Abstract
Social insects live in closely related family groups but face risks of intrusion and infection by pathogenic and parasitic microbes. To cope with the microbes invading their nests and feeding sites, social insects produce various types of antimicrobial substances. Subterranean termites occupy microbe-rich decaying wood and soil at high density, expanding their nest area by exploring and feeding on wood outward from the royal chamber (room for kings and queens). Although antimicrobial agents have been identified in many termite species, few studies have investigated those used by foraging workers in decaying wood under development, which is richer in microbes than the well-sterilized royal chamber and its surroundings. Here, we report that phenylacetic acid, an antifungal aromatic compound, is secreted by foraging workers of the Japanese subterranean termite Reticulitermes speratus. The compound was detected by gas chromatography–mass spectrometry analysis of ethyl acetate extracts of shelter papers infested with the workers, and antimicrobial tests demonstrated that it inhibits the germination and/or mycelial growth of the entomopathogenic fungi (Metarhizium anisopliae and Beauveria bassiana) and the termite egg-mimicking fungus Athelia termitophila. Our study provides new insights into the antimicrobial defense mechanisms of termites, including by combining different types of antimicrobial substances secreted by different castes, and thus the survival strategy of entomopathogenic and parasitic fungi in termite nests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The remarkable diversity and ecological success of social insects have been attributed to their ability to cope with infectious microbes invading their nests and feeding sites (Traniello et al. 2002; Cremer et al. 2018). Because social insects live in dense clusters with closely related family members susceptible to infections by the same pathogen (Cremer et al. 2007), infectious diseases can easily spread between group members, in contrast to solitary insects (Meunier 2015). Because larger social groups tend to have higher disease burdens, social insects have evolved prophylaxis and control measures to maintain hygiene in response to the potentially intense selection pressures posed by parasites and pathogens (Anderson and May 1979; Fefferman et al. 2007; Cremer and Sixt 2008).
Termites are social insects that form colonies of hundreds of thousands of close relatives, maintaining dense nests in microbe-rich wood and soil (Tsunoda et al. 1999). Maintaining a protected environment in their nests, by preventing pathogen invasion and growth, is thus a critical task of termites (Cremer et al. 2007). While physical removal of pathogens such as allogrooming is a basic hygiene behavior (Eggleton 2011), termites have developed the ability to produce and secrete a variety of antimicrobial substances. In the evolutionary transition in termites from one-piece nesting life type (the nest itself provides food for termites) to multiple-site nesting life type (nests of a single colony are interconnected by belowground tunnels and aboveground shelter tubes) (Abe 1987; Shellman-Reeve 1997), termite workers have experienced increased selective pressure for immune substances such as gram-negative-binding proteins (GNBPs), as they forage pathogen-rich environments outside of the nest. Such selective pressure could have enhanced social mechanisms of defense that afforded effective front-line external protection (Korb et al. 2015; Bulmer and Stefano 2022). Besides allogrooming and secretion of antimicrobial proteins, various antimicrobial organic compounds are used as protective substances: for example, α-pinene and limonene from Nasutitermes species (Zhao et al. 2004), trinervitane and n-hexanoic acid from Zootermopsis species (Rosengaus et al. 2004), and naphthalene, an antiseptic and an anthelmintic (Middleditch et al. 1981) found in the inner wall of the nest of Coptotermes formosanus Shiraki (Chen et al. 1998). Antifungal and antibacterial proteins (lysozymes, termicin, spingerin, and Gram-negative-binding proteins) have also been identified in many termite species (Bulmer and Crozier 2006; Terrapon et al. 2014; Mitaka et al. 2017a). However, as none of these substances is effective against all pathogenic microorganisms, termites must have multiple antimicrobial substances with different antimicrobial spectra to combat the various types of pathogenic microorganisms in the environment.
Reticulitermes termites including Reticulitermes speratus are classified as multiple-site nesting termites (Yanagihara et al. 2018). In a colony of R. speratus, a royal chamber, i.e., a room of kings and queens, is located deep in one of the nest trees (Yanagihara et al. 2018) and this chamber is kept at low risk of pathogen infection due to the antifungal protection provided by the antifungal volatile queen pheromone (mixture of n-butyl-n-butyrate and 2-mehtyl-1-butanol) (Matsuura and Matsunaga 2015) and antibacterial egg recognition pheromone (lysozyme) (Matsuura et al. 2007). On the other hand, the foraging area, i.e., the area away from the royal chamber and where soldiers protect nest entrance (Yanagihara et al. 2018) and workers often forage for new wood food (Crosland et al. 1997), are considered to be microbe-rich environment compared with the sanitized royal chamber. Therefore, workers and soldiers in the foraging area are at higher risk of disease transmission than those around the royal chamber. Previous studies revealed that soldier pheromone (β-elemene) secreted from soldiers suppress the growth of entomopathogenic fungi, Metarhizium anisopliae and Beauveria bassiana (Mitaka et al. 2017b), and mellein contained in bodies of workers and soldiers in the foraging area also suppress the growth of these fungi (Mitaka et al. 2019). The antifungal activity of these compounds is not so strong and only retards mycelial growth of entomopathogenic fungi. Nevertheless, foraging areas where workers come and go are usually clean and few areas of microbial growth are found (Cremer et al. 2007). This suggests that some antimicrobial substance may be present not only in the body of individual termite but also in the inside of the nest.
