Abstract
Due to agriculture and logging, Costa Rica has lost many primary forests, making reforestation an important task. To judge the progress of reforestation, it is important to follow the reassembly of organismal communities within restored habitats. The COBIGA project near La Gamba, in the Golfo Dulce region of Costa Rica, aims at reforestation of lowland sites with native tree species. Ants, as ubiquitous and highly abundant terrestrial organisms, have a substantial influence on tropical ecosystems. The multiple roles include scavenging, predation, herbivory, and mutualistic interactions. We examined ant community responses to reveal the status of community regeneration and functional integrity. We compared the composition and diversity of the ant assemblages at three different age reforestation sites (2, 8, and 10 years old) with those at an old-growth forest as a reference site. By offering canned tuna fish at ground level along replicated transects, we observed 43 ant species representing six functional groups during the 2 months of sampling. Most of the observed ant species were omnivorous, but old-growth forests harbored a substantial number of other functional groups, such as generalized predators, arboreal predators, and arboreal omnivores. In contrast, the youngest reforestation site harbored a severely impoverished ant assemblage comprising mostly generalized polygynous and polydomous ant species from lower trophic levels. The within-site heterogeneity of the ant assemblages increased from the youngest to the oldest forest. In addition, our results show the importance of monitoring the progress of forest recovery to avoid the spread of invasive species into primary habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical forests are globally important habitats. They harbor half of the Earth’s terrestrial species (Singh et al. 2019) and store large amounts of carbon (Berenguer et al. 2014). The conversion and the degradation of habitats are among the major factors influencing biodiversity decline (Newbold et al. 2015). Ants are one of the most abundant groups of terrestrial organisms on Earth (they are considered to make up to 25% of animal biomass: Fayle and Klimeš 2022). Due to their high abundance in tropical ecosystems, they are considered major contributors to maintaining multiple ecosystem services (Griffiths et al. 2018). Ants employ many different feeding strategies, including large numbers of omnivores and scavengers, but many ant species also consume honeydew or extrafloral nectars, seeds, and fungi (Hölldobler and Wilson 1990; Lach et al. 2010). In many habitats, they are the most important predators of other invertebrates (Hölldobler and Wilson 1990). For these reasons, ants are often used as bioindicators in land management, and the status of ant assemblages serves as a proxy for conditions in tropical ecosystems. This line of research was pioneered by the classification of ants into functional groups, which were initially established in Australia (Andersen 1995). Several studies have shown that the colonization of ant communities during ecosystem recovery reflects general patterns of diversity in other invertebrates (Andersen and Majer 2004). Since then, a significant number of studies on forest recovery have been performed with ants as the focal group. Globally, dominant ant taxa prevalent in complex environments often belong to the subfamily Dolichoderinae (Andersen 2000). Another large ant group comprises generalized Myrmicinae, which are considered subdominant and have high food exploitation capacity; however, their worker body size is smaller, they occupy smaller foraging territories, and they often show broader environmental tolerance than dolichoderines (Andersen 1995). Generalized Myrmicinae are usually prevalent in habitats under moderate stress and disturbance levels. The last major group is formed by opportunists which occupy wider environmental ranges but have poor competitive abilities and are more prevalent in stressed, dynamic, and disturbed habitats (Andersen and Majer 2004). The influence of environmental disturbance on ant communities is usually indirect and caused by stress due to changes in habitat structure, microclimate, and food availability (Andersen 1995).
Studies from the Neotropical region conducted in lowland rainforests revealed that certain functional groups are more sensitive to increased disturbance levels and are less prominent in disturbed habitats (Leal et al. 2012; Rabello et al. 2021; Ryder Wilkie et al. 2009). In anthropogenically altered systems, functional groups of ants, such as specialized predators or cryptic leaf litter species, are known to exhibit decreased richness and numbers because of their sensitivity to habitat disturbance. Bihn et al. (2010) revealed that the decrease in functional diversity in young secondary forests is mostly due to the absence of rare, functionally unique ants. This impoverishment is also fostered by breaking competitive hierarchies among ant species due to changes in the abundance of dominant ants to which generalized and opportunistic species are sensitive (Andersen and Majer 2004). The influence of environmental disturbance on ant communities is usually indirect and caused by stress due to changes in habitat structure, microclimate, and food availability (Andersen 1995). Primary tropical forests harbor greater ant species richness than secondary forests, but assemblage richness and complexity increase during succession with time since disturbance (Silva et al. 2007). The response of community structure to a changing environment depends on the existing local to regional species pool and the species’ ability to colonize new habitats under changing conditions (Gibb et al. 2015) as well as on elevation. The time needed for complete recovery of ant fauna has previously been estimated to be between 25 and 40 years (Dunn 2004); however, two recent studies evaluating secondary forest recovery based on ant community properties have shown that full succession will take less than 40 years (Hethcoat et al. 2019; Hoenle et al. 2022).
The present study aimed to compare the ant assemblages of replanted rainforests in the Golfo Dulce region in SW Costa Rica at different successional stages. The area is one of the most humid regions in Mesoamerica, with a very intense rainy season from August to November and a nondistinctive dry period compared to other regions in February and March. The mean annual temperature is 27.4 °C, and the annual precipitation is 6241 mm at the La Gamba field station (Weissenhofer et al. 2019a, b). Tall lowland forests survive in two national parks (Corcovado and Piedras Blancas), which are surrounded by a cultivated landscape compromising a mix of cattle pastures, oil palm plantations, and various types of secondary growth. The Esquinas forests near the Pacific coast used to be connected with the Fila Cruces mountain range, which is a part of the Mesoamerican Biological Corridor that stretches from southern Mexico to Panama (Holland 2012). Today, however, the connection between lowlands and mountain forests is interrupted by farmland and pastures.
