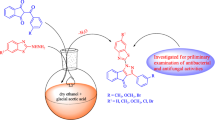
Abstract
In an attempt to design and synthesize effective antimicrobial agents using click chemistry, mono- and di-alkyne-substituted monoboc protected o-phenylenediamines were reacted with different substituted aryl azides which yielded 18 new compounds (4a–4k and 5a–5f, 5l). Structures of all newly synthesized compounds were established by 1H and 13C NMR analysis. The intermediate compound 1 was also confirmed by X-ray crystallography. The title compounds were screened for their antibacterial activity against Gram +ve bacteria (Staphylococcus aureus and Enterococcus faecalis), Gram −ve bacteria (Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa), and their antifungal profile were tested on (Candida tropicalis, Candida albicans, Candida krusei, and Cryptococcus neoformans) as well as on molds such as (Aspergillus niger, Aspergillus fumigatus). The compounds 4k and 5f both showed maximum potency against S. aureus (ATCC 25323) strain with MIC value of 6.25 µg/ml, which is comparable with standard drug ciprofloxacin (MIC 6.25 µg/ml) while remaining compounds showed moderate to weak activity. Further, all compounds showed average antifungal activity in the range of 100–200 µg/ml.



Similar content being viewed by others
References
Agarwal A, Singh MK, Awasthi SK (2011a) 1-Prop-2-ynyl-1H-benzimidazol-2-amine. Acta Cryst E67:o3213–o3214
Agarwal A, Singh MK, Singh S, Bhattacharya S, Awasthi SK (2011b) N-(Prop-2-yn-1-yl)-1,3-benzothiazol-2-amine. Acta Cryst E67:o2637–o2638
Andre A, Gerard N (1969) 1-(Carboxyalkyl)-2-methyl-3-[substituted benzoyl (and thiobenzoyl)]indoles Ger. Offen (Roussel–UCLAF) DE 1901167 A 19691211
Bag SS, Matsumoto K, Talukdar S, Kundu R (2013) Triazolyl donor/acceptor chromophore decorated unnatural nucleosides and oligonucleotides with duplex stability comparable to that of a natural adenine/thymine pair. J Org Chem 78:278–291
Banu KM, Dinakar A, Ananthanarayanan C (1999) Synthesis, characterization, antimicrobial studies and pharmacological screening of some substituted 1,2,3-triazoles. Indian J Pharm Sci 61:202–205
Biagi G, Calderone V, Giorgi I, Livi O, Martinotti E, Martelli A, Nardi A (2004) 1,5-Diarylsubstituted 1,2,3-triazoles as potassium channel activators. VI. Farmaco 59:397–404
Bickoff EM, Livingston AL, Guggolz J, Thompson CR (1952) Comparative evaluation of antioxidants for carotene. J Am Oil Chem Soc 29:445–446
Bouabdallah I, Barek LAM, Zyad A, Ramdani A, Zidane I, Melhaoui A (2006) Anticancer effect of three pyrazole derivatives. Nat Prod Res 20:1024–1030
Chan DPY, Shoichet MS, Owen SC (2013) Double click: dual functionalized polymeric micelles with antibodies and peptides. Bioconjug Chem 24:105–113
Chen M, Lu S, Yuan G, Yang S, Du X (2000) Synthesis and antibacterial activity of some heterocyclic β-enamino ester derivatives with 1,2,3-triazole. Heterocycl Commun 6:421–426
Danoun S, Baziard-Mouysset G, Stigliani JL, Payard M, Selkti M, Viossat B, Tomas A (1998) Addition of diazomethane to 3 and 4-nitrophthalodinitriles. Heterocycl Commun 4:45–51
Diana GD, Nitz TJ (1993) Preparation of [(1,2,4-oxadiazolylphenoxy)alkyl]isoxazoles and their use as antiviral agents. Eur Pat Appl EP 566199(A1):19931020