In addition to pathogenic microorganisms, termite-egg-mimicking fungus ‘termite ball’ are also parasitic in egg piles in the nests of Reticulitermes termites (Yashiro and Matsuura 2007). Termite balls are the sclerotia of Athelia termitophila (Maekawa et al. 2020). They mimic termite eggs both morphologically (Matsuura 2005) and chemically (Matsuura et al. 2009) and thereby be protected from desiccation and other microorganisms by egg-caring behavior of workers. Once the care of workers becomes absent, termite balls start to germinate (Matsuura et al. 2000; Komagata et al. 2022) and can consume surrounding eggs (Matsuura and Matsunaga 2015). Since the interaction is parasitic, in that it is beneficial for the fungus but costly for the host termites, the germination and growth of termite balls are usually inhibited throughout the nests in the filed colonies (Matsuura 2006). The volatile queen pheromone, which is emitted by queens and eggs (Matsuura et al. 2010), is known to be able to inhibit germination and mycelial growth of termite balls (Matsuura and Matsunaga 2015). However, the queen pheromone should be absent or in very low concentration in foraging area far from the royal chamber, and β-elemene and mellein emitted by workers and soldiers have no inhibitory effect against termite balls (Mitaka et al. 2017b, 2019). Therefore, it is expected that compounds other than the queen pheromone inhibit growth of entomopathogenic microorganisms and termite balls in the interior surface of foraging areas.
In this study, ethyl acetate extracts of secretions on shelter papers infested with R. speratus workers were analyzed by gas chromatography–mass spectrometry (GC–MS), which led to the identification of phenylacetic acid. Tests of its activity against the growth of the egg-parasitic fungus A. termitophila and the entomopathogenic fungi M. anisopliae and B. bassiana were conducted. The antibacterial activities of this compound against several species of entomopathogenic (Bacillus subtilis, Bacillus thuringiensis, and Serratia marcescens) and opportunistic (Micrococcus luteus, and Pseudomonas aeruginosa) bacteria were examined as well, based on previous laboratory bioassays demonstrating the pathogenicity of these entomopathogen to termites (Shimizu and Yamaji 2002; Singha et al. 2010; Omoya and Kelly 2014).
Materials and methods
Termite collection
Five R. speratus colonies were collected from secondary forests in Japan as follows: colony TI003 was collected from Ryuo-cho, Shiga Prefecture, in April 2018; colony 190124C was collected from Amarube-cho, Kameoka, Kyoto Prefecture, in January 2019; colony 190409B was collected from Shimoyada-cho, Kameoka, Kyoto Prefecture, in April 2019; and colonies 200622A and 200622B were collected from Ohmiya-Shakadani, Kita-ku, Kyoto, Kyoto Prefecture, in June 2020.
Ethics
No specific permits were required for the described field activities. Specific permission was also not required to access or sample the termite colonies, as they were collected from unprotected public lands. This study did not involve endangered or protected species. However, in Japan, phenylacetic acid is classified as a raw material for stimulant production; we obtained the necessary designation certificate by the governor of Kyoto Prefecture to allow its purchase from FUJIFILM Wako Pure Chemical Corp., Osaka, Japan.
Preparation of shelter papers
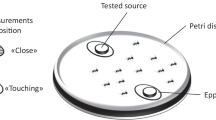
Substances secreted by termite workers into the inner walls of their nest were trapped using shelter papers (Fig. 1A). Two hundred workers from a colony were placed in a 35 mm dish lined with a filter paper (30 mm diameter, 0.26 mm thickness; Advantec No. 2; Toyo Roshi Kaisha, Ltd, Tokyo, Japan), with 20 dishes prepared from each of the five colonies (TI003, 190124C, 190409B, 200622A, and 200622B). The papers were moistened with 150 µL distilled water and the dishes were incubated at 25 °C. Shelter papers were collected from each dish every 24 h for 7 days and preserved in a freezer at −20°C for use in the analyses.
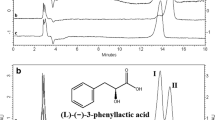
Chemical profile of the shelter papers infested by workers of R. speratus. A Schematic drawing of the rearing dish used to prepare the shelter papers. B Representative gas chromatography result of the extract prepared from termite shelter papers (colony 200622B). Numbers associated with arrows indicate different compounds: 1-(2-butoxyethoxy)-ethanol (5), phenylacetic acid (6), 2-(2-butoxyethoxy)acetic acid (8), palmitic acid (18), n-tricosane (30), 2-methyltetracosane (36), and n-pentacosane (38). Peaks without arrows mean colony-specific compounds. C Mass spectrum and structure of phenylacetic acid are shown
To extract the chemical compounds (including a variety of molecules ranging from non-polar to highly polar molecules) released by the termites, the shelter papers (n = 200) from each colony were placed in 200 mL glass jars and extracted with 200 mL ethyl acetate (≥ 99.5% purity, FUJIFILM Wako Pure Chemical Corp.) for 24 h. As a control, unused filter papers (n = 200) were also extracted under the same condition. The resulting extracts were concentrated to 1 mL using a rotary evaporator and then subjected to GC–MS analyses for chemical identification.
GC–MS analysis
GC–MS analyses were performed on a JMS-Q1500GC mass spectrometer (JEOL Ltd., Tokyo, Japan) combined with an Agilent Technologies 7890B GC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a DB-1MS column (30 m × 250 μm × 0.25 μm, Agilent Technologies). The column temperature was held at 50 °C for 5 min, then increased from 50 to 300 °C at 20 °C/min, and held for 5 min. Then, one µL sample was manually injected using an injector in splitless mode, helium as the carrier gas (1 mL/min), and an injection port temperature maintained at 250 °C. MS data were obtained under the following conditions: 50 μA ionization current, 70 eV ionization energy, 2 kV accelerating voltage, a 40–500 m/z scan range, and 2,300 amu/s scan rate. Both GC and MS systems were controlled using an msPrimo system controller ver. 1.06 (JEOL Ltd.). The data were analyzed using the software Escrime ver. 2.04 (JEOL Ltd., https://www.jeol.co.jp/en/products/detail/JMS-Q1500GC.html). Candidate compounds were predicted based on the Kovats index information from the NIST Chemistry WebBook, Standard Reference Database Number 69 (https://doi.org/https://doi.org/10.18434/T4D303) and the mass spectrum similarities from the EI MS library of the NIST/EPA/NIH Mass Spectral Library 2011 (NIST11). Phenylacetic acid was identified according to its Kovats index and mass spectrum, based on a comparison with the commercial standard. Kovats indices of candidate compounds was calculated using the retention times of phenylacetic acid standard (98% purity, FUJIFILM Wako Pure Chemical Corp.) and a mixture of C9–C33 n-alkanes’ standards. The concentration of phenylacetic acid in each extract was calculated by comparison with an external standard curve (regression R2 = 0.94). Since the samples used for GC–MS analysis were adjusted for concentration to be 0.2 filter paper equivalents/μL, the amount of phenylacetic acid per filter paper was calculated based on the calculated concentration of it in each extract. Finally, we calculated the phenylacetic acid secreted by one worker over a 24-h period, taking into account that the phenylacetic acid contained in the filter paper was secreted by 200 workers over a 24-h period.
