Abstract
Studies on the origin and evolutionary history of closely related plants help to understand patterns of diversity of the mountain flora in addition to providing the basis for their identification. The genus Tephroseris includes three endemic taxa with small and disjoint distributions in the high mountains of the Iberian Peninsula and on the Maritime Alps. Tephroseris balbisiana is native to the Southwestern Alps, Tephroseris elodes to Sierra Nevada, and Tephroseris coincyi to Sierra de Gredos. These taxa have been treated under different combinations of species or subspecies due to limited morphological differentiation, but comprehensive studies have not been published so far. By combining information from phylogeny, molecular dating and genome size, we demonstrated the taxonomic distinctiveness between T. balbisiana and the two Iberian taxa. Although the lack of variability in plastid DNA hampered the precise estimation of the diversification events, some of the recovered patterns suggested a recent divergence of T. balbisiana, T. elodes and T. coincyi dating back to the Pleistocene (0.5–2.8 Mya). However, niche modeling supported a geographical overlap between the three taxa during the Last Glacial Maximum (LGM). Moreover, the fragmentation of their ancient larger distribution range, particularly in the lower elevations of the Iberian Peninsula, and migration to glacial refuges in the south-western Alps, provide the most plausible explanations for the current disjoint distribution within the Mediterranean mountains. Furthermore, based on the evidence we gathered, we inferred that the alpine T. balbisiana, as well as the Iberian taxa, should be considered as three distinct subspecies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Mediterranean Basin hosts major centres of plant endemism and speciation with an exceptionally rich and diversified flora (Medail and Quézel 1999; Thompson 1999; Cañadas et al. 2014). Notably, this floristic richness thrives thanks to unique climatic and habitat conditions of this area. The unstable climate of the Pleistocene in the Cenozoic has left a fundamental imprint on the distribution and abundance of species within the Mediterranean Basin by favoring genetic differentiation, reproductive isolation and, ultimately, ecological divergence (Givnish 2010). Repeated events of speciation in allopatry followed by secondary contacts have been documented in the Iberian Peninsula (Cantabrian Range, Sistema Central, and Baetic Cordillera), the Alps (Vargas 2003; Medail and Quézel 1997; Taberlet et al. 1998; Médail and Diadema 2009; Goczał et al. 2020), and Pyrenees and Balearic Islands (Médail and Baumel 2018; Bobo-Pinilla et al. 2022).
Genetic structure and speciation processes of the mountain flora were mainly dominated by two events (Parisod 2022). First, glacial survival during Quaternary climatic oscillations which would have prompted the in situ persistence and isolation of populations in stable climatic refugia i.e. the ice-free mountains encircled by the Mediterranean sea (Sandel et al. 2011; Christie et al. 2014; Harrison and Noss 2017; Molina-Venegas et al. 2017). Second, glacial-induced migrations to lowland or to peripheral refugia which would have allowed secondary contacts between isolated populations, followed by recolonization of alpine habitats. As a consequence of the repeated climatic oscillations, many colonization routes developed across the Southwestern Europe mountains (Vargas 2003; Dixon et al. 2009; Alárcon et al. 2012; Harrison and Noss 2017). For example, mountain ranges of the Iberian Peninsula and interjacent Pyrenees were interconnected during long glacial stages and contemporary alpine flora is the result of migration from lower altitudes after glacial retreat. However, the different cycles of range contraction and expansion experienced by mountain plants during the late Quaternary have also favored range fragmentation or the formation of temporary corridors for taxa migration (Loidi et al. 2015; Padilla-Garcia et al. 2021). Several closely related species or subspecies are now disjointly distributed across all major mountain ranges of the Mediterranean Basin (Dixon et al. 2009; Alárcon et al. 2012; Greiner et al. 2012).
The genus Tephroseris (Rchb.) Rchb. comprises approximately 50 (Nordenstam 2007) perennial species with one exception— the annual T. palustris. Its core distribution is in the temperate and boreal zone of Europe and Asia, with a few species in the NW part of North America (Wang et al. 2009; Nordestam and Pelser 2011). The plants of the genus are herbaceous, with erect, up to 1 m high stem not branching in the lower part; yellow to orange capitula in arranged pseudoumbels or less common in panicles (Kadereit et al. 2021). The genus grows from coastal cliffs to alpine regions (up to 2500 m a.s.l.); they prefer open habitats including wet (peat bogs, the shores of lakes, ditches, or streams), mesotrophic (grasslands, tall-herb subalpine plant communities and open forests) as well as dry ones (xerophytic or rocky meadows and slopes) (Kadereit et al. 2021). Despite their close morphological resemblance, Tephroseris can be easily distinguished from Senecio L. species without the need for microscopic examination by two easily observable characteristics: their capitula lacking outer supplementary bracts, and their styles branched with continuous stigmatic areas. Phylogenetically, the genus Tephroseris is independent from Senecio and more closely related to Nemosenecio (Kitam.) B.Nord. and Sinosenecio as shown by previous molecular studies (Pelser et al. 2007; Wang et al. 2009).
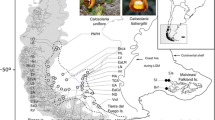
So far, three taxa of Tephroseris were identified in the Southwestern mountains of the Mediterranean Basin: Tephroseris balbisiana (DC.) Holub, Tephroseris elodes (DC.) Holub and Tephroseris coincyi (Rouy) B. Nord. The distribution of T. balbisiana is centred on the Mercantour-Argentera Massif, the crystalline massif located in the Maritime Alps at the border between France and Italy. Few populations can be found in northern Italy on Mount Monviso (Cottian Alps) and on the northern Apennines (Pignatti et al. 2017; Bartolucci et al. 2018). On the other hand, T. coincyi inhabits a very small area of the western part of the Central System, Sierra of Villafranca and Sierra de Gredos (Ávila province, Central-western Spain) and a small spot in the Sanabria lake area, while T. elodes has only a few known localities in the south face of Sierra Nevada (southern edge of the Baetic Cordillera; southern Spain).
These short-lived perennial hemicryptophytes are typical of the high-altitude mountain flora and, all together, prefer soils with average moisture to well moistened. They belong to the vegetation of the wet grasslands and of small watercourses. Tephroseris balbisiana is found mainly in subalpine tall-herb communities, also known as megaforbs, a species-rich habitat distributed along water courses at elevations as high as 2100 m a.s.l.. The community consists of a mix of tall, broad-leaved herbs thriving on soils with a relatively high nutrient status; most of these herbs are otherwise rare in open grasslands due to their sensitivity to extreme drought and grazing. However, there can be significant variations within T. balbisiana habitats, mainly influenced by factors such as geography, altitude, and changing soil moisture conditions throughout the vegetative season (Bono 1967; Noble and Diadema 2011). In contrast, T. coincyi inhabits wet siliceous soils with a permanently high level of edaphic humidity throughout the year. As a result, it grows exclusively on edges of streams, mostly abandoned hay meadows, peaty meadows and small peat bogs (Martinez-Garcia 2008; Martínez-García et al. 2012). The plant can be found at altitudes ranging between 1500 and 1800 m a.s.l., although a few subpopulations are located at 1300 m and others at 1900 m a.s.l. Meanwhile, T. elodes belong to the oromediterranean belt and can be found only in a few watercourses within dense low-growing grasslands linked to acidic and moisture soils (hygrophilous meadow reed communities). These are communities growing on rich soils with a high-water table throughout most of the year, although not waterlogged (Molero and Marfil 2017). With only a few known localities and rapidly declining population numbers in recent decades, T. elodes is severely threatened (Gutiérrez et al. 2019; Peñas et al. 2019).