Dixit SK, Mishra N, Sharma M, Singh S, Agarwal A, Awasthi SK, Bhasin VK (2012) Synthesis and in vitro antiplasmodial activities of fluoroquinolone analogs. Eur J Med Chem 51:52–59
Eicher T, Hauptmann S (2003) The Chemistry of Heterocycles:Structure, Reactions, Syntheses and Applications, 2nd edn. Wiley-VCH, Weinheim
Friedemann MN, Robinson SW, Gerhardt G (1996) o-Phenylenediamine-modified carbon fiber electrodes for the detection of nitric oxide. Anal Chem 68:2621–2628
Huang Y-B, Tang G-R, Jin G-Y, Jin G-X (2007) Binuclear nickel and copper complexes with bridging 2,5-diamino-1,4-benzoquinonediimines: synthesis, structures, and catalytic olefin polymerization. Organometallics 27:259–269
Hueber-Becker F, Nohynek GJ, Meuling WJ, Benech-Kieffer F, Toutain H (2004) Human systemic exposure to a [14C]-para-phenylenediamine-containing oxidative hair dye and correlation with in vitro percutaneous absorption in human or pig skin. Food Chem Toxicol 42:1227–1236
Kahl T, Schröder KW, Lawrence FR, Marshall WJ, Höke H, Jäckh R (2007) “Aniline” in Ullmann’s Encyclopedia of Industrial Chemistry. John Wiley and Sons, New York. doi:10.1002/14356007.a02_3032
Kang IJ, Lee MH (2006) Quantification of para-phenylenediamine and heavy metals in henna dye. Contact Dermat 55:26–29
Kolb HC, Sharpless KB (2003) The growing impact of click chemistry on drug discovery. Drug Discov Today 8:1128–1137
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed 40:2004–2021
Krüger RH, Boissiére C, Klein-Hartwig K, Kretzschmar HJ (2005) New phenylenediamine antiozonants for commodities based on natural and synthetic rubber. Food Addit Contam 22:968–974
Lilienkampf A, Mao J, Wan B, Wang Y, Franzblau SG, Kozikowski AP (2009) Structure–activity relationships for a series of quinoline-based compounds active against replicating and nonreplicating Mycobacterium tuberculosis. J Med Chem 52:2109–2118
Mahawer C, Singh MK, Agarwal A (2013) Crystal structure of {4-[3-(4-Fluoro-phenyl)-acryloyl]-phenyl}-carbamic acid tert-butyl ester. Proc Natl Acad Sci India A. doi:10.1007/s40010-013-0077-5
Manfredini S, Vicentini CB, Manfrini M, Bianchi N, Rutigliano C, Mischiati C, Gambari R (2000) Pyrazolo-triazoles as light activable DNA cleaving agents. Bioorg Med Chem 8:2343–2346
Meier R (1986) Preparation of fluorinated phenylalkyltriazoles as anticonvulsants and pharmaceutical compositions containing them. Eur Pat Appl EP 199262(A2):19861029
Nielsen SF, Larsen M, Boesen T, Schonning K, Kromann H (2005) Cationic chalcone antibiotics. Design, synthesis and mechanism of action. J Med Chem 48:2667–2677
Niemann GJ, Dekker J (1966) Activity against rust and powdery mildew of some para-phenylenediamines and related compounds. Neth J Plant Pathol 72:213–221
Obaseiki-Ebor EE, Odukoya K, Telikepalli H, Mitscher LA, Shankel DM (1993) Antimutagenic activity of extracts of leaves of 4 common edible vegetable plants in Nigeria (West-Africa). Mutat Res 302:109–117
Passannanti A, Diana P, Barraja P, Mingoia F, Lauria A, Cirrincione G (1998) Pyrrolo[2,3-d][1,2,3]triazoles as potential antineoplastic agents. Heterocycles 48:1229–1235
Patai S (1994) Chemistry of Triple-Bonded Functional Groups. Wiley, New York
Pauling L, Brockway LO (1934) A study of the methods of interpretation of electron-diffraction photographs of gas molecules, with results for benzene and carbon tetrachloride. J Chem Phys 2:867–881