Antifungal tests
The inhibitory effect of phenylacetic acid on mycelial growth of A. termitophila, M. anisopliae, and B. bassiana was tested. The strain of A. termitophila used in this bioassay was isolated from the nest of R. speratus as described in a previous study (Mitaka et al. 2019). M. anisopliae (NBRC31961) and B. bassiana (NBRC103721) strains were provided by the Biological Resource Center (NBRC, National Institute of Technology and Evaluation, Tokyo, Japan) and cultured on potato-dextrose agar (PDA) plates at 28 °C. The assay was performed by placing a 5 mm diameter plug of growing mycelia from each fungal culture in the center a Petri dish (90 × 15 mm) containing phenylacetic acid at concentrations of 0, 50, 500, and 5,000 ng/μL (Fig. 2A). The Petri dishes were wrapped with two layers of Parafilm and incubated at 28 °C for 14 days. Five replicates were prepared for each treatment of each fungus. The size of the mycelia was measured by taking vertical photographs of each dish every 7 days after inoculation using a digital camera (tg-6, Olympus, Japan). The colony area in cm2 was determined by counting the total number of pixels in the fungal colony area using ImageJ software (US National Institutes of Health, Bethesda, MD, USA) (Schneider et al. 2012).
Inhibitory effect of phenylacetic acid against the mycelial growth of fungi. A Experimental set-up. Phenylacetic acid was added to the PDA medium (90 mm diameter, 5 mm height) to achieve a final concentration of 0, 50, 500, and 5,000 ng/μL of phenylacetic acid. B The results of antifungal tests. Values denote the mean ± standard error of mean of five replications. Different letters indicate significant differences (ANOVA followed by Tukey’s HSD test, P < 0.05). Phenylacetic acid inhibited A. termitophilas, B. bassiana, and M. anisopliae at a concentration ≥ 500 ng/μL, 5,000 ng/μL, and 50 ng/μL, respectively
The inhibitory effect of phenylacetic acid on the spore germination rate of entomopathogenic fungi (M. anisopliae and B. bassiana) was also tested by modifying a previously conducted method (Hywel-Jones and Gillespie 1990). We did not assess A. termitophila, because this species does not produce amorphous spores under culture conditions (Maekawa et al. 2020). Spores of fungi plates were washed by pipetting 10 mL of 0.05% Tween 80 onto the plate and scraping off the spore with a spatula. The spore suspension was transferred to a 15-mL Eppendorf tube, shaken, and filtered through sterilized gauze to remove aggregates. The spore concentration was determined with a haemocytometer (Thoma chamber; Erma, Saitama, Japan) and diluted to a final concentration of 1–2 × 107 spores/mL. The 100 μL spore suspension was spread on to each of five replicate PDA agar plates (90 × 15 mm) including phenylacetic acid at concentrations of 0, 50, 500, or 5,000 ng/μL. After seeding, the plates were incubated at 28 °C for 24 h, and 300 spores on the plates were randomly observed with the inverted microscope at × 200 magnification (DM IL LED, Leica, Tokyo, Japan). Spores whose germ tubes were more than half the length of the spore diameter were classified as germinated. Germination rates were calculated as follows: the number of germinated spores / the number of total spores observed (= 300).
Antibacterial tests
The antibacterial activity of phenylacetic acid against B. subtilis, B. thuringiensis, M. luteus, P. aeruginosa, and S. marcescens was tested as described previously (Zhu et al. 2011). Strains of B. subtilis (NBRC3009), B. thuringiensis (NBRC13865), M. luteus (NBRC16250), and P. aeruginosa (NBRC3080) were provided by the NBRC. The S. marcescens strain used in the bioassay was isolated from the nest of R. speratus following a procedure developed in a previous study (Inagaki and Matsuura 2018) and cultured on lysogeny broth (LB, Nakarai Tesque, Kyoto, Japan) agar plates at 30 °C.
The agar diffusion assay was performed by spreading 100 µL bacterial inoculum (1–2 × 108 CFUs/mL) on an LB agar plate. Then, 40 µL aqueous phenylacetic acid at concentrations of 0, 50, 500, and 5,000 ng/μL (equivalent to 0, 2,000, 20,000, and 200,000 ng of phenylacetic acid) was added to a 7 mm (diameter) well punched in the center of the plate. As the negative control, distilled water was added to the well. Ten replicates were prepared for each treatment of each bacterium. The plates were wrapped with two layers of Parafilm and incubated at 30 °C for 24 h. After incubation, the major and minor axes of the inhibition zone were measured using a digital caliper, and the means of two measurements were used to calculate the apparent area of the inhibition zone. Because this area included the area of the well, the practical area of the inhibition zone was calculated by subtracting the area of the well from the apparent area.