The COBIGA reforestation project started in 2006 in response to dramatic deforestation in Costa Rica that occurred until the 1990s (Weissenhofer et al. 2019a, b). Earlier, the cultivation of bananas played a major role in regional deforestation (Hernandez and Witter 1996); however, in recent years, the expansion of oil palm cultivation has become a major threat (Höbinger et al. 2012). To counteract such damage, selected former pastures and farms are currently reforested with a range of multiple native tree species based on historical documentation from primary forest inventories. Given the global increase in the area of secondary and otherwise regenerating forests in the tropics, it is important to extend conservation research into such habitats because of the ever-increasing importance of these forests for biodiversity preservation as well as for ecosystem services and functions (Chazdon 2014). Thus far, vegetation development in the reforested areas of the COBIGA project has been investigated and documented in detail (Hietz et al. 2019). Using ants as bioindicators of forest recovery became widely used because of their abundance in the tropical region, and is particularly well documented in Australia (Andersen and Sparling 1997; Grimbacher and Hughes 2002; Jackson and Fox 1996; Piper et al. 2009; Majer et al. 2013; Andersen et al. 2003). In the Neotropical region, similar studies were conducted in Brazil (Bihn et al. 2008; Gomes et al. 2014; Schmidt et al. 2013; Silva et al. 2007), Colombia (Kattan et al. 2006; Hethcoat et al. 2019; Narváez-Vásquez et al. 2021), Mexico and Ecuador (Roche-Ortega et al. 2013; Wilkie et al. 2009; Hoenle et al. 2021). Studies in Cosa Rica remain sparse and especially Golfo Dulce region lacks documentation on this topic. An initial study has addressed the utilization of newly reforested habitats by various groups of animals (Schulze et al. 2019). However, no regional case study has been conducted on ants as essential ecosystem engineers. The only local ant study available demonstrated that, compared with those in old-growth forests, spontaneously regenerating secondary forests harbor rather rich but still impoverished ant assemblages, whereas ant communities in oil palm plantations are severely depauperate (Falk et al. 2019). There is a knowledge gap in the evaluation of attributes shaping the re-establishment of ant communities regarding forest recovery trajectories. A recent study on the re-establishment of epigaeic ant communities in secondary forests of different restoration types in Paraná, Brazil, revealed that ant assemblage composition of any secondary forest diverges irreversibly. This suggests that reforestation leads to building up new community structures that differ from those prior to anthropogenic alteration (Nickele et al. 2023). Studying forest recovery can be challenging due to the long scale. Therefore, chronosequences are often be used as surrogates of forest recovery (Chazdon 2014). Yet, there are only a few studies that evaluated tropical ant communities through a chronosequence of reforestation sites (Silva et al. 2007; Bihn et al. 2008; Hoenle et al. 2021; Hoenle et al. 2023).
This study investigated differences in ant community composition at three successional stages in planted secondary forests compared to old-growth forests. Specifically, we tested the following hypotheses:
-
1.
Old-growth forest harbors the highest species richness, with the highest number of omnivores but also includes functional specialists, i.e., specialized predators or cryptic litter species.
-
2.
The species assemblage compositions differed among the four habitats, with the most homogenized community occurring at the youngest reforestation site and the most heterogeneous communities occurring in the old-growth forest. The youngest reforestation site harbors mostly generalized opportunists and has very low compositional variation, which increases with forest age, while the oldest reforestation site should more closely resemble the composition of the old-growth forest with more functional specialists present.
Materials and methods
Sampling sites
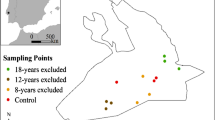
The sampling sites were located in the Golfo Dulce region of southwestern Costa Rica, near the village of La Gamba (Table 1). One primary forest stand and three reforestation sites were included at different stages of succession. The old-growth forest reference site (Finca Torre) is situated directly on the border of Piedras Blancas National Park and contains tall near-natural ravine, ridge, and slope forests (terminology according to Binz et al. 2015). The 10-year-old reforestation site (La Bolsa) is a mixture of primary and secondary forests as well as an abandoned pasture and cropland. Here, sampling was performed on the abandoned former agricultural area that was reforested between 2010 and 2012, and the area is now covered by dense secondary forest. Here, sampling was performed on the abandoned former agricultural area reforested between 2010 and 2012; 6065 trees had been planted, and the area is now covered by dense secondary forest. The 8-year-old (Finca Amable) reforestation site was formerly a pasture stocked with cultivated Russell River grass (Paspalum paniculatum) and surrounded by oil palm plantations (Elaeis guineensis) from two sides. It also borders the Río Bonito River and the old-growth forests of the National Park Piedras Blancas to the north, with a substantial area of that site being flooded during the rainy season. Between 2013 and 2016, 10,700 trees had been planted at the site. The youngest reforestation site (Finca Eduardo) comprises a former pasture, drainage ditches, and oil palm plantations. Here, reforestation started only in 2020. It is surrounded by palm oil plantations from two sites and connects the forests of the Fila Gamba with the eastern foothills of the National Park Piedras Blancas.
