthesis and in vitro antitumor evaluation of some Indeno [1,2-c]-pyrazol(in)es substituted with sulfonamide, sulfonylurea (thiourea) pharmacophores, and some derived thiazole ring systems. Bioorg Med Chem 14:6475–6485
Sortino M, Delgado P, Juarez S, Quiroga J, Abonia R, Insuasty B, Nogueras M, Rodero L, Garibotto FM, Enriz RD, Zacchino SA (2007) Synthesis and antifungal activity of (Z)-5-arylidenerhodanines. Bioorg Med Chem 15:484–494
Volynets GP, Bdzhola VC, Golub AG, Synyugin AR, Chekanov MA, Kukharenko OP, Yarmoluk SM (2013) Rational design of apoptosis signal-regulating kinase 1 inhibitors: discovering novel structural scaffold. Eur J Med Chem 61:104–115
WHO/Cancer-World Health Organisation (2013) http://www.who.int/topics/cancer/en/. Accessed 1 Nov 2013
Yadav VR, Prasad S, Sung B, Aggarwal BB (2011) The role of chalcones in suppression of NF-KB-mediated inflammation and cancer. Int Immunopharmacol 11:295–309
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans W, Speck W (1987) Salmonella mutagenicity tests: III. Results from the testing of 225 chemicals. Environ Mutagen 9:1–110
Acknowledgments
Authors are grateful to Dr. A. D. Taranalli, Principal and Dean Faculty of pharmacy, for providing necessary facilities for the research. Abounded thanks are due to Dr. Raghu for providing the Schrodinger software and also for the encouragement and motivation to complete the computational studies. Authors are also grateful to NMR Research center, IISC, Bangalore, India, for providing the spectral data.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alegaon, S.G., Alagawadi, K.R., Vinod, D. et al. Synthesis, pharmacophore modeling, and cytotoxic activity of 2-thioxothiazolidin-4-one derivatives. Med Chem Res 23, 5160–5173 (2014). https://doi.org/10.1007/s00044-014-1087-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1087-9