Statistical analysis
All statistical analyses were performed using R software v. 4.2.2 (R Core Team 2016). Fungal colony area (the size of mycelia) was compared using an analysis of variance (ANOVA) followed by Tukey’s HSD test (P < 0.05). Spore germination rate was compared using a generalized linear mixed model (GLMM). The germination rate was regarded as the response variable, assuming a binomial distribution. Concentrations were used as an explanatory variable. Replications were used as a random effect. Likelihood ratio tests (LRTs) were conducted to test the significance of each explanatory variable, and Tukey’s HSD tests were conducted to test for differences between levels of a significant fixed effect. In the antibacterial tests, the area of the inhibition zone was compared using an ANOVA followed by Tukey’s HSD test (P < 0.05).
Results
Chemical profile of termite shelter papers
We detected a total of 60 compounds (1 − 60) from the extracts of filter papers (including both shelter papers and unused filter papers) by GC–MS. Among these, only seven compounds were consistently detected in the extracts of the shelter papers derived from the five R. speratus colonies (Fig. 1B and Table 1): 1-(2-butoxyethoxy)-ethanol (5), phenylacetic acid (6), 2-(2-butoxyethoxy) acetic acid (8), palmitic acid (18), n-tricosane (30), 2-methyltetracosane (36), and n-pentacosane (38). 2-(2-butoxyethoxy) acetic acid was also detected in the extract of unused filter papers (Table 1). Therefore, a total of six compounds (5, 6, 18, 30, 36, and 38) was considered to be derived from termites. 1-(2-butoxyethoxy)-ethanol (5) is commonly used as solvents or preservatives in siloxane (used as stationary phase in GC columns) production (Scherer et al. 2019), suggesting that this compound was contaminant. Palmitic acid (18) is used for an aggregation pheromone in R. speratus (Mitaka et al. 2020), and n-tricosane (30), 2-methyltetracosane (36), and n-pentacosane (38) are cuticular hydrocarbon components in R. speratus (Takematsu and Yamaoka 1999; Mitaka et al. 2020). Therefore, it is unlikely that these five compounds function as antimicrobial compound. However, only phenylacetic acid is reported to be an antimicrobial compound able to inhibit the growth of fungi and bacteria (Kim et al. 2008; Zhu et al. 2011; Fernández-Marín et al. 2015). We, therefore, focused on this compound. The amount of phenylacetic acid extracted per shelter paper was 1991.569 ± 1231.247 ng (mean ± SEM). Because 200 workers were used in this procedure, we estimated that the amount of phenylacetic acid secreted in 24 h was 9.958 ng/worker.
Estimation of the phenylacetic acid concentration in the foraging area
Field colonies of R. speratus contain a median and maximum of 24,500 and 451,800 workers, respectively (Takata et al. 2023). In the subterranean termite Coptotermes formosanus, 20% of the workers are estimated to be foragers, and the foraging worker rate is maintained regardless of changes in total colony size and demographic composition (Lee et al. 2022). Assuming the same proportion for R. speratus, the median and maximum numbers of foraging workers in field colonies of R. speratus are 4,900 and 90,360, respectively. If all foraging workers pass through an area of the same size as the shelter paper (706.858 mm2 [≈ 15 mm × 15 mm × π]) where they secrete phenylacetic acid for 24 h, the median and maximum concentrations of phenylacetic acid soaked into the spot would be 48,794.200 ng (= 9.958 ng per worker × 4,900 workers) and 899,804.900 ng (= 9.958 ng per worker × 90,360 workers), respectively.
Antifungal activity of phenylacetic acid
To test the inhibitory effects of phenylacetic acid on fungal mycelial growth, colonies of entomopathogenic fungi (M. anisopliae and B. bassiana) and the egg-mimicking termite ball fungus (A. termitophila) were exposed to authentic phenylacetic acid at different concentrations (Fig. 2A). Inhibitory effects against M. anisopliae were observed at 50–5,000 ng/μL (ANOVA, df = 3, F3,16 = 160.2, P < 0.001, Tukey’s HSD test, P < 0.05; Fig. 2B), against A. termitophila at 500–5,000 ng/μL (ANOVA, df = 3, F3,16 = 162.6, P < 0.001, Tukey’s HSD test, P < 0.05; Fig. 2B), and against B. bassiana at only 5,000 ng/μL (ANOVA, df = 3, F3,16 = 113.2, P < 0.001, Tukey’s HSD test, P < 0.05; Fig. 2B).
The inhibitory effect of phenylacetic acid on the spore germination of entomopathogenic fungi (M. anisopliae and B. bassiana) was also tested by exposing the spores to the compound at different concentrations. The inhibitory effect was observed at 50–5,000 ng/μL in both M. anisopliae (LRT, df = 3, χ2 = 7179.3, P < 0.001, Tukey’s HSD test, P < 0.05; Fig. 3) and B. bassiana (LRT, df = 3, χ2 = 4970.1, P < 0.001, Tukey’s HSD test, P < 0.05; Fig. 3).
Inhibitory effect of phenylacetic acid against the spore germination of entomopathogenic fungi B. bassiana and M. anisopliae. Phenylacetic acid was added to the PDA medium (90 mm diameter, 5 mm height) to achieve a final concentration of 0, 50, 500, and 5,000 ng/μL of phenylacetic acid. Values denote the mean ± standard error of mean of five replications. Different letters indicate significant differences (GLMM followed by Tukey’s HSD test, P < 0.05). Phenylacetic acid inhibited both B. bassiana and M. anisoplicae at a concentration ≥ 50 ng/μL
Antibacterial activity
To determine whether phenylacetic acid inhibits bacterial growth, colonies of entomopathogenic bacteria (B. subtilis, B. thuringiensis and S. marcescens), a typical Gram-positive bacterium (M. luteus), and a typical Gram-negative bacterium (P. aeruginosa) were exposed to authentic phenylacetic acid at different concentrations. The compound inhibited the growth of all bacteria only at the highest tested concentration of 5,000 ng/μL (ANOVA, df = 3, F3,36 = 84.30 [B. subtilis], 128.60 [B. thuringiensis], 110.50 [M. luteus], 204.30 [P. aeruginosa], or 192.00 [S. marcescens], P < 0.001, Tukey’s HSD test, P < 0.05; Fig. S1).