In a previous study, a strong genetic similarity between the three taxa was suggested (Adamo et al. 2020). This relatedness was supported by both recent (Aedo 2019) and past botanical treatments (Rouy 1890; Cufodontis 1933; Chater and Walters 1976; Tutin et al. 1980). In 2019, Aedo treated T. coincyi as T. elodes subsp. coincyi (Rouy), remarking that the two can be differentiated by leaf margins which are sometimes more coarsely dentate-serrate in the former and with a broader lamina. However, Kadereit et al. (2021) proposed that morphological traits were not sufficient for the recognition of two subspecies and that both cannot be distinguished morphologically from T. balbisiana. Based on results of ITS and ETS sequence analysis, these authors included the two Iberian species in T. balbisiana (Kadereit et al. 2021), irrespective of their different distributions and habitats. More recently, Sánchez-Villegas et al. (2022) have recombined the two Iberian taxa as T. balbisiana (DC.) Holub subsp. coincyi (Rouy) P. Vargas and Luceño, and T. balbisiana (DC.) Holub subsp. elodes (Boiss. ex DC.) P. Vargas and Luceño, recognizing that both possess basal leaves attenuated and even decurrent on the petiole. In contrast, T. balbisiana exhibits truncated and even cordate basal leaves, with deeply toothed leaf margins. These are more often sinuate or sparsely toothed in T. coincyi and sometimes entire in T. elodes (Sánchez-Villegas et al. 2022).
Patterns of diversity of high-mountain flora are intimately linked between neighboring mountain ecosystems, such as Southwestern Alps, Pyrenees, Sierra Nevada, Apennines, Carpathians, Dinarids, and Balkans, where most of the actual alpine lineages found shelter during glaciations (Loidi et al. 2015; Kadereit 2023). It is essential for the studies to be able to predict hypothetical ancestral areas where species or their populations were in contact and how they diverged, allowing the reconstruction of possible evolutionary scenarios in order to understand patterns of diversity of the mountain flora (Parisod 2022; Nieto Feliner et al. 2023).
The main goals of this work are: (i) to better analyze the relationships within T. balbisiana, T. coincyi and T. elodes based on phylogeny and genome size; (ii) to reconstruct the origin of the three taxa and of their populations inhabiting mountain spots of the Mediterranean Basin using molecular dating and DIYABC analysis; (iii) to study the potential geographical contact and subsequent divergence between the three taxa using Species Distribution Modeling (SDM).
Materials and methods
Molecular analysis
Leaf material of 67 accessions of Tephroseris (19 species including 6 outgroups) have been collected in nature, sampled from herbarium specimens or accessed from GenBank (www.ncbi.nlm.nih.gov/nucleotide/, last accessed 31st January 2022) (see Table 1). DNA was extracted using the NucleoSpin Plant II Kit (Macherey–Nagel, Allentown, PA, USA) following the manufacturer’s protocol.
Polymerase chain reactions were carried out for two DNA regions previously identified as the most informative by Kadereit et al. (2021): for nuclear ribosomal ITS, we used the forward primer ITS7A (Jayasena et al. 2017) and the reverse primer ITS4 (White et al. 1990); for nuclear ribosomal ETS, we employed the forward primer 18S-ETS (Baldwin and Markos 1998) and the reverse primer AST1 (Markos and Baldwin 2001). PCR reactions were carried out in 25 µL volumes containing 1 μL DNA template, 5 × FIREPol® Master Mix (Solis BioDyne, Tartu, Estonia) 0.4 µM of each primer, and 2 µL bovine serum albumin (10 mg/mL). PCR cycles started with an initial denaturation step at 95 °C for 4 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 52–58 °C for 30 s, elongation at 72 °C for 1 min, and final elongation step of 5 min. The PCR products were cleaned with NucleoSpin Gel and PCR Clean-up (Macherey–Nagel, Allentown, PA, USA) following the manufacturer’s protocol and sequenced in both directions with the same primers as used for the PCRs by BioFab research s.r.l. (Rome, Italy).
Chromatograms were paired and manually checked using SeqTrace (Stucky 2012), and sequences aligned with Muscle v5 (Edgar 2004), then poorly aligned positions and divergent regions were eliminated using Gblock v0.91b (Talavera and Castresana 2007) allowing smaller final blocks and less strict flanking positions after the alignment steps ETS and ITS alignments were concatenated using SeaView v5.0.5 (Gouy et al. 2010).
Phylogenetic reconstructions were carried out with the Maximum Likelihood (ML) algorithm using RAxML v.8.2.12 (Stamatakis 2014) and with bayesian inference (BI) using MrBayes v3.2.6 (Huelsenbeck and Ronquist 2001). The concatenated alignment was found to fit the GTR + G4 substitution model using JModelTest2 (Darriba et al. 2012), ten independent RAxML bootstrapping were stopped automatically, and two independent MrBayes runs were stopped after 10,000,000 states, with a 10% burning, raising average standard deviation of split frequencies < 0.01.
The time tree was calculated using BEAST2 v2.6.6 (Bouckaert et al. 2019). Absolute age estimation analyses relied on three calibration points, strict molecular clocks, a Yule birth/death model, and a GTR + G4 substitution model. A first calibration (Nemosenecio-Tephroseris MRCA) point was imposed at 3.16Mya using a log-normal distribution, the second calibration point was imposed at 8.3Mya (4.3–10.3 Mya 95% HPD [Highest Posterior Density] interval) using a log-normal distribution for the Nemosenecio-Sinosenecio MRCA. The third calibration point was imposed at 32.1 Mya (31.6–39.6 Mya 95% HPD interval) using a log-normal distribution for the Doronicum-Miricacalia MRCA. Calibration dates were extracted from the TimeTree database (Kumar et al. 2017).
We conducted a MCMC analysis with 100 million generations with a sampling frequency of 1000 generations, the convergence of the strict molecular clock parameters was confirmed on the software TRACER v1.6 (http://tree.bio.ed.ac.uk/software/tracer/); all parameters had ESS > > 200 (after a burn-in of 10% was removed). Maximum clade credibility trees were summarized from the MCMC trees in the program TreeAnnotator v.1.8. of the software BEAST burning 100,000 initial states.
DIYABC analysis
In order to assess the evolutionary history of the three lineages, an Approximate Bayesian Computation (ABC) analysis was performed using the software DIYABC v2.1 (Cornuet et al. 2014). DIYABC calculates the posterior probabilities of alternative scenarios by simulating a large number of data sets in each case. The logistic regression procedure (Fagundes et al. 2007) estimates the occurrence of each scenario among the simulated data sets that are closest to the observed data. The aim of this approach was to compare the different phylogeographic hypotheses that could be used to explain the present distribution of the three studied lineages. Seven different sets of scenarios were designed in order to test the proposed phylogeographical hypothesis. Due to the lack of ancestral information, prior distributions of the parameters were chosen as an initial approach with a large interval. Population sizes were set equally in all cases except for founder events. Divergence times were unrestricted to allow the program to set the most likely value. The JC69 model of nucleotide evolution (Jukes et al. 1969) was chosen, and the Uniform Mutation rate was set to [10–9−10–7]. One million data sets were simulated for each scenario (Cornuet et al. 2008, 2010). The best scenario was chosen by calculating the posterior probabilities of each one by performing a polychotomous weighted logistic regression on the 1% of simulated data sets closest to the observed data set (Cornuet et al. 2008, 2010). Subsequent distributions of parameters were evaluated under the best scenario using a local linear regression on the 1% closest simulated data sets with a logit transformation. Confidence in the choice of scenario was tested by evaluating Type I and Type II error rates (Cornuet et al. 2010). The similarity between real data and simulated data sets was assessed for the best scenario to test the model adequacy using the posterior distribution of the parameter values.