Ant sampling
Fieldwork took place from 1st July to 9th September 2022. Within each site, three 100 m transects were delimited to facilitate setting out the bait traps, and four traps were placed along each transect. Each trap was not exactly located on the same spot every day but was always placed within the frame of each transect and at the same distance from the next neighboring trap. Transects were placed approximately in the middle of each site to maintain similar distances from the edges, but the traps (and not the transects) served as units of analysis. Every trap was set 10 m apart from its nearest neighbor, and transects within a site were located 50 m apart from each other. Sampling was conducted only in the morning between 06:00 and 12:00 h and only on rain-free days. If rain started during the sampling, ant baiting was stopped, and the process was repeated the next day. At each site, sampling was repeated for 10 days (randomly distributed over the sampling period), yielding approximately 120 sampling points at each site. Sites were visited in random order. As bait, we used canned tuna fish in soy oil, which is always of the same brand. This herb has proven to be a good bait for tropical ants (Gotelli et al. 2011; Yanoviak and Kaspari 2000). One tablespoon of tuna fish was placed on a transparent plastic plate (diameter 10 cm) and dug into the litter or topsoil so that ants foraging on the ground could easily reach it. Protein-rich baits are considered to attract mostly omnivorous species, but since these are the most prevalent fraction of many ant communities, the use of these baits and traps is a suitable method for estimating the influence of these ants on the ecosystem. The traps were exposed for approximately two hours, and voucher specimens were collected into vials filled with 70% ethanol every hour for further analysis. On each visit, ants were sampled twice, 60 and 120 min after bringing out the baits, but were monitored more frequently to capture also solitary foragers that might not remain for a longer time in the trap (Fig. 1).
Species identification
Specimens collected from the traps were identified on the grounds of morphological traits using the internet sources Antwiki (2022), Antweb (2022) and Longino (2022). All the samples were identified to the species level if possible. The nomenclature of the ant species followed Antwiki (2022). Twelve ant samples were chosen for DNA barcoding because of difficulties with morphology-based identification. DNA was extracted using an Analytik Jena Kit and an innuPREP DNA Micro Kit and processed for Sanger sequencing on an AB 3730 DNA Analyzer from Applied Biosystems. The primers standard LepF and LepR were used. The obtained COI sequences (with a length of 658 bp) were entered into the BOLD systems repository (http://www.boldsystems.org) and subjected to a BLAST search. Most of the sequences were matched to samples affiliated with a valid scientific name, but four taxa were assigned only the working name for the respective species. These species are marked in boldface in the table of identified species.
Statistical data analysis
To capture the differences between each reforestation habitat and sampling points at a smaller spatial scale, we used the data aggregated at each individual trap (12 replicates per site) as sampling units in the subsequent analyses. Species richness per forest site was calculated using the iNext package in R (Chao et al. 2014; Hsieh et al. 2022). This was conducted based on the incidences of each species at each trap. Species accumulation curves were subsequently created with ggplot2 (Wickham 2016). Community composition was first analyzed using non-metric multidimensional scaling (NMDS). As a data frame, we used a single incidence measure for each trap based on all samples at that location, which means the incidence of a species in an individual trap was a sum of all replicate observations in that particular trap. Samples taken after 60 and 120 min were treated separately, meaning that if a certain ant species occurred twice (or remained after both 60 and 120 min), it was considered twice as common as it occurred in this trap. The analysis was conducted using the metaMDS function of the vegan R package (Oksanen et al. 2020). Bray–Curtis distances were used to perform these computations. Additionally, a betadisper function was used to check for homogeneity of variances between the groups. A PERMANOVA (with the adonis function in R) was subsequently performed to determine the significance of each analysis.
Results
Species richness
Altogether, 43 ant species represented by approximately 15,000 workers were identified. As a result of DNA barcoding, we identified four species that apparently are not yet taxonomically described with a scientific name, but which are covered by the barcoding database www.boldsystems.org (viz. Azteca ACG6526, Pheidole MAS01, Pheidole MAS026, Pheidole EPEM191). Based on the classification by Groc et al. (2014), ant genera were assigned to six functional groups. The percentage distribution of functional groups among the sampling sites is shown in Fig. 2. The broadest representation of different functional groups was observed in the old-growth forest, in which generalized omnivores composed of the highest percentage, but generalized ground-dwelling predators, arboreal omnivores, and arboreal predators made up further sizeable fractions.
Bar plot of functional ant groups identified at each reforestation site according to the classification by Groc et al. (2014), with the percentage contribution of each group to locally observed species richness (AO—arboreal omnivore, AP—arboreal predator, GGP—generalized ground-dwelling predator, GO—generalized omnivore, RHP—raid hunting predator, and LC—leaf cutter)
The oldest reforestation site showed a very high representation of generalized omnivores compared to other functional groups but already covered almost all the functional groups observed in the old-growth forest. In contrast, the 8-year-old forest covered only three functional groups, 80% of which were generalized omnivores. Finally, the 2-year-old forest harbored only two functional groups, with generalized omnivores covering more than 90% of the identified species (Fig. 2). According to the iNEXT results (Fig. 3), the 2-year-old reforestation site reached full saturation, suggesting that extending the sampling effort would likely not have increased the recorded number of ant species further. In the three other habitats, increased sampling effort using the same bait traps might have added a few species, but the extrapolation curves are largely asymptotic. Additionally, the ant species richness in the 8-year-old forest was very close to that in the 10-year-old forest. At a standardized number of 200 sampling units and judging from the 95% confidence intervals of the richness estimates, the four reforestation sites fell into three groups concerning their overall ant species richness: old-growth forest, 8-year-old + 10-year-old (mid-aged reforestations), and 2-year-old (the youngest reforestation).