Discussion
In R. speratus colonies, foraging areas are a more microbe-rich environment than the royal chamber, such that foraging workers were predicted to secrete substances that inhibit microbial growth. Our GC–MS analysis revealed that foraging workers of R. speratus secrete phenylacetic acid (Fig. 1), initially identified as a plant-growth-promoting substance in the 1930s but later shown to possess substantial antimicrobial activity in bacteria, fungi, algae, land plants, and insects (Fernández-Marín et al. 2015; Cook 2019). However, its antimicrobial effects against the entomopathogenic microorganisms of termites had not been investigated. The amount of phenylacetic acid secreted by R. speratus (9.958 ng per worker per 24 h according to the quantification done after GC–MS measurement) is sufficient to suppress the mycelial growth of M. anisopliae and A. termitophila in their colonies. Assuming that all foraging workers in the field continually secrete it over a foraging area equivalent to the area of one shelter paper (706.858 mm2), then after 24 h, the cumulative median and maximum amounts of phenylacetic acid will be 48,794 ng and 899,805 ng, respectively. In PDA media with phenylacetic acid in our antifungal tests (Fig. 2A), the amount of this compound in an area equivalent to one shelter paper (assuming a thickness of 1 mm, that is, a volume of 706.858 mm3 = 706.858 μL) is 35,343 ng for the 50 ng/μL treatment, 353,429 ng for the 500 ng/μL treatment, and 3,534,290 ng for the 5,000 ng/μL treatment, respectively. The results of mycelial growth tests demonstrated that phenylacetic acid inhibited mycelial growth of M. anisopliae at the 50–5,000 ng/μL treatments, A. termitophila at the 500–5,000 ng/μL treatments, and B. bassiana at the 5,000 ng/μL treatment in antifungal tests (Fig. 2B). This means that field termites would suppress the mycelial growth of M. anisopliae if the number of foraging workers were above the median, and the growth of both M. anisopliae and A. termitophila if the number of foraging workers reaches the maximum. The mycelial growth of B. bassiana would not be suppressed even at the maximum number of foraging workers. However, phenylacetic acid inhibited not only the spore germination of M. anisopliae but also that of B. bassiana at ≥ 50 ng/μL, especially at ≥ 500 ng/μL (Fig. 3), suggesting that termites would suppress the spore germination of M. anisopliae and B. bassiana if the number of foraging workers reached the medium, and strongly suppress if the foraging workers reached the maximum.
Since the foraging worker rate is usually kept constant in termite colonies (Lee et al. 2022), it is unlikely that the number of foraging workers in a large colony changes throughout the year unless there is an accident that causes a drastic decrease in colony size, such as the loss of many workers at once. Therefore, it is considered that the foraging areas in a large colony always accumulate sufficient phenylacetic acid to suppress the spore germination and mycelial growth of M. anisopliae and B. bassiana and suppress the mycelial growth of A. termitophila. On the other hand, in the antibacterial tests, phenylacetic acid resulted in inhibition zones for all tested strains at the 5,000 ng/μL treatment, although the size of the zone was very small (≤ 180 mm2, Fig. S1). Even with the maximum number of foraging workers secreting the compound in the field, bacterial growth would barely be suppressed. These results suggest that phenylacetic acid functions as an effective antimicrobial substance only in the developing range of the foraging area with high worker traffic, not in the undeveloped area with few workers.
R. speratus releases a variety of antimicrobial substances with different effects on microbial growth. Mellein, secreted by foraging workers and soldiers, inhibits the mycelial growth of B. bassiana but not that of A. termitophila (Mitaka et al. 2019). Several termite pheromones also have antimicrobial effects. For example, lysozyme, a component of termite egg recognition pheromone, has broad antibacterial activity against Gram-positive bacteria including Bacillus species (Matsuura et al. 2007), while queen pheromone, a mixture of n-butyl-n-butylate and 2-methyl-1-butanol, inhibits the germination and mycelial growth of M. anisopliae, B. bassiana, Isaria farinose, Sclerotium tuliparum, Athelia rolfsii, and A. termitophila (Matsuura and Matsunaga 2015). However, queen pheromone is secreted only by queens and eggs (Matsuura 2012). The internal structure of Reticulitermes termite nests is multi-layered (Yanagihara et al. 2018) and the radius of the foraging area can range from one meter to tens or even hundreds of meters (Vargo and Husseneder 2009). Therefore, the effective range of the antifungal activity of queen pheromone would be limited to the area around the royal chamber and its side egg chamber. In this work, phenylacetic acid secreted from foraging workers was shown to inhibit the mycelial growth of M. anisopliae and A. termitophila, with suppression involving the entire termite nest where many colony members are present and active. Our results suggest that each caste of R. speratus makes use of multiple antimicrobial substances in combination to inhibit the growth of pathogenic microorganisms.