Flow cytometric analyses
One to 15 plants per population (altogether 66 plants) of T. balbisiana, T. coincyi and T. elodes were analyzed by flow cytometry using the fluorochrome 4′, 6-diamidino-2-phenylindole (DAPI). We used fresh plant material from seedlings grown from seeds collected at the studied sites (Table S1). Each plant was analyzed separately to ensure the accuracy of relative DNA content estimations. Fresh material of Bellis perennis L. (2C = 3.38 pg; Schönswetter et al. 2007) was added for internal standardization. The nuclei isolation and staining procedure followed the simplified two-step protocol (Doležel et al. 2007) with some modifications. First, the seedling (without the root part) was chopped with intact leaf tissue of an internal standard in 0.5 ml of ice-cold Otto I buffer (0.1 M citric acid, 0.5% Tween 20). Next, the crude nuclear suspension was filtered through 42-μm nylon mesh. For staining, 0.5 ml of a solution containing Otto II buffer (0.4 M Na2HPO4·12H2O), 2-mercaptoethanol (2 μl/ml) and DAPI (4 μg/ml) was added to the flow-through fraction. Samples were analyzed after 10 min incubation at room temperature. The fluorescence of 3000 particles was recorded, and only histograms with symmetrical peaks with a coefficient of variance (CV) of the standard and sample G1 peaks below 3% were considered. Flow cytometric analyses were carried out using a Cyflow ML instrument or Cyflow Space instrument (Partec, Munster, Germany) equipped with a UV-LED as an excitation source. Flow cytometric histograms were evaluated using FloMax software v2.7d (Partec, Munster, Germany). The relative DNA content was calculated as the ratio of G1 peak of standard Bellis perennis and G1 peak of the Tephroseris sample (refer to from now on as the ratio sample/standard). The relationship between chromosome numbers and relative DNA content was verified using chromosome counts (Table S1).
A newly generated dataset of relative nuclear DNA content was compared with previously published data for hexaploid Tephroseris taxa (169 individuals; Olšavská et al. 2015; Skokanová et al. 2019). Box-and-whisker plots and scatter plots were used to depict variation in the relative DNA content of the taxa studied; the Tukey–Kramer test (Tukey’s test for unequal sample size) was used to test for differences in relative DNA content between taxa. Analyses were carried out using STATISTICA 12 (StatSoft Inc. 2013).
Chromosome counting
Root tip meristems from seedlings were used for karyological analyses. The root tips were pre-treated in a 0.002 M water solution of 8-hydroxyquinoline at 4 °C for about 16 h (overnight), fixed in a 1:3 mixture of 98% acetic acid and 96% ethanol for 1–24 h, washed in distilled water, macerated in 1N HCl at 60 °C for 5 min and then washed in distilled water. Tip squashes were made using the cellophane square technique (Murin 1960). Permanent slides were stained with a 7% solution of Giemsa Stain–Modified Solution (Fluka Analytical) in Sorensen phosphate buffer, dried and observed in a drop of immersion oil using a Leica DM1000 microscope equipped with an HDCE-X5 camera and ScopeImage 9.0 software and the number of chromosomes were counted.
Species distribution models (SDMs)
We assembled a dataset of 169 occurrences of T. balbisiana, 33 occurrences of T. coincyi and 41 occurrences of T. elodes based on literature data, herbarium collections, personal observations, and original data from GBIF (www.gbif.org; last access December 2021) and Silene-Flore (http://flore.silene.eu, exported on December 2018). Occurrences for all the species were manually checked by the authors based on their own knowledge of the field, moreover, the GBIF occurrences were treated with the CoordinateCleaner R package v2.0-20 (Zizka et al. 2020) in order to remove occurrences falling into the sea, corresponding to any herbarium or research institution and other potentially problematic coordinates that might be biased by rasterized coordinates.
Due to the low number of occurrences available for the Iberian species and based on the molecular phylogeny results (see below, paragraph Phylogeny and molecular clock), we merged T. coincyi and T. elodes occurrences in a single SDM in the case of the past distributions, where models did not converge in a satisfactorily way.
We calibrated species distribution models within the accessible area (Owens et al. 2013), namely the geographic extent hypothesized to fall within the long-term dispersal and colonization potential for the three modeled taxa over their evolutionary history. In the lack of precise information on the current and past dispersal ability of these species, we included all European territories between the Atlantic Ocean and the Western Alps to inspect their potential past and current interactions.
We used the 19 “standard” bioclimatic variables as predictors, all variables at a resolution of 2.5 min. Climatic variables were downloaded from WorldClim2 (Fick and Hijmans 2017) for the “current” period (1970–2000), the Last Interglacial (LIG; ~ 120,000–140,000 years ago) (Otto-Bliesner et al. 2009) and the Last Glacial Maximum (LGM, ~ 22,000 years ago). In the LGM case, we used the three models CCSM4 (Gent and Danabasoglu 2011), MPI-ESM-P (Xu et al. 2018) and MIROC-ESM (Watanabe et al. 2011). We decided to focus on climatic predictors, excluding soil features. This choice was guided by the high collinearity level observed between climate and pedology in mountain ranges (Adamo et al. 2020), moreover, it has been observed that when assessing climate change impact, the number of predictor variables in SDMs should be kept reasonably small in order to obtain models of higher quality (Brun et al. 2020). Hydrological systems topography or habitats were not used as predictors or to calibrate the SDMs. Habitats occupied by our species are sized at a local scale, while the calibration of the model is at regional scale.
The current climate is important for species conservation, but also to visually check the goodness of fit of our models, since the great majority of the species stations, are actually included in the analysis. Niche distributions in past cold and warm periods were modeled in order to ascertain potential contacts between the three taxa.
We explored predictors’ importance on both presence and pseudo-absence points using a Principal Component Analysis (PCA); we tested collinearity between predictors (Braunisch et al. 2013) using Pearson correlation, setting the threshold for collinearity at |r|> 0.7 (Zuur and Ieno 2016). We excluded collinear variables based on our expert opinion, moreover, we used PC1 and PC2 as proxies for predictors prioritization (Guisan et al. 2017).
To model species distribution, we used four different probabilistic models found in the ‘biomod2’ package in R (Thuiller et al. 2021): Generalized Additive Model (GAM), Generalized Boosting Model (GBM), RandomForest (RF) and Classification Tree Analysis (CTA). We ran the models with two different sets of pseudo‐absence points ten times more abundant than presence points. Each model was then calibrated using 70% of each presence/pseudo‐absence dataset and evaluated with the 30% remaining. We evaluated the model’s predictive accuracy using two different indices: the True Skill Statistics (TSS, Allouche et al. 2006) and the area under the receiver operating characteristic curve (ROC, Hanley and McNeil 1982).
We projected the model into the accessible area to obtain a graphical representation of the current potential bioclimatic niche suitability of T. balbisiana, T. elodes, and T. coincyi, and the past bioclimatic potential niche of T. balbisana and of T. elodes together with T. coincyi. In the case of the LGM prediction, we settled at zero suitability for the areas covered by glaciers (and nunataks) using the ice distribution by Ehlers et al. (2011).