Community composition analysis
Species incidences were used for computing a distance-based analysis of the ant community composition among all 48 traps across the four reforestation sites. First, a non-metric multidimensional scaling was computed to check for compositional differences in the ant assemblages between sites (Fig. 4). As expected, the ant communities differed significantly among the sampling sites, with old-growth forest showing the highest diversity in ant composition (i.e., dispersion among individual traps) and the youngest reforestation site showing the lowest compositional diversity. A PERMANOVA test showed that all these differences were highly significant (Fig. 4). Additionally, a multivariate test for within-group dispersion was computed. The results revealed significant compositional differences within the groups, with the old-growth forest exhibiting the highest compositional diversity and the youngest reforestation site exhibiting the lowest, thus revealing the graphical patterns in the ordination plot. The distances of the individual bait trap sites from the centroid of each reforestation site reflect the compositional complexity of the ant assemblages at each reforestation site (Fig. 5).
NMDS ordination plot of the ant assemblages per bait trap position based on Bray‒Curtis distances. A gradient in species composition and within-site dispersion is visible, starting from the right (old-growth forest) across 10 years and 8 years to the youngest reforestation site (stress: 0.21). The data used were species incidences with individual traps per site as sampling units. PERMANOVA: F = 5.382, df = 3, p = 0.001.
Discussion
Overall, we collected and identified 43 species from 24 genera and seven subfamilies. As expected, the primary forest harbored the most species, but the oldest secondary forest had a species number close to that of the old-growth forest, even though the functional group composition was more unbalanced there. A study conducted in the Amazon basin using pitfall traps and conducted for a shorter period resulted in a similar species number (Santos et al. 2021). Bait sampling has many limitations because it captures only ground-dwelling omnivorous ants whose diet includes protein-based food products and who forage during the day. This means that this sampling method can hardly record groups, such as leaf-cutting ants, highly specialized predators (i.e., army ants), arboreal ants, and ants inhabiting myrmecophytes. In practice, only a few arboreal ants were captured in the traps while foraging on the ground. Pitfall traps capture more species than baited traps because they are left overnight and are exposed for only a few hours. Almost all arboreal species captured are known to nest on the ground and forage into the canopy from there, which can explain their presence in our traps. Another limitation of the study was no habitat replications, so we could not compare the species composition and richness of multiple reforestation sites of the same age. As mentioned before, studies on the recovery of ant assemblages in secondary forests remain sparse in the region, making it important to conduct such research in the context of this regional reforestation program. This study, therefore, provides a piece of valuable information on ant assemblages in selected reforestation sites and can be further compared with research conducted on multiple reforestation sites of the same age in Costa Rica and elsewhere.
There were only three ant species that occurred at all the sampling sites. Their biology confirms that they all survived in disturbed habitats and had very generalized habitat requirements (Achury et al. 2018). These species included Nylanderia steinheili (A. Forel), a species native to the Neotropics that is common in open areas (Williams and Lucky 2020). The current spread of this invasive species beyond its native range can be fostered by temperature increases and the transport of goods (Williams and Lucky 2020). Another species found at all the sampling sites was the fire ant, Solenopsis geminata (J. Fabricius), whose native range includes Central and South America and whose abundance in disturbed areas tends to increase globally (Gotzek et al. 2015). Currently, Monomorium floricola is a globally distributed tramp ant in tropical countries (Wetterer 2010a, b). The occurrence of these three weedy invasive species at reforestation sites seems understandable, whereas the fact that they were also present in the old-growth forest might be caused by human impacts on this forest stand, which is situated directly at the margin of the national park area. Surprisingly, Monomorium pharaonis (C. Linnaeus) was present only in the old-growth forest and showed a very high abundance there. M. pharaonis was first found in the West Indies but is currently extremely widespread worldwide (Wetterer 2010a, b). Its occurrence in the old-growth forest is likely connected to human activity there. These observations indicate that this forest stand, even though characterized as an old-growth forest by its vegetation, suffers from faunal changes induced by nearby human activities. Other studies have shown that invasive ants tend to colonize the edges of forests due to habitat fragmentation resulting in more extreme conditions that are not favorable for native species (Crist 2009).
Looking at functional groups rather than species, as expected, old-growth forests covered all the functional groups identified at all the sampling sites (Fig. 2). In contrast, the youngest reforestation site covered only two functional groups, with generalized omnivores representing more than 95% of the observed ants. This was expected because it has very young trees, its vegetation is still dominated by herbs, and the whole site was still mostly an open area at the time of ant sampling. The 8-year-old reforestation site included an additional functional group, viz. generalized predators. This site had significantly more canopy coverage than did the 2-year-old forest but was still not as dense as an old-growth forest. Additionally, it is prone to flooding due to its proximity to a river, which can influence ant species richness, especially in the rainy season. According to Baccaro et al. (2013), the number of specialized ant species decreases in the face of increasing water table levels, while the number of generalized species remains the same. Additionally, the activity of leaf-cutting ants and the number of nests decrease in proximity to high water levels (Sendoya et al. 2014). At this relatively young reforestation site, many specialized ant species were not expected to be detected, but recurrent flooding during the rainy season could further influence the ratio of more specialized generalized species.