Antimicrobial activity of phenylacetic acid was also reported in Atta leaf-cutting ants, and these ants secrete this compound from the metapleural gland to inhibit the spore germination and growth of pathogenic fungi (Fernández-Marín et al. 2015). In that study, B. bassiana and Metarhizium brunneum were isolated from the ant nests but only the mycelial growth of B. bassiana was inhibited by phenylacetic acid. This contrasts with our bioassays, in which the growth of M. anisopliae but not B. bassiana was suppressed (Fig. 2). Thus, susceptibility to phenylacetic acid likely varies among fungal species within the same genus and among strains of the same species. Parallel to the competition between pathogens and termites, parasites and their host termites seem to be engaged in a coevolutionary arms race, in which parasites eventually gain resistance to antimicrobial substances. In open field environments, the lower relatedness among parasites within infected hosts leads to higher levels of within-host competition, which selects for higher parasite virulence (Frank 1994). The coexistence of multiple strains of parasitic fungi within the nests of social insects gives parasitic fungi a distinct advantage in the coevolutionary arms race between hosts and parasites (Yashiro et al. 2011). In response, termites are likely to use multiple antimicrobial substances, alone, or in combination, against parasitic microorganisms that may be costly to the colony. The process of acquiring resistance to termite-secreted antimicrobial substances in entomopathogenic and parasitic fungi by species or strain should be investigated in the future.
Although the site of the biosynthesis of phenylacetic acid in the termite body is still unknown, it is probably in the gut tissues. A previous study reported that Reticulitermes flavipes fed high amounts of xylan, a hemicellulose contained in wood, increased the production of phenylacetic acid in the gut (Brasseur et al. 2016). In the phenylalanine biosynthesis pathway of many organisms, phenethylamine is produced and subsequently metabolized to phenylacetic acid (Ramos and Filloux 2007). Thus, amino acid metabolism by gut symbionts of R. speratus may include the production of phenylacetic acid, which is then excreted. Its presence in termite feces may help maintain nest hygiene. In Zootermopsis nevadensis, acetate produced by gut symbionts and excreted by the termite suppresses the growth of S. marcescens (Inagaki and Matsuura 2018). The mechanism of action of acetate and other weak acids is considered to involve cytoplasmic acidification of pathogenic microorganisms, in turn inhibiting enzyme activity and amino acid transport (Hillenga et al. 1995; Lambert and Stratford 1999; Weber et al. 2012). Phenylacetic acid is also a weak acid such that it presumably inhibits pathogen activity by a similar mechanism. Further studies are needed to determine the site of phenylacetic acid biosynthesis and the mechanism underlying its inhibition of fungal growth.
In summary, R. speratus secrete phenylacetic acid into their nest materials, where it inhibits not only the mycelial growth of A. termitophila and M. anisopliae but also the spore germination of M. anisopliae and B. bassiana. Thus, this compound is one of several antimicrobial compounds, such as the antimicrobial components of pheromones (Matsuura et al. 2007; Mitaka et al. 2017b) and the other antimicrobial agent (Mitaka et al. 2019), released by termites to protect their colonies. The simultaneous use of multiple antimicrobial substances enables termites to cope with a wide variety of parasitic microorganisms. Our work contributes to a more in-depth understanding of the defense mechanisms of termites against pathogenic and parasitic microorganisms.
Data availability
The data used in this study are available in the electronic supplementary materials.
References
Abe T (1987) Evolution and Coadaptation in Biotic Communities. In: Connell J, Hidaka T (eds) Kawano S. Tokyo Press, Tokyo, pp 125–148
Anderson RM, May RM (1979) Population biology of infectious diseases: Part I. Nature 280:361–367. https://doi.org/10.1038/280361a0
Brasseur C, Bauwens J, Tarayre C et al (2016) GC×GC-TOFMS for the analysis of metabolites produced by termites (Reticulitermes flavipes) bred on different carbon sources. Separations 3:19. https://doi.org/10.3390/separations3020019
Bulmer MS, Crozier RH (2006) Variation in positive selection in termite GNBPs and Relish. Mol Biol Evol 23:317–326. https://doi.org/10.1093/molbev/msj037
Bulmer MS, Stefano AM (2022) Termite eusociality and contrasting selective pressure on social and innate immunity. Behav Ecol Sociobiol 76:4. https://doi.org/10.1007/s00265-021-03090-5
Chen J, Henderson G, Grimm CC et al (1998) Naphthalene in Formosan subterranean termite carton nests. J Agric Food Chem 46:2337–2339. https://doi.org/10.1021/jf9709717
Cook SD (2019) An historical review of phenylacetic acid. Plant Cell Physiol 60:243–254. https://doi.org/10.1093/pcp/pcz004
Cremer S, Sixt M (2008) Analogies in the evolution of individual and social immunity. Philos Trans R Soc B Biol Sci 364:129–142. https://doi.org/10.1098/rstb.2008.0166
Cremer S, Armitage SAO, Schmid-Hempel P (2007) Social immunity. Curr Biol 17:R693–R702. https://doi.org/10.1016/j.cub.2007.06.008
Cremer S, Pull CD, Fürst MA (2018) Social immunity: emergence and evolution of colony-level disease protection. Annu Rev Entomol 63:105–123. https://doi.org/10.1146/annurev-ento-020117-043110