Results
Phylogeny and molecular clock
RAxML, MrBayes, and BEAST topologies were almost identical, but with different branch supports, thus we reported the single consensus tree obtained with MrBayes. In the BI analysis of combined ITS and ETS sequences (Fig. 1), three major clades were resolved. The “integrifolia clade” (node support 87/1.0 for Maximum Likelihood and Bayesian analysis, respectively) which contains T. integrifolia and T. palustris sequences as sisters to the former. All the subspecies of T. integrifolia were included in this single clade. The “longifolia clade” with T. longifolia, T. helenitis, T. papposa, and T. crispa which are not monophyletic. The clade support was high only in the case of the Bayesian analysis (52/1.0, respectively). The “longifolia clade” included two sequences from samples collected on the northern Apennines by Adamo et al. (2020) that are regarded as belonging to T. balbisiana; here named Tephroseris sp. BAL4 and BAL5. The “T. balbisiana clade” (node support 85/1.0) contains the sequences of T. balbisiana, T. coincyi and T. elodes. Inside it, we retrieved a well-supported separation between T. balbisiana (node support 87/1.0) and the two Iberian taxa (node support 91/1.0). According to this finding, here we propose to recognize them as separate subspecies of T. balbisiana (see Discussion for more details). Iberian taxa, however, formed a polytomy including among the others, sequences of T. elodes samples collected in the field for this study and the sequence (Tep3) of the specimen at the Berlin Herbarium (Kadereit et al. 2021). Our phylogeny strongly supported four clades: longifolia, integrifolia, T. balbisiana, and Asian (all nodes supports were 1.00 in the bayesian analysis). We found a good support for the relationship between the longifolia and integrifolia clades (0.95), while the node for the T. balbisiana and Asian clades was weakly supported (31/0.59).
Tephroseris genus phylogeny based on ITS and ETS regions. The tree topology corresponds to results of the Bayesian inference. Numbers at nodes over the branches report maximum likelihood bootstrap values and posterior probabilities, respectively. Node supports are reported when bootstrap was higher than 70 or posterior probability higher than 0.85. Square bracketed bold values below nodes correspond to the 95% HPD intervals, namely, the most probable split time between taxa. 95% HPD intervals are reported only for supported nodes. Labels correspond to accepted name of taxa, followed by an ID corresponding to those in Table 1. We identified “integrifolia clade” and “longifolia clade” as highly supported clades (continuous line), while “Asian clade” and “T. balbisiana clade” were not appropriately separated, thus, since the inner nodes were well supported, we choose to keep the separation as a partially supported clade (dashed line)
Time tree analysis reports ranges of time in million of years only for the nodes showing supports higher than 70/0.75 (Fig. S1). Tephroseris genus separated from the sister genus Nemosenecio at about 6.73 [8.29–5.33] Mya. Divergence of the integrifolia and longifolia clades from the Asian and T. balbisiana clades dated at 4.61 [5.88–3.55] Mya. The node at the separation between the Asian and T. balbisiana clades was unsupported according to ML and Bayesian analysis. However, we were able to date the separation between T. balbisiana subsp. balbisiana and the clade with T. balbisiana subsp. coincyi and T. balbisiana subsp. elodes at 2.56 Mya [3.7–1.6] (Fig. S1).
Results of the DIYABC analysis
Of all the scenarios tested, the three with the highest statistical support were Scenario 4 which received the highest posterior probability [P4 = 0.28 (95% CI 0.23–0.31)], followed by Scenario 3 and Scenario 1 [P3 = 0.18 (CI 0.14–0.20) and P1 = 0.17 (CI 0.14–0.19)](Fig. 2). Scenario 4 supported the derivation of T. balbisiana and T. elodes from a common ancestral population. This event would have been followed by the more recent divergence of T. coincyi. Scenarios 3 showed T. elodes as diverged from an ancestral population of T. balbisiana (Scenario 3), while T. balbisiana as diverged from an ancestral population of T. elodes (Scenario 1).
Hypothetical coalescent scenarios tested in DIYABC for the evolution of the three endemic Tephroseris. Statistically, Scenario 4 was the most probable, assuming the presence of a common ancestor for the Alpine and the Iberian branches. Scenario 1 and Scenario 3 had lower, but similar probabilities. Scenario 4 assumes that T. balbisiana and T. elodes diverged at the same time from an ancestral population and that the Iberian T. coincyi had diverged only later. All the remaining Scenarios had a low probability to be actual (P < 0.15)
For Scenario 4, Type II error rate (the probability that a Scenario was selected when it was not the true Scenario for the simulated data) and Type I error rate (the probability that it was not selected when it was the actual Scenario) were 26% and 41%, respectively, owing to the high similarity among scenarios.
Karyological analyses and relative DNA content
The chromosome number 2n = 48 was newly reported for T. elodes and T. balbisiana, and 2n = 48 + 1B for T. coincyi (Fig. S2, Table S1). Relative DNA content estimates of T. balbisiana, T. coincyi and T. elodes corresponded to a hexaploid level 2n ~ 6x ~ 48 (Table S1). Relative DNA contents significantly differed between the two Iberian populations (P.to Peňa Negra and Sierra Nevada) and were significantly higher than those measured in populations sampled for T. balbisiana (Tukey–Kramer, P < 0.001) which, conversely, did not significantly differ (Fig. S3) pointing to a single species. Interestingly, the Iberian species, T. coincyi and T. elodes, showed relative DNA contents significantly higher also with respect to all the remaining European hexaploid Tephroseris (data obtained from Olšavská et al. 2015 and Skokanová et al. 2019) as represented by box-and-whisker plots (Tukey–Kramer test, P < 0.05) (Fig. 3). Notably, according to this last comparison, T. balbisiana and T. longifolia subsp. gaudini did not differ for their DNA contents (Fig. 3).
Relative DNA contents of the hexaploid Tephroseris taxa (see Table S1 for details). Statistically significant differences (Tukey–Kramer, P < 0.05) were reported by different small letters. Grey boxplots correspond to bibliographic data (Olšavská et al. 2015; Skokanová et al. 2019), while black boxplots are from this study
Climatic features of the three mountain Tephroseris
We built models based on five non-collinear variables, namely Isothermality (bio3), Temperature Seasonality (bio4), Mean Temperature of Wettest Quarter (bio8), Annual Precipitation (bio12), and Precipitation of Warmest Quarter (bio18). PCA highlighted the overlap between bioclimatic hypervolumes of T. coincyi and T. elodes, while T. balbisiana belongs to a clearly independent bioclimatic hypervolume (Fig. S4). All models converged pointing to bio8, the mean temperature for the 3 months with the highest cumulative precipitation, as the most important predictor of the distribution of the two Iberian species. Conversely, the most important predictor of T. balbisiana distribution was isothermality (bio3) in GAM, GBM, and RF, while in CTA the most important variable was the mean temperature of the wettest quarter (bio8), followed by bio3 (Table S2).
Coherently with PCA observations, predicted models showed that the three taxa have similar but not identical climatic features. As expected in consideration of their mountain ranges, habitats of the three taxa are characterized by marked temperature changes over the course of the year (bio4) (Fig. S5), and this was true especially in the case of T. elodes habitat which would show major climatic extremes. This finding is supported also by the observation that T. coincyi and T. balbisiana habitats would be characterized by narrower diurnal temperature oscillations (bio3) than in the habitat of T. elodes. Mean temperatures in the wettest quarter (bio8) are around zero or lower for all the three taxa. Total annual precipitation (bio12) resulted particularly important for T. balbisiana, in fact, this species shows a peak of suitability for sites characterized by ~ 1000 mm of rain per year, a value which can be significantly lower in the case of the Iberian taxa. Consequently, precipitations in the warmest quarter of the year (bio18; the less rainy quarter in all three cases) range between 150 and 180 mm in T. balbisiana habitats, while the two Iberian species would prefer drier summers (Fig. S5).