Three species were characteristic of this site because their abundance was the highest there or because they were even exclusive to that site. Two Pheidole species (Pheidole boliviana (E. Wilson) and Pheidole MAS011) exhibited a very high abundance in this 8-year-old forest, with the undescribed species P. MAS011 occurring only there. Pheidole is one of the most speciose animal genera and consists of 900 taxonomically defined species, many of which are still undescribed. It is very diverse and abundant around the world but is particularly prevalent in tropical regions (Wilson 2003). Pheidole species identified in my study were all generalized omnivores. Dolichoderus curvilobus (J. Lattke) was very abundant in the old-growth forest and the oldest reforestation site but did not occur in any younger forest sites. This species is an arboreal species whose main food source is nectar. They build large colonies and exhibit aggressive behavior when defending their nest (Longino 2022). They were very abundant and were present almost daily in my traps at the sites where they occurred. Another surprising but rather coincidental observation was made for two species of Pseudomyrmex. Ants of this largely arboreal genus inhabit hollow stems and twigs of various plants. Pseudomyrmex perboscii is known to inhabit cavities of Pithecellobium sp. (Ward 1989), whereas Pseudomyrmex simplex prefers dead twigs or stalks of various plants, such as Baccharis halimifolia, Cladium jamaicense, Laguncularia racemosa, Metopium toxiferum, and Nectandra coriacea (Ward 1985). Our results show that the abundance of certain ant species cannot only be explained through their feeding and nesting habits but also their morphology and colony-building type need to be taken into account. As far as trait information is available, most ant species present in the youngest reforestation sites are characterized by polydomy and occupy low trophic levels. With the increase in forest age, the species richness increases leading to a community comprising more specialized species, including those at higher trophic levels. According to multiple studies plant diversity of the habitat is positively correlated with ant species richness, but species composition is more informative for assessing forest recovery (Solar et al. 2016; Segat et al. 2017).
The community composition of ants is influenced by both biotic and abiotic factors (Boet et al. 2020). The major biotic factor is considered to be competition between ants (Cerdá et al. 2013). It is very difficult to “capture” competition, but resource limitation is key to understanding competition and the co-occurrence of species (Law and Parr. 2020). Several studies have shown that arboreal ant species co-occur less frequently than expected by chance. Dominant ant species do not co-occur due to competitive exclusion (Sanders et al. 2007). According to Law and Parr (2020), canopy ant assemblages exhibit greater nitrogen limitation, and dominant species monopolize resources high in protein. This shortage could explain why some arboreal species occurred in my protein bait traps on the ground. A similar study on ground-dwelling ants in a very simple banana agroecosystem showed that certain species had a greater probability of co-occurring, while others excluded each other (Carval et al. 2016). As mentioned before, the history of land use might influence the recovery speed of habitats. This difference could explain the different results of several studies performed in the Neotropical region. Our results showed that the oldest reforestation site starts to serve as a reminder of the community of primary forest ten years after replanting, but the ant assemblage still remains intermediate between 8-year-old and old-growth forests. This finding is in line with other studies conducted in the Neotropical region. Hoenle et al. (2021) reported near complete recovery of ant communities after approximately 34 years of succession in a tropical forest in Ecuadorian Chocó, whereas according to Rocha-Ortega and Favila (2013), the process of recovering ant communities was still ongoing in a 27-year-old secondary forest. Even after 50 years of succession, the ant assemblage of a secondary forest significantly differed from that of an old-growth forest (Bihn et al. 2008). These differences in the time required for reassembly might suggest that the history of previous land use of sites to be rehabilitated plays a role in the recovery process (Bihn et al. 2010). Older reforestation sites harbored some species that were not present in the old-growth forest. However, in contrast to the findings of Nickele et al. (2023) according to which all secondary forests differed significantly in their ant community compositions from the primary forest, their community composition shared similarities with the old-growth forest. This suggests these assemblages might resemble the old-growth forest ant fauna when reaching full recovery.
Our results showed that the community of ground-dwelling ants in the oldest secondary forest is already at an advanced level of recovery, yet this process is still ongoing. Its composition still differs from that of old-growth forests. Our results are in line with studies performed in secondary forests at different successional stages that revealed variation in recovery times, but all more recent authors expect full recovery within less than 30 years (Hoenle et al. 2021; Rocha-Ortega and Favila 2013; Wilkie et al.2009).
The observed abundance of invasive ant species even in old-growth forests and adjacent sites raises an important issue that might also arise from similar reforestation projects elsewhere. In the case of our study, these ants are not expected to cause danger to other native fauna since they were all part of the natural Central American species pool. However, due to their competitive potential, conservation reforestation projects should closely observe whether invasive ants might generally benefit during the early stages of restoration. Promoting the recovery of near-natural ant communities is an important goal due to the important roles of these communities in tropical ecosystems. However, the dominance of ecosystems dominated by a few weedy ants with high invasion potential might well counteract this aim. Homogenous ant communities at young reforestation sites include only species with very low trophic levels and therefore lack higher trophic level ants, which play multiple important roles in the community. The succession of ant assemblages during reforestations is not accidentally arrested by the dominance of invasive species in the long run; these communities have the potential to recover fully and regain all the multiple functions that ants usually provide in tropical forests. Hence, monitoring invasive ants' prevalence might be a more rewarding target during similar reforestation campaigns.