Raut CN, Bagul SM, Janrao RA, Vaidya SD, Kumar VBS, Mahulikar PP (2010) Synthesis of some novel N-alkyl/acyl/aroyl 2-(chroman/6-bromochroman-2-yl)-1H-benzimidazoles using ionic liquids and their antibacterial activity. J Heterocycl Chem 47:582–588
Robertson KN, Knop O, Cameron TS (2003) C–H···H–C interactions in organoammonium tetraphenylborates: another look at dihydrogen bonds. Can J Chem 81:727–743
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB (2002) A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed 41:2596–2599
Sachin HP, Khan MHM, Bhujangaiah NS (2009) Surface modification of mild steel by orthophenylenediamine and its corrosion study. Int J Electrochem Sci 4:134–143
Sanghvi YS, Bhattacharya BK, Kini GD, Matsumoto SS, Larson SB, Jolley WB, Robins RK, Revankar GR (1990) Growth inhibition and induction of cellular differentiation of human myeloid leukemia cells in culture by carbamoyl congeners of ribavirin. J Med Chem 33:336–344
Sheldrick GM (2008) A short history of SHELX. Acta Cryst A64:112–122
Sheremet EA, Tomanov RI, Trukhin EV, Berestovitskaya VM (2004) Synthesis of 4-aryl-5-nitro-1,2,3-triazoles. Russ J Org Chem 40:594–595
Singh MK, Agarwal A, Awasthi SK (2011a) Benzyl N-(3-chloro-4-fluorophenyl)carbamate. Acta Cryst E 67:o1137
Singh MK, Agarwal A, Mahawara C, Awasthi SK (2011b) tert-Butyl N-{2-[bis(prop-2-yn-1-yl)amino]phenyl}carbamate. Acta Cryst E 67:o1382
Singh S, Singh MK, Agarwal A, Awasthi SK (2011c) 2-(4-Chlorophenyl)chromen-4-one. Acta Cryst E 67:o3163
Singh S, Singh MK, Agarwal A, Hussain F, Awasthi SK (2011d) (2E)-1-(4-Aminophenyl)-3-(2,4-dichlorophenyl)prop-2-en-1-one. Acta Cryst E67:o1616–o1617
Singh MK, Tilak R, Nath G, Awasthi SK, Agarwal A (2013) Design, synthesis and antimicrobial activity of novel benzothiazole analogs. Eur J Med Chem 63:635–644
Varma RS (1999) Solvent-free synthesis of heterocyclic compounds using microwaves. J Heterocycl Chem 36:1565–1571
Wiegand I, Hilpert K, Hancock REW (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175
Yasuo S, Akio A, Yashiro F, Koshida S, Wakao M, Nishimura T, Kusumoto S, Sobel M (2006) Immobilization and clustering of structurally defined oligosaccharides for sugar chips: an improved method for surface plasmon resonance analysis of protein–carbohydrate interactions. Bioconjug Chem 17:1125–1135
Zabula AV, Hahn FE (2008) Mono- and bidentate benzannulated N-heterocyclic germylenes, stannylenes and plumbylenes. Eur J Inorg Chem 33:5165–5179
Zeng XH, Liu M, Ding MW, He HW (2010) Facile synthesis of 2-alkylthio-5,6,7,8-tetrahydrobenzothieno[2,3-d]pyrimidin-4(3H)-ones. Synth Commun 40:1453–1460
Acknowledgments
AA is thankful to (UGC), New Delhi, India (Scheme No. 34-3212008) and Banaras Hindu University Varanasi, UP, India, respectively, for financial support. MKS is thankful to Banaras Hindu University, Varanasi, India for financial support. This work was in partly supported by GN and RT by the Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, UP, India.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, M.K., Gangwar, M., Kumar, D. et al. In vitro antimicrobial activity of o-phenylenediamine-tert-butyl-N-1,2,3-triazole carbamate analogs. Med Chem Res 23, 4962–4976 (2014). https://doi.org/10.1007/s00044-014-1063-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1063-4