Current and past bioclimatic potential niches
Model’s predictive accuracy estimated with ROC and TSS were interpreted using the classification of Araújo et al. (2005), and indicated excellent model performances (Fig. S6), resulting in scores higher than 0.8 for all the estimations associated to restrained standard deviations. Projections of the potential distribution of the three taxa into the current climate conditions over-impress the known distribution of the three taxa, highlighting a core area extending over the Ligurian and the Maritime Alps as well as over Sierra Nevada and Sierra de Gredos, where T. balbisiana, T. elodes, and T. coincyi, respectively, grow. In the Lago de Sanabria area, where T. coincyi can also be found, we predicted lower niche suitability, if compared to the above-described core ranges (Fig. S7).
The model predicted a few areas with high niche suitability, where the species are not currently present or documented, namely: Baza-Los Filabres range mountain for T. elodes and a series of elevated regions in the North direction for T. coincyi.
To project the potential distribution in the past of the three Tephroseris taxa, we merged T. elodes and T. coincyi occurrences. At LIG conditions, the Iberian taxa showed scattered occurrences across the Iberian Peninsula and very similar to their actual distribution. T. balbisiana optimal distributions corresponded to the French northwestern Alps, with the addition of a restricted area of niche suitability located on the Pyrenees (Fig. 4).
Distribution prediction of Tephroseris balbisiana (the three upper maps) and the Iberian Tephroseris (all together in the three lower maps) for the Last Interglacial period (LIG; first column), the Last Glacial Maximum (LGM; second column), and current time (third column). Color gradient axis on the left of figures goes from gray to green and represents growing level of habitat suitability for Tephroseris. Pale blue areas in the LGM models represent maximum ice shield expansion during LGM. North arrow indicates the orientation in the maps, while the scale bar refers to km; for simplicity, these frames are reported only once in the T. balbisiana current SDM
Tephroseris distributions during LGM differed from those projected for LIG and current climate conditions. According to the models, niche suitably would have generally expanded and moved toward areas at lower altitudes in the French Central Massif and in the Iberian Peninsula, probably dominated by warm temperatures and lower precipitations. T. balbisiana optimum of distribution shifted in a southeastern direction and it was mainly centered on the Northern Apennines (Fig. 4). Expansion of distributions at lower elevations in the Iberian Peninsula would have allowed for the establishment of interconnections between populations at all major sites presently occupied by T. elodes (Sierra Nevada) and by T. coincyi in the Central System (Sierra of Villafranca and Sierra de Gredos) (Fig. 4). Moreover, Tephroseris distribution would have shown also a shift toward the lower elevations bordering the Pyrenees and to the Massif Central of France. Following the linear arrangement of the main mountains of Southwestern Europe, Tephroseris distribution would have further extended to the Southern Alps and the Northern Apennines (Fig. 4). This finding is likely to enforce the preference for climatic patterns of Southern Europe and particularly for the climate of coastal (sea-mitigated) mountain ranges which could have behaved as peripheral refugia during the LGM (Fig. 4). During the last 20,000 years, a substantial reduction of T. balbisiana, T. elodes and T. coincyi ranges occurred (~ 92%, ~ 97% and ~ 97%, respectively). T. balbisiana distribution shifted from the Northern Apennines to the current distribution on the Southwestern Alps by the end of the LGM. In addition, the habitat of the Iberian species would have contracted into the current disjointed ranges (pale blue area; Fig. 5).
Loss (red) and gain (green) of habitat suitability predicted for T. balbisana and the two Iberian taxa, comparing the habitat predicted for the LGM with current suitability. A shift can be observed in the T. balbisiana distribution from the Northern Apennines to the current distribution on the Southwestern Alps (green dots). According to the model, Iberian species would have lost a very large portion of their species range because of ice retreat and have contracted into current disjointed ranges (pale blue area)
Discussion
By combining molecular and genome size data with information on ecological niche evolution, we assessed the taxonomic distinctiveness and the diversification history of three Tephroseris endemic to the Mediterranean mountains. Overall, we find that results of phylogeny and molecular dating may be congruent with a recent divergence of T. balbisiana, T. elodes and T. coincyi, as already reported for the other European taxa (Kadereit et al. 2021). Climatic preferences and patterns of niche evolution supported a geographical overlap of the three taxa in LGM. Limits of the nuclear DNA sequences and the lack of variability in plastid DNA weaken our ability to date precise sequences of events in their diversification. However, some of the recovered patterns were congruent between analyses, allowing us to draw some general conclusions on the evolutionary patterns and taxonomic asset of Tephroseris within the Mediterranean mountains.
Phylogenetic relationships
Based on rDNA spacers (ITS and ETS), European species of Tephroseris grouped into two supported clades separated by the Asian clade, largely confirming previous studies (Skokanová et al. 2019). The novelty of our analysis was the clade with T. balbisiana, T. coyinci and T. elodes and their separate placement from all the other European taxa (Fig. 1). Tephroseris balbisiana genetic asset had never been deeply inspected so far. Using sequences from different populations living in Mercantour-Argentera Massif at the core of the Maritime Alps and on the main mountains of Iberia, we were able to show a very well-supported separation between the sequences of T. balbisiana and the two Iberian taxa and to propose the recognition of the three taxa as separated subspecies. Only three individuals were used before to analyze the entire “balbisiana” clade (Kadereit et al. 2021), and this approach might have led the authors to conclude that these sequences corresponded to a single species with a largely fragmented range. In our study, the exclusion from the “balbisiana” clade of two sequences (BAL5 and BAL4; Fig. 1) originating from the Northern Apennines (almost probably erroneously ascribed in previous studies to T. balbisiana; Bonafede et al. 2013) would stress the distinctiveness of the populations of the Maritime Alps and enforce the role of this geographic area as a center of endemism for the genus.
Relative genome size and chromosome numbers
Results of this study showed important differences between the Iberian and the Alpine Tephroseris, pointing to the existence of at least two distinct genome size lineages (Fig. 3 and Fig. S3). No other Tephroseris species, in fact, has a genome size as high as T. coincyi and T. elodes, a finding that might imply the evolutionary isolation of the Iberian populations. However, the significant differences in genome size found between the two Iberian taxa would argue for their recognition as two subspecies. Overlapping genome sizes found between populations of the Maritime Alps supported, on the other side, the existence of a single genetic pool on this mountain range. Moreover, the fact that T. balbisiana genome size lies between those of T. l. subsp. brachychaeta and T. l. subsp. gaudinii would imply a relatedness of the alpine species to the T. longifolia aggregate of species (Adamo et al. 2020). If this finding was proven, an older dating of T. balbisiana with respect to T. coincyi and T. elodes will find support.