Data availability
All raw data underlying this paper are available open access under this permanent URL: https://phaidra.univie.ac.at/o:1800158
References
Achury R, Suarez AV (2018) Richness and composition of ground-dwelling ants in tropical rainforest and surrounding landscapes in the Colombian inter-Andean valley. Neotrop Entomol 47:731–741. https://doi.org/10.1007/s13744-017-0565-4
Andersen AN (1995) A classification of Australian ant communities, based on functional groups which parallel plant life-forms in relation to stress and disturbance. J Biogeogr 22:15–29. https://doi.org/10.2307/2846070
Andersen AN (2000) A global ecology of rain forest ants: functional groups in relation to stress and disturbance. In: Agosti D, Majer JD, Alonso L, Shultz T (eds) Ants: standard methods for measuring and monitoring biodiversity. Smithsonian Institution Press, Washington DC, pp 25–34. https://doi.org/10.2307/2846070
Andersen AN, Majer JD (2004) Ants show the way down under: invertebrates as bioindicators in land management. Front Ecol Environ 2(6):291–298. https://doi.org/10.1890/1540-9295(2004)002[0292:ASTWDU]2.0.CO;2
Andersen AN, Sparling GP (1997) Ants as indicators of restoration success: relationship with soil microbial biomass in the Australian seasonal tropics. Restor Ecol 5(2):109–114. https://doi.org/10.1046/j.1526-100X.1997.09713.x
AntWeb. Version 8.83.5. California Academy of Science, online at https://www.antweb.org. Accessed 1 July 2022
AntWiki, online at https://www.antwiki.org. Accessed 30 Nov 2022
Baccaro FB, Rocha IF, del Aguila BE, Schietti J, Emilio T, Pinto JLPDV, Lima AP, Magnusson WE (2013) Changes in ground-dwelling ant functional diversity are correlated with water-table level in an Amazonian terra firme forest. Biotropica 45(6):755–763. https://doi.org/10.1111/btp.12055
Berenguer E, Ferreira J, Gardner TA, Aragão LEOC, De Camargo PB, Cerri CE, Barlow J (2014) A large-scale field assessment of carbon stocks in human-modified tropical forests. Global Change Biol 20(12):3713–3726. https://doi.org/10.1111/gcb.12627
Bihn JH, Verhaagh M, Brändle M, Brandl R (2008) Do secondary forests act as refuges for old growth forest animals? Recovery of ant diversity in the Atlantic forest of Brazil. Biol Conserv 141(3):733–743. https://doi.org/10.1016/j.biocon.2007.12.028
Bihn JH, Gebauer G, Brandl R (2010) Loss of functional diversity of ant assemblages in secondary tropical forests. Ecology 91(3):782–792. https://doi.org/10.1890/08-1276.1
Binz H, Schulze CH, Linsenmair KE (2015) Effects of topography on forest butterfly assemblages in the Pacific lowlands of Costa Rica. Ecotropica 20:1–14
Boet O, Arnan X, Retana J (2020) The role of environmental vs. biotic filtering in the structure of European ant communities: a matter of trait type and spatial scale. PLoS ONE 15(2):e0228625. https://doi.org/10.1371/journal.pone.0228625
Carval D, Cotté V, Resmond R, Perrin B, Tixier P (2016) Dominance in a ground-dwelling ant community of banana agroecosystem. Ecol Evol 6:8617–8631. https://doi.org/10.1002/ece3.2570
Cerdá X, Arnan X, Retana J (2013) Is competition a significant hallmark of ant (Hymenoptera: Formicidae) ecology. Myrmecol News 18(1):131–147
Chazdon RL (2014) Second growth. University of Chicago Press, Chicago
Crist TO (2009) Biodiversity, species interactions, and functional roles of ants (Hymenoptera: Formicidae) in fragmented landscapes: a review. Myrmecol News 12:3–13
Dunn RR (2004) Recovery of faunal communities during tropical forest regeneration. Conserv Biol 18(2):302–309. https://doi.org/10.1111/j.1523-1739.2004.00151.x
Falk M, Schulze CH, Fiedler K (2019) Ground-dwelling ant assemblages severely degrade in oil-palm plantations—a case study from the Golfo Dulce region, SW Costa Rica. Acta ZooBot Aust 156:115–133
Fayle TM, Klimeš P (2022) Improving estimates of global ant biomass and abundance. Proc Natl Acad Sci 119(42):e2214825119. https://doi.org/10.1073/pnas.2214825119
Gibb H, Stoklosa J, Warton DI, Brown AM, Andrew NR, Cunningham SA (2015) Does morphology predict trophic position and habitat use of ant species and assemblages? Oecologia 177:519–531. https://doi.org/10.1007/s00442-014-3101-9
Gotzek D, Axen HJ, Suarez AV, Helms Cahan S, Shoemaker D (2015) Global invasion history of the tropical fire ant: a stowaway on the first global trade routes. Mol Ecol 24(2):374–388. https://doi.org/10.1111/mec.13040
Griffiths HM, Ashton LA, Walker AE, Hasan F, Evans TA, Eggleton P, Parr CL (2018) Ants are the major agents of resource removal from tropical rainforests. J Anim Ecol 87(1):293–300. https://doi.org/10.1111/1365-2656.12728
Grimbacher PS, Hughes L (2002) Response of ant communities and ant-seed interactions to bush regeneration. Ecol Manag Restor 3(3):188–199. https://doi.org/10.1046/j.1442-8903.2002.00112.x