Crosland MWJ, Lok CM, Wong TC et al (1997) Division of labour in a lower termite: the majority of tasks are performed by older workers. Anim Behav 54:999–1012. https://doi.org/10.1006/anbe.1997.0509
Eggleton P (2011) An introduction to termites: biology, taxonomy and functional morphology. In: Bignell DE, Roisin Y, Lo N (eds) Biology of termites: a modern synthesis. Springer, Netherlands, Dordrecht, pp 1–26
Fefferman NH, Traniello JFA, Rosengaus RB, Calleri DV (2007) Disease prevention and resistance in social insects: modeling the survival consequences of immunity, hygienic behavior, and colony organization. Behav Ecol Sociobiol 61:565–577. https://doi.org/10.1007/s00265-006-0285-y
Fernández-Marín H, Nash DR, Higginbotham S et al (2015) Functional role of phenylacetic acid from metapleural gland secretions in controlling fungal pathogens in evolutionarily derived leaf-cutting ants. Proc R Soc B Biol Sci 282:20150212. https://doi.org/10.1098/rspb.2015.0212
Frank SA (1994) Kin selection and virulence in the evolution of protocells and parasites. Proc R Soc B Biol Sci 258:153–161. https://doi.org/10.1098/rspb.1994.0156
Hillenga DJ, Versantvoort H, van der Molen S et al (1995) Penicillium chrysogenum takes up the penicillin G precursor phenylacetic acid by passive diffusion. Appl Environ Microbiol 61:2589–2595. https://doi.org/10.1128/aem.61.7.2589-2595.1995
Hywel-Jones NL, Gillespie AT (1990) Effect of temperature on spore germination in Metarhizium anisopliae and Beauveria bassiana. Micol Res 94:389–392. https://doi.org/10.1016/S0953-7562(09)80363-8
Inagaki T, Matsuura K (2018) Extended mutualism between termites and gut microbes: nutritional symbionts contribute to nest hygiene. Sci Nat 105:52. https://doi.org/10.1007/s00114-018-1580-y
Kim Y-S, Lee I-K, Seok S-J, Yun B-S (2008) Chemical constituents of the fruiting bodies of Clitocybe nebularis and their antifungal activity. Mycobiology 36:110–113. https://doi.org/10.4489/MYCO.2008.36.2.110
Komagata Y, Fukasawa Y, Matsuura K (2022) Low temperature enhances the ability of the termite-egg-mimicking fungus Athelia termitophila to compete against wood-decaying fungi. Fungal Ecol 60:101178. https://doi.org/10.1016/j.funeco.2022.101178
Korb J, Poulsen M, Hu H et al (2015) A genomic comparison of two termites with different social complexity. Front Genet 6:9. https://doi.org/10.3389/fgene.2015.00009
Lambert RJ, Stratford M (1999) Weak-acid preservatives: modelling microbial inhibition and response. J Appl Microbiol 86:157–164. https://doi.org/10.1046/j.1365-2672.1999.00646.x
Lee S-B, Chouvenc T, Mizumoto N et al (2022) Age-based spatial distribution of workers is resilient to worker loss in a subterranean termite. Sci Rep 12:7837. https://doi.org/10.1038/s41598-022-11512-1
Maekawa N, Yokoi H, Sotome K et al (2020) Athelia termitophila sp. nov. is the teleomorph of the termite ball fungus Fibularhizoctonia sp. Mycoscience 61:323–330. https://doi.org/10.1016/j.myc.2020.08.002
Matsuura K (2005) Distribution of termite egg-mimicking fungi (“termite balls”) in Reticulitermes spp. (Isoptera: Rhinotermitidae) nests in Japan and the United States. Appl Entomol Zool 40:53–61. https://doi.org/10.1303/aez.2005.53
Matsuura K (2006) Termite-egg mimicry by a sclerotium-forming fungus. Proc R Soc B Biol Sci 273:1203–1209. https://doi.org/10.1098/rspb.2005.3434
Matsuura K (2012) Multifunctional queen pheromone and maintenance of reproductive harmony in termite colonies. J Chem Ecol 38:746–754. https://doi.org/10.1007/s10886-012-0137-3
Matsuura K, Matsunaga T (2015) Antifungal activity of a termite queen pheromone against egg-mimicking termite ball fungi. Ecol Res 30:93–100. https://doi.org/10.1007/s11284-014-1213-7
Matsuura K, Tanaka C, Nishida T (2000) Symbiosis of a termite and a sclerotium-forming fungus: sclerotia mimic termite eggs. Ecol Res 15:405–414. https://doi.org/10.1046/j.1440-1703.2000.00361.x
Matsuura K, Tamura T, Kobayashi N et al (2007) The antibacterial protein lysozyme identified as the termite egg recognition pheromone. PLoS ONE 2:e813. https://doi.org/10.1371/journal.pone.0000813
Matsuura K, Yashiro T, Shimizu K et al (2009) Cuckoo fungus mimics termite eggs by producing the cellulose-digesting enzyme β-glucosidase. Curr Biol 19:30–36. https://doi.org/10.1016/j.cub.2008.11.030
Matsuura K, Himuro C, Yokoi T et al (2010) Identification of a pheromone regulating caste differentiation in termites. Proc Natl Acad Sci 107:12963–12968. https://doi.org/10.1073/pnas.1004675107
Meunier J (2015) Social immunity and the evolution of group living in insects. Philos Trans R Soc B Biol Sci 370:20140102. https://doi.org/10.1098/rstb.2014.0102
Middleditch BS, Missler SR, Hines HB (1981) Naphthalene. In: Middleditch BS, Missler SR, Hines HB (eds) Mass Spectrometry of Priority Pollutants. Springer, US, Boston, MA, pp 129–130
Mitaka Y, Kobayashi K, Matsuura K (2017a) Caste-, sex-, and age-dependent expression of immune-related genes in a Japanese subterranean termite, Reticulitermes speratus. PLoS ONE 12:e0175417. https://doi.org/10.1371/journal.pone.0175417
Mitaka Y, Mori N, Matsuura K (2017b) Multi-functional roles of a soldier-specific volatile as a worker arrestant, primer pheromone and an antimicrobial agent in a termite. Proc R Soc B Biol Sci 284:20171134. https://doi.org/10.1098/rspb.2017.1134
Mitaka Y, Mori N, Matsuura K (2019) A termite fungistatic compound, mellein, inhibits entomopathogenic fungi but not egg-mimicking termite ball fungi. Appl Entomol Zool 54:39–46. https://doi.org/10.1007/s13355-018-0589-1