The chromosome count of 2n = 48 was newly reported for T. balbisiana. Similarly, the re-examination of T. elodes from Sierra Nevada revealed chromosome number of 2n = 48, which contradicts the previous record 2n = 40 (Blanca and Cueto 1992) from the same site/mountain range. When Kadereit et al. (2021) revised the voucher specimen of the record by Blanca and Cueto (1992), they ruled out its incorrect identification. However, the possibility of miscalculation or aneuploidy should be considered. Previously, the chromosome counts of 2n = 40 for any Tephroseris taxa have been considered unusual because the lowest chromosome number reported for many species of the genus was 2n = 48 (Kochjarová 2005; Altınordu et al. 2014; Kadereit et al. 2021). Nevertheless, aneuploidy has been known in the genus such as the chromosome number of 2n = 46 has been reported for T. integrifolia subsp. aurantiaca (Liu and Yang 2011).
Climate preferences and climatic niche evolution
Our results showed that niche suitability in Tephroseris retreated to high altitudes during the interglacial period (LIG was characterized by a climate warmer than present day) and, converse, niche expanded toward lower altitudes during the Last Glacial Maximum (LGM; cold climate, approximately 22,000 years ago). Similar altitudinal variations of species niche have been documented for the flora of the Maritime Alps, of the mountain massifs of the Iberian Peninsula and in Pyrenees (Martín-Bravo et al. 2010; Peredo et al. 2009; Guerrina et al. 2022). In the Iberian Peninsula, high temperatures during LIG, impaired the survival of cold-adapted species at low altitudes. For example, temperature increase during LIG has forced Iberian populations of Erodium to migrate northward or to remain isolated on high mountains (Alárcon et al. 2012). On the contrary, given the limited ice extent in Central Iberia, some cold-adapted endemics as Androsace vitaliana (L.) Lapeyr. underwent a massive range expansion during the LGM and migrated to lowlands (Boucher et al. 2012). Diversification in this taxon would have involved long-distance dispersal during the Pleistocene trough connections existing between the Alps and the mountain systems of the Iberian Peninsula bypassing the Pyrenees (Dixon et al. 2009). Our projections of SDMs at the LGM, however, tell a slightly different story when comparing T. balbisiana and the two Iberian taxa. In response to LGM, the niche suitability of the cold-adapted Iberian taxa significantly increased due to improved temperature trends at lower altitudes. Additionally, the minimal extent of ice during this period preserved the original distribution of Tephroseris populations in the higher mountains of Central Iberia. Furthermore, the extension of the ice shield forced the populations of T. balbisiana to shift from the western Alps toward southern ranges such as glacial refuges of the Maritime Alps and, possibly, the Northern Apennines. Conversely, the populations of Tephroseris of the Iberian Peninsula experienced a more extensive range expansion. Therefore, it seemed that their habitat suitability extended toward Pyrenees, the Central Massif, possibly even the Southwestern Alps (Fig. 4).
Our results showed that the three taxa have similar climatic niches, specifically preferring habitats with high levels of soil humidity during the vegetative stage. Tephroseris balbisiana has a higher requirement for total annual rainfall compared to the Iberian Tephroseris, which, in contrast, thrives in soils with consistently high levels of edaphic humidity. These features could have evolved after the establishment of Tephroseris populations on their current habitats at the end of the LGM. Consistent with the findings of Pellisier et al. (2013) and of Wasof et al. (2015), geographically isolated populations tend to conserve their ecological niche over time spans of millennia. This tendency is particularly pronounced in species that prefer cold temperatures and moist soils, which could also apply to Tephroseris after fragmentation of its range across the Mediterranean mountains.
Post-glacial contraction has been mainly explained by intolerance of cold-adapted species to warmer temperatures and the increase in competition by larger plants (Birks 2008; Martín-Bravo et al. 2010). According to our model, a considerable reduction of the niche suitability was predicted for the Iberian species which, after ice retreat, would have lost a very large portion of their range and contracted into current disjointed ranges (Fig. 5).
Population divergence history and molecular dating
It is likely that LGM saw the spread of Tephroseris within a large geographic area, potentially corresponding to the mountains within current Mediterranean bioclimatic region. This interpretation seems to find support by results of the DIYABC analysis which showed derivation of both Iberian and Alpine Tephroseris from a common ancestor no longer extant (Scenario 4, Fig. 2). From the end of the LGM to the early Holocene (11,000 years before present), because of the warming of the Mediterranean climate, Tephroseris range would have ultimately undergone a strong contraction and a multiple fragmentation during the retreat of the ice (Figs. 4 and 5). This occurrence would have led to the current distribution of three schizo-endemic taxa: T. balbisiana, T. coincyi and T. elodes, isolated into their relict mountain ranges (Diadema et al. 2005; Schmitt et al. 2010). The geographical divergence within Mediterranean Tephroseris in relatively recent times as a result of fragmentation processes separating populations from Iberia and the Alps is seemingly in contrast with the results of our molecular clock which pointed to the split from the ancestor of the “T. balbisiana” clade dating well before the Quaternary glacial series (1.6–3.7 Mya; Fig. S1). However, results of our molecular dating are congruent with previous literature. (Skokanová et al. 2019). As hypothesized by Skokanová et al. (2019) and by Kadereit et al. (2021), in fact, main diversification events within the European taxa of Tephroseris likely took place in the Pleistocene (0.5–2.8 Mya) under the action of glacial cycles which led to migration and subsequent isolation into southern refugia of central and subalpine European populations. According to our phylogenetic data and Skokanová et al. (2019) and Kadereit et al. (2021), T. balbisiana group diversification would have happened in (Pliocene-) early Pleistocene. After that, Pleistocene glacial circles forced the populations to “get closer” geographically (and ecologically) as in our LGM projections, and had compatible contacts (once or more times). Geographical separations in interglacial altering periods (as in our LIG projection, and others before), probably also happened even if with low genetic divergences because of conservative ecological niches. This interpretation supports main diversification events within “integrifolia” and within “balbisiana” at about 1.6–3.8 Mya and those within “longifolia” clade, slightly earlier (about 2.17–4.27 Mya). We are aware, however, that the complete lack of variability found in plastid DNA for the three taxa could have limited a precise dating of diversification events (Pelser et al. 2010), forcing our analysis to be circumscribed to the ETS and ITS spacers.
Alternatively, the Iberian Tephroseris would not have expanded to reach the south-western Alps during the LGM. Indeed, Pyrenees together with Sierra Nevada and the Alps are regarded as the sole three main areas covered by ice in Southern Europe during the LGM (Ehlers et al. 2011). As addressed by the result of DIYABC, Iberian and Alpine populations extending their range during the LGM (Fig. 4 LGM), would have come in contact and genetically admixed on the Pyrenees (likely, all three Scenarios of Fig. 2 are compatible with this view). Pyrenees have been regarded as a geological barrier to postglacial colonization from southern Iberian refugial areas as well as a migratory northward route (Taberlet et al. 1998). Yet, according to the projection model performed by Garcia-Ruiz et al. (2003), ice covering is estimated to have descended as down as 900 m a.s.l. in the Pyrenees and 1200 m a.s.l. in the Pre-Pyrenees. Thus, “bridging” populations of Tephroseris would have occurred in the low-altitude western and eastern edges of the Pyrenean range (Padilla-Garcia et al. 2021) (Fig. 5). The persistence of mountain populations in the Central Pyrenees is likely to have been hampered by the harsh conditions at the higher elevations, possibly explaining also the current absence of Tephroseris from these mountains.
According to this scenarios, evolutionary processes at the base of Tephroseris diversification would infer the importance of the biogeographical relationships between different areas of the Mediterranean Basin (Western Alps, Pyrenees, and Iberian Massifs) hosting different processes of migration and isolation. These last evolutionary events, yet to be demonstrated, would find support in the results of genome sizing conducted on the three taxa.