Groc S, Delabie JH, Fernandez F, Leponce M, Orivel J, Silvestre R, Dejean A (2014) Leaf-litter ant communities (Hymenoptera: Formicidae) in a pristine Guianese rainforest: stable functional structure versus high species turnover. Myrmecol News 19:43–51
Hernandez CE, Witter SG (1996) Evaluating and managing the environmental impact of banana production in Costa Rica: a systems approach. Ambio 25(3):171–178
Hethcoat MG, King BJ, Castiblanco FF, Ortiz-Sepúlveda CM, Achiardi FCP, Edwards FA, Medina C, Gilroy JJ, Haugaasen T, Edwards DP (2019) The impact of secondary forest regeneration on ground-dwelling ant communities in the Tropical Andes. Oecologia 191:475–482. https://doi.org/10.1007/s00442-019-04497-8
Hietz P, Kleinschmidt S, Mala B, West Z, Schwarzfurtner K (2019) Biomass accumulation and carbon sequestration in a reforestation project in La Gamba, Costa Rica. Acta ZooBot Aust 156:61–77
Höbinger T, Schindler S, Seaman BS, Wrbka T, Weissenhofer A (2012) Impact of oil palm plantations on the structure of the agroforestry mosaic of La Gamba, southern Costa Rica: potential implications for biodiversity. Agrofor Syst 85:367–381. https://doi.org/10.1007/s10457-011-9425-0
Hoenle PO, Donoso DA, Argoti A, Staab M, Von Beeren C, Blüthgen N (2022) Rapid ant community reassembly in a neotropical forest: recovery dynamics and land-use legacy. Ecol Appl 32(4):e2559. https://doi.org/10.1002/eap.2559
Hoenle PO, Staab M, Donoso DA, Argoti A, Blüthgen N (2023) Stratification and recovery time jointly shape ant functional reassembly in a neotropical forest. J Anim Ecol 92(7):1372–1387. https://doi.org/10.1111/1365-2656.13896
Holland MB (2012) Mesoamerican biological corridor. In: Hilty JA, Chester CC, Cross MS (eds) Climate and conservation. Island Press/Center for Resource Economics, London, pp 56–66. https://doi.org/10.5822/978-1-61091-203-7_5
Hölldobler B, Wilson EO (1990) The ants. Belknap Press, Cambridge
Hsieh TC, Ma KH, Chao A (2022) iNEXT: interpolation and extrapolation for species diversity. R package version 3.0.0. http://chao.stat.nthu.edu.tw/wordpress/software_download/
Jackson GP, Fox BJ (1996) Comparison of regeneration following burning, clearing or mineral sand mining at Tomago, NSW: II. Succession of ant assemblages in a coastal forest. Aust J Ecol 21(2):200–216. https://doi.org/10.1111/j.1442-9993.1996.tb00600.x
Kattan GH, Correa D, Escobar F, Medina C (2006) Leaf-litter arthropods in restored forests in the Colombian Andes: a comparison between secondary forest and tree plantations. Restor Ecol 14(1):95–102. https://doi.org/10.1111/j.1526-100X.2006.00109.x
Lach L, Parr C, Abbott K (2010) Ant ecology. Oxford University Press, Oxford
Law SJ, Parr C (2020) Numerically dominant species drive patterns in resource use along a vertical gradient in tropical ant assemblages. Biotropica 52(1):101–112. https://doi.org/10.1111/btp.12743
Leal IR, Filgueiras BKC, Gomes JP, Iannuzzi L, Andersen AN (2012) Effects of habitat fragmentation on ant richness and functional composition in Brazilian Atlantic forest. Biodivers Conserv 21:1687–1701. https://doi.org/10.1007/s10531-012-0271-9
Longino JT (2022) Costa Rica project, online https://ants.biology.utah.edu. Last accessed 30 October 2022
Majer JD, Heterick B, Gohr T, Hughes E, Mounsher L, Grigg A (2013) Is thirty-seven years sufficient for full return of the ant biota following restoration? Ecol Process 2:1–12. https://doi.org/10.1186/2192-1709-2-19
Narváez-Vásquez A, Gaviria J, Vergara-Navarro EV, Rivera-Pedroza L, Löhr B (2021) Ant (Hymenoptera: Formicidae) species diversity in secondary forest and three agricultural land uses of the Colombian Pacific Coast. Rev Chilena Entomol 47(3):1
Newbold T, Hudson LN, Hill SL, Contu S, Lysenko I, Senior RA, Börger L, Bennett DJ, Choimes A, Collen B, Day J, De Palma A, Díaz S, Echeverria-Londoño S, Edgar MJ, Feldman A, Garon M, Harrison ML, Alhusseini T, Ingram DJ, Itescu Y, Kattge J, Kemp V, Kirkpatrick L, Kleyer M, Correia DL, Martin CD, Meiri S, Novosolov M, Pan Y, Phillips HR, Purves DW, Robinson A, Simpson J, Tuck SL, Weiher E, White HJ, Ewers RM, Mace GM, Scharlemann JP, Purvis A (2015) Global effects of land use on local terrestrial biodiversity. Nature 520(7545):45–50. https://doi.org/10.1038/nature14324
Nickele MA, Reis Filho W, Penteado SDRC, de Queiroz EC, da Silva LCR, Camargo TS, Pie MR (2023) Assessing forest restoration effectiveness in the Seasonal Semideciduous Forest in the Upper Paraná Atlantic Forest ecoregion using epigaeic ant assemblages. J Trop Ecol 39:e37. https://doi.org/10.1017/S0266467423000275
Oksanen J, Kindt R, Legendre P, O'Hara B, Simpson GL, Solymos P, Stevens MHH, Wagner H (2020) Vegan: community ecology package (R package version 2.5-7)