Mitaka Y, Matsuyama S, Mizumoto N et al (2020) Chemical identification of an aggregation pheromone in the termite Reticulitermes speratus. Sci Rep 10:7424. https://doi.org/10.1038/s41598-020-64388-4
Omoya FO, Kelly BA (2014) Variability of the potency of some selected entomopathogenic bacteria (Bacillus spp. and Serratia spp.) on termites, Macrotermes bellicosus (Isoptera: Termitidae) after exposure to magnetic fields. Int J Trop Insect Sci 34:98–105. https://doi.org/10.1017/S1742758414000253
R Core Team (2016) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria
Ramos J-L, Filloux A (2007) Pseudomonas a model system in biology. Springer, New York
Rosengaus RB, Traniello JFA, Lefebvre ML, Maxmen AB (2004) Fungistatic activity of the sternal gland secretion of the dampwood termite Zootermopsis angusticollis. Insectes Soc 51:259–264. https://doi.org/10.1007/s00040-004-0749-x
Scherer N, Marcseková K, Posset T, Winter G (2019) New studies on leachables in commercial scale protein drug filling lines using stir bar sorptive extraction coupled with TD-GC–MS and UPLC/QTOF-MS/MS analytics. Int J Pharm 555:404–419. https://doi.org/10.1016/j.ijpharm.2018.11.033
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Shellman-Reeve JS (1997) The spectrum of eusociality in termites. In: Choe JC, Crespi BJ (eds) The Evolution of Social Behavior in Insects and Arachnids. Cambridge University Press, Cambridge, pp 52–93
Shimizu S, Yamaji M (2002) Pathogenicity of entomopathogenic fungi to the termite, Reticulitermes speratus. Jpn J Appl Entomol Zool 46:89–91. https://doi.org/10.1303/jjaez.2002.89
Singha D, Singha B, Dutta BK (2010) In vitro pathogenicity of Bacillus thuringiensis against tea termites. J Biol Control 24:279–281
Takata M, Yabe K, Noro T et al (2023) A method for estimating colony size using queen fecundity in termites under field conditions. Sci Nat 110:35. https://doi.org/10.1007/s00114-023-01865-6
Takematsu Y, Yamaoka R (1999) Cuticular hydrocarbons of Reticulitermes (Isoptera : Rhinotermitidae) in Japan and neighboring countries as chemotaxonomic characters. Appl Entomol Zool 34:179–188. https://doi.org/10.1303/aez.34.179
Terrapon N, Li C, Robertson HM et al (2014) Molecular traces of alternative social organization in a termite genome. Nat Commun 5:3636. https://doi.org/10.1038/ncomms4636
Traniello JFA, Rosengaus RB, Savoie K (2002) The development of immunity in a social insect: evidence for the group facilitation of disease resistance. Proc Natl Acad Sci 99:6838–6842. https://doi.org/10.1073/pnas.102176599
Tsunoda K, Matsuoka H, Yoshimura T, Tokoro M (1999) Foraging populations and territories of Reticulitermes speratus (Isoptera: Rhinotermitidae). J Econ Entomol 92:604–609. https://doi.org/10.1093/jee/92.3.604
Vargo EL, Husseneder C (2009) Biology of subterranean termites: insights from molecular studies of Reticulitermes and Coptotermes. Annu Rev Entomol 54:379–403. https://doi.org/10.1146/annurev.ento.54.110807.090443
Weber SS, Kovalchuk A, Bovenberg RAL, Driessen AJM (2012) The ABC transporter ABC40 encodes a phenylacetic acid export system in Penicillium chrysogenum. Fungal Genet Biol 49:915–921. https://doi.org/10.1016/j.fgb.2012.09.003
Yanagihara S, Suehiro W, Mitaka Y, Matsuura K (2018) Age-based soldier polyethism: old termite soldiers take more risks than young soldiers. Biol Lett 14:20180025. https://doi.org/10.1098/rsbl.2018.0025
Yashiro T, Matsuura K (2007) Distribution and phylogenetic analysis of termite egg-mimicking fungi “termite balls” in Reticulitermes termites. Ann Entomol Soc Am 100:532–538. https://doi.org/10.1603/0013-8746(2007)100[532:DAPAOT]2.0.CO;2
Yashiro T, Matsuura K, Tanaka C (2011) Genetic diversity of termite-egg mimicking fungi “termite balls” within the nests of termites. Insectes Soc 58:57–64. https://doi.org/10.1007/s00040-010-0116-z
Zhao C, Rickards RW, Trowell SC (2004) Antibiotics from Australian terrestrial invertebrates. Part 1: antibacterial trinervitadienes from the termite Nasutitermes triodiae. Tetrahedron 60:10753–10759. https://doi.org/10.1016/j.tet.2004.08.065
Zhu Y-J, Zhou H-T, Hu Y-H et al (2011) Antityrosinase and antimicrobial activities of 2-phenylethanol, 2-phenylacetaldehyde and 2-phenylacetic acid. Food Chem 124:298–302. https://doi.org/10.1016/j.foodchem.2010.06.036
Acknowledgements
We thank Daisuke Sakata for collecting termites; Takahiro Kusukawa for providing GC-MS facilities; Toshiharu Akino for laboratory support.
Funding
Open Access funding provided by Nagoya University. This study was partially supported by Japan Society for the Promotion of Science (JSPS) KAKENHI grants (18J00399 to Y.M., 18J13513 to T.I., 25221206 and 18H05268 to K.M.).
Author information
Authors and Affiliations
Contributions
M.N., T.I., Y.M. and K.M. designed experiments. Y.M. and T.I. collected termites. Y.M. performed GC–MS analysis. M.N., Y.M. and T.I. performed antimicrobial tests. M.N. and Y.M. wrote the manuscript. All authors provided feedbacks and edits.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakashima, M., Mitaka, Y., Inagaki, T. et al. An antifungal compound secreted by termite workers, phenylacetic acid, inhibits the growth of both termite egg-mimicking fungus and entomopathogenic fungi. Insect. Soc. (2024). https://doi.org/10.1007/s00040-024-00966-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00040-024-00966-3