Proposed taxonomic treatment and conservation consequences
Phylogenetic studies have shown that the European species of Tephroseris are characterized by low evolutionary rate, particularly in relation to plastidial DNA (Adamo et al. 2020; Kadereit et al. 2021). Despite sequencing the plastome of T. balbisiana, T. coincyi, T. elodes and other species of the genus, we found no significant sequence variability (data not shown). Since plastidial markers failed to provide any evolutionary insights, with the results of our molecular phylogeny, chromosome counting and genome size analyses, we consider it more appropriate to support the nomenclatural combinations proposed by Vargas and Luceño (Sanchez-Villegas et al. 2022). They suggested combinations based on a few but solid distinctive morphological traits (see before in this paper), proposing a separation of T. coincyi and T. elodes at the rank of subspecies. Here, we propose the recognition of T. balbisiana (DC.) Holub subsp. balbisiana as the nominal subspecies comprising all the populations ranging on the Maritime Alps and of the following subspecies which can accommodate the two Iberian taxa: Tephroseris balbisiana (DC.) Holub subsp. coincy (Rouy) P. Vargas and Luceño, and Tephroseris balbisiana (DC.) Holub subsp. elodes (Boiss. ex DC.) P. Vargas and Luceño.
Reevaluation of the status of the taxa and implementation of appropriate conservation polices are required. From a biodiversity conservation perspective, the current geographic isolation of the Iberian lineages, lasting since LGM, is particularly noteworthy in light of the potential adaptive or extinction rates facing future climate changes.
Data availability
The sequences are publicly available on NCBI. Coordinates used in species distribution models are not published due to the rarity of the plants studied, but can be requested to the authors.
References
Adamo M, Mammola S, Noble V, Mucciarelli M (2020) Integrating multiple lines of evidence to explore intraspecific variability in a rare endemic alpine plant and implications for its conservation. Plants 9:1160
Aedo C (2019) Tephroseris (Rchb.) Rchb. Flora Iber XVI (III) Compos (partim) 1498–1503
Alarcón M, Vargas P, Sáez L, Molero J, Aldasoro JJ (2012) Genetic diversity of mountain plants: two migration episodes of Mediterranean Erodium (Geraniaceae). Mol Phylogenet Evol 63:866–876
Allouche O, Tsoar A, Kadmon R (2006) Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J Appl Ecol 43:1223–1232
Altınordu F, Martin E, Hamzaoğlu E, Çetin Ö (2014) New chromosome counts, karyotype analyses and asymmetry indices in some taxa of genus Senecio L. and related genera Tephroseris (Rchb.) Rchb. and Turanecio Hamzaoğlu belong to tribe Senecioneae (Asteraceae) from Turkey. Plant Syst Evol 300:2205–2216
Araujo MB, Pearson RG, Thuiller W, Erhard M (2005) Validation of species–climate impact models under climate change. Glob Change Biol 11:1504–1513
Baldwin BG, Markos S (1998) Phylogenetic utility of the external transcribed spacer (ETS) of 18S–26S rDNA: congruence of ETS and ITS trees of Calycadenia (Compositae). Mol Phylogenet Evol 10:449–463
Bartolucci F, Peruzzi L, Galasso G et al (2018) An updated checklist of the vascular flora native to Italy. Plant Biosyst 152:179–303
Birks HH (2008) The late-quaternary history of arctic and alpine plants. Plant Ecol Divers 1:135–146
Blanca G, Cueto M (1992) In Numeros cromosomaticos de plantas occidentales. Ann Jard Bot Madrid 50:83
Bobo-Pinilla J, Salmerón-Sánchez E, Mendoza-Fernández AJ et al (2022) Conservation and phylogeography of plants: from the Mediterranean to the rest of the world. Diversity 14:78
Bonafede F, Vigonelli M, Alessandrini A (2013) Tephroseris balbisiana (DC.) Holub. In: Actaplantarum Notes 1. ArabAFenice, 82
Bono G (1967) Nota sui raggruppamenti a “Senecio balbisianus” DC e “Peucedanum ostruthium” Koch. del versante italiano del Massiccio cristallino dell’Argentera. Nuovo Giorn Bot Ital NS 101:409
Boucher FC, Thuiller W, Roquet C et al (2012) Reconstructing the origins of high-alpine niches and cushion life form in the genus Androsace sl (Primulaceae). Evol Int J Org Evol 66:1255–1268
Bouckaert R, Vaughan TG, Barido-Sottani J et al (2019) BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLOS Comput Biol 15:e1006650. https://doi.org/10.1371/journal.pcbi.1006650
Braunisch V, Coppes J, Arlettaz R et al (2013) Selecting from correlated climate variables: a major source of uncertainty for predicting species distributions under climate change. Ecography (cop) 36:971–983. https://doi.org/10.1111/j.1600-0587.2013.00138.x
Brun P, Thuiller W, Chauvier Y, Pellissier L, Wüest RO, Wang Z, Zimmermann NE (2020) Model complexity affects species distribution projections under climate change. J Biogeogr 47:130–142
Cañadas EM, Fenu G, Peñas J et al (2014) Hotspots within hotspots: endemic plant richness, environmental drivers, and implications for conservation. Biol Conserv 170:282–291. https://doi.org/10.1016/j.biocon.2013.12.007
Chater AO, Walters SM (1976) Senecio L. Flora Eur 4:191–205
Christie C, Caetano S, Aeschimann D, Kropf M, Diadema K, Naciri Y (2014) The intraspecific genetic variability of siliceous and calcareous Gentiana species is shaped by contrasting demographic and re-colonization processes. Mol Phylogenet Evol 70:323–336
Cornuet J-M, Santos F, Beaumont MA et al (2008) Inferring population history with DIYABC: a user-friendly approach to approximate Bayesian computation. Bioinformatics 24:2713–2719
Cornuet J-M, Ravigné V, Estoup A (2010) Inference on population history and model checking using DNA sequence and microsatellite data with the software DIYABC (v1. 0). BMC Bioinf 11:1–11
Cornuet J-M, Pudlo P, Veyssier J et al (2014) DIYABC v2.0: a software to make approximate Bayesian computation inferences about population history using single nucleotide polymorphism, DNA sequence and microsatellite data. Bioinformatics 30:1187–1189
Cufodontis G (1933) Kritische Revision von Senecio sect. Tephroseris Beih Reper Spec Nov Regni Veg 70:
Darriba D, Taboada GL, Doallo R, Posada D (2012) JModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772
Diadema K, Bretagnolle F, Affre L, Yuan YM, Médail F (2005) Geographic structure of molecular variation of Gentiana ligustica (Gentianaceae) in the Maritime and Ligurian regional hotspot, inferred from ITS sequences. Taxon 54:887–894
Dixon CJ, Schönswetter P, Vargas P, Ertl S, Schneeweiss GM (2009) Bayesian hypothesis testing supports long-distance Pleistocene migrations in a European high mountain plant (Androsace vitaliana, Primulaceae). Mol Phylogenet Evol 53:580–591
Doležel J, Greilhuber J, Suda J (2007) Estimation of nuclear DNA content in plants using flow cytometry. Nat Protoc 2:2233–2244
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32(5):1792–1797. https://doi.org/10.1093/nar/gkh340
Ehlers J, Ehlers J, Gibbard PL, Hughes PD (2011) Quaternary glaciations-extent and chronology: a closer look. Elsevier
Fagundes NJR, Ray N, Beaumont M et al (2007) Statistical evaluation of alternative models of human evolution. Proc Natl Acad Sci 104:17614–17619
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315. https://doi.org/10.1002/joc.5086
García-Ruiz JM, Valero-Garcés BL, Martí-Bono C, González-Sampériz P (2003) Asynchroneity of maximum glacier advances in the central Spanish Pyrenees. J Q Sci 18:61–72
Gent PR, Danabasoglu G (2011) Response to increasing Southern Hemisphere winds in CCSM4. J Clim 24:4992–4998
Givnish TJ (2010) Ecology of plant speciation. Taxon 59:1326–1366
Goczał J, Oleksa A, Rossa R et al (2020) Climatic oscillations in Quaternary have shaped the co-evolutionary patterns between the Norway spruce and its host-associated herbivore. Sci Rep 10:1–14
Gouy M, Guindon S, Gascuel O (2010) SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224
Greiner R, Vogt R, Oberprieler C (2012) Phylogenetic studies in the polyploid complex of the genus Leucanthemum Mill (Compositae, Anthemideae) based on cpDNA sequence variation. Plant Syst Evol 298:1407–1414
Guerrina M, Theodoridis S, Minuto L et al (2022) First evidence of post-glacial contraction of Alpine endemics: Insights from Berardia subacaulis in the European Alps. J Biogeogr 49:79–93
Guisan A, Thuiller W, Zimmermann NE (2017) Habitat suitability and distribution models: with applications in R. Cambridge University Press
Gutiérrez Carretero L, Fuentes Carretero J, Cueto Romero M, et al (2019) Top ten de las plantas más amenazadas de Andalucia Oriental: taxones endémicos y no endémicos. Acta Bot Malacitana 44:5–33. https://doi.org/10.24310/abm.v44i0.5636
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36
Harrison S, Noss R (2017) Endemism hotspots are linked to stable climatic refugia. Ann Bot 119:207–214
Huelsenbeck JP, Ronquist F (2001) MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Jayasena AS, Fisher MF, Panero JL et al (2017) Stepwise evolution of a buried inhibitor peptide over 45 My. Mol Biol Evol 34:1505–1516. https://doi.org/10.1093/molbev/msx104
Jukes TH, Cantor CR et al (1969) Evolution of protein molecules. Mamm Protein Metab 3:21–132
Kadereit JW, Laux P, Dillenberger MS (2021) A conspectus of Tephroseris (Asteraceae: Senecioneae) in Europe outside Russia and notes on the decline of the genus. Willdenowia 51:271–317
Kadereit JW (2023) Adaptive evolutionary divergence of populations persisting in warming cold-stage refugia: candidate examples from the periphery of the European Alps. Alp Bot 133:1–10. https://doi.org/10.1007/s00035-022-00291-0
Kochjarová J (2005) Scilla bifolia group in the Western Carpathians and adjacent part of the Pannonian lowland: annotated chromosome counts. Preslia 77:317–326
Kumar S, Stecher G, Suleski M, Hedges SB (2017) TimeTree: a resource for timelines, timetrees, and divergence times. Mol Biol Evol 34:1812–1819
Liu Y, Yang QE (2011) Cytology and its systematic implications in Sinosenecio (Senecioneae-Asteraceae) and two closely related genera. Plant Syst Evol 291:7–24
Loidi J, Campos JA, Herrera M, Biurrun I, García-Mijangos I, García-Baquero G (2015) Eco-geographical factors affecting richness and phylogenetic diversity patterns of high-mountain flora in the Iberian Peninsula. Alp Bot 125:137–146
Markos S, Baldwin BG (2001) Higher-level relationships and major lineages of Lessingia (Compositae, Astereae) based on nuclear rDNA internal and external transcribed spacer (ITS and ETS) sequences. Syst Bot 26:168–183
Martìn-Bravo S, Valcárcel V, Vargas P, Luceño M (2010) Geographical speciation related to Pleistocene range shifts in the western Mediterranean mountains (Reseda sect. Glaucoreseda, Resedaceae). Taxon 59:466–482
Martinez-Garcia F (2008) Catálogos de amenaza vs catálogos de protección. El ejemplo de Senecio coincyi. Conserv Veg 12:18
Martinez-Garcia F, Guerrero-Garcia S, Pérez-Garcia F (2012) Evaluation of reproductive success and conservation strategies for Senecio coincyi (Asteraceae), a narrow and threatened species. Aust J Bot 60:517–525
Médail F, Baumel A (2018) Using phylogeography to define conservation priorities: the case of narrow endemic plants in the Mediterranean Basin hotspot. Biol Conserv 224:258–266
Médail F, Diadema K (2009) Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J Biogeogr 36:1333–1345. https://doi.org/10.1111/j.1365-2699.2008.02051.x
Médail F, Quezel P (1997) Hot-spots analysis for conservation of plant biodiversity in the Mediterranean Basin. Ann Missouri Bot Gard 84:112. https://doi.org/10.2307/2399957
Medail F, Quezel P (1999) Biodiversity hotspots in the Mediterranean Basin: setting global conservation priorities. Conserv Biol 13:1510–1513
Molero J, Marfil JM (2017) Betic and Southwest Andalusia. The Vegetation of the Iberian Peninsula. Springer, New York, pp 143–247
Molina-Venegas R, Aparicio A, Lavergne S, Arroyo J (2017) Climatic and topographical correlates of plant palaeo-and neoendemism in a Mediterranean biodiversity hotspot. Ann Bot 119:229–238
Murín A (1960) Substitution of cellophane for glass covers to facilitate preparation of permanent squashes and smears. Stain Technol 35:351–353
Nieto Feliner G, Cellinese N, Crowl AA, Frajman B (2023) Editorial: understanding plant diversity and evolution in the Mediterranean Basin. Front Plant Sci 14:1152340
Noble V, Diadema K (2011) La flore des Alpes-Maritimes et de la Principauté de Monaco. Naturalia Publications, Turriers
Nordenstam B (2007) Senecioneae. In: Kadereit JW, Jeffrey C (eds) The families and genera of vascular plants, flowering plants. Eudicots. Asterales, vol 8. Springer, Berlin, pp 208–241
Nordenstam B, Pelser PB (2011) Notes on the generic limits of Sinosenecio and Tephroseris (Compositae-Senecioneae)
Olšavská K, Šingliarová B, Kochjarova J et al (2015) Exploring patterns of variation within the central-European Tephroseris longifolia agg.: karyological and morphological study. Preslia 87:163–194
Otto-Bliesner BL, Joussaume S, Braconnot P, et al (2009) Modeling and data syntheses of past climates: paleoclimate modeling intercomparison Project Phase II Workshop; Estes Park, Colorado, 15--19 Sept= 2008
Owens HL, Campbell LP, Dornak LL et al (2013) Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol Modell 263:10–18
Padilla-García N, Machon N, Segarra-Moragues JG, Martínez-Ortega MM (2021) Surviving in southern refugia: the case of Veronica aragonensis, a rare endemic from the Iberian Peninsula. Alp Bot 131:161–175
Parisod C (2022) Plant speciation in the face of recurrent climate changes in the Alps. Alp Bot 132:21–28
Pellissier L, Bråthen KA, Vittoz P et al (2013) Thermal niches are more conserved at cold than warm limits in arctic-alpine plant species. Glob Ecol Biogeogr 22:933–941
Pelser PB, Nordenstam B, Kadereit JW, Watson LE (2007) An ITS phylogeny of tribe Senecioneae (Asteraceae) and a new delimitation of