Piper SD, Catterall CP, Kanowski JJ, Proctor HC (2009) Biodiversity recovery during rainforest reforestation as indicated by rapid assessment of epigaeic ants in tropical and subtropical Australia. Aust Ecol 34(4):422–434. https://doi.org/10.1111/j.1442-9993.2009.01943.x
Rabello AM, Parr CL, Queiroz AC, Braga DL, Santiago GS, Ribas CR (2021) Taxonomic and functional approaches reveal different responses of ant assemblages to land-use changes. Basic Appl Ecol 54:39–49. https://doi.org/10.1016/j.baae.2021.04.001
Rocha-Ortega M, Favila ME (2013) The recovery of ground ant diversity in secondary Lacandon tropical forests. J Insect Conserv 17:1161–1167. https://doi.org/10.1007/s10841-013-9597-1
Sanders NJ, Lessard JP, Fitzpatrick MC, Dunn RR (2007) Temperature, but not productivity or geometry, predicts elevational diversity gradients in ants across spatial grains. Global Ecol Biogeogr 16(5):640–649. https://doi.org/10.1111/j.1466-8238.2007.00316.x
Schulze CH, Leidinger P, Paces B, Páez AFR (2019) The importance of reforested and naturally regenerating young forest patches as secondary habitats for forest birds in the Biological Corridor La Gamba, Costa Rica. Acta ZooBot Aust 156:79–98
Segat JC, Vasconcellos RLF, Silva DP, Baretta D, Cardoso EJBN (2017) Ants as indicators of soil quality in an on-going recovery of riparian forests. For Ecol Manag 404:338–343. https://doi.org/10.1016/j.foreco.2017.07.038
Sendoya SF, Silva PS, Farji-Brener AG (2014) Does inundation risk affect leaf-cutting ant distribution? A study along a topographic gradient of a Costa Rican tropical wet forest. J Trop Ecol 30(1):89–92. https://doi.org/10.1017/S026646741300076X
Silva RR, Feitosa RSM, Eberhardt F (2007) Reduced ant diversity along a habitat regeneration gradient in the southern Brazilian Atlantic Forest. For Ecol Manag 240(1–3):61–69. https://doi.org/10.1016/j.foreco.2006.12.002
Singh D, Slik JWF, Jeon YS, Tomlinson KW, Yang X, Wang J, Kerfahi D, Porazinska DL, Adams JM (2019) Tropical forest conversion to rubber plantation affects soil micro- and mesofaunal community and diversity. Sci Rep 9:5893. https://doi.org/10.1038/s41598-019-42333-4
Solar RR, De C, Barlow J, Andersen AN, Schoereder JH, Berenguer E, Ferreira JN, Gardner TA (2016) Biodiversity consequences of land-use change and forest disturbance in the Amazon: a multi-scale assessment using ant communities. Biol Conserv 197:98–107. https://doi.org/10.1016/j.biocon.2016.03.005
Ward PS (1985) The Nearctic species of the genus Pseudomyrmex (Hymenoptera: Formicidae). Quaest Entomol 21:209–246
Ward PS (1989) Systematic studies on Pseudomyrmecine ants: revision of the Pseudomyrmex oculatus and P. subtilissimus species groups with taxonomic comments on other species. Quaest Entomol 25:393–468
Weissenhofer A, Picado Zuñiga A, Barrrantes Ramírez W, Acevedo Mairena H, Huber W (2019) Forest conservation and restoration in southwestern Costa Rica: the biological corridors COBIGA and AMISTOSA. Acta ZooBot Aust 156:47–60
Weissenhofer A, Picado-Zuñiga A, Ramírez WB (2019) Forest conservation and restoration in southwestern Costa Rica: the biological corridors COBIGA and AMISTOSA. Acta ZooBot Aust 156:47–60
Wetterer JK (2010) Worldwide spread of the flower ant, Monomorium floricola (Hymenoptera: Formicidae). Myrmecol News 13:19–27
Wetterer JK (2010) Worldwide spread of the pharaoh ant, Monomorium pharaonis (Hymenoptera: Formicidae). Myrmecol News 13:115–129
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York. https://ggplot2.tidyverse.org
Wilkie KTR, Mertl AL, Traniello JF (2009) Diversity of ground-dwelling ants (Hymenoptera: Formicidae) in primary and secondary forests in Amazonian Ecuador. Myrmecol News 12:139–147
Williams JL, Lucky A (2020) Nonnative and invasive Nylanderia crazy ants (Hymenoptera: Formicidae) of the world: integrating genomics to enhance taxonomic preparedness. Ann Entomol Soc Am 113(4):318–336. https://doi.org/10.1093/aesa/saz039
Wilson EO (2003) Pheidole in the New World: a dominant, hyperdiverse ant genus, vol 1. Harvard University Press, New York
Acknowledgements
Dr. Brigitte Gottsberger for performing DNA barcoding of the unidentified species and the La Gamba Research Station staff for all the help on the spot. We thank the National System of Conservation Areas (SINAC—Sistema Nacional de Áreas de Conservación) for Granting Permit No. SINAC-ACOSA-D-PI-R-030-2022. to conduct this research.
Funding
Open access funding provided by University of Vienna. The project was partially financed through a KWA scholarship to MK from the University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Appendix
Appendix
See Table 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karolak, M., Fiedler, K. Reassembly of ground-dwelling ant communities in reforestation plots in SW Costa Rica. Insect. Soc. (2024). https://doi.org/10.1007/s00040-024-00975-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00040-024-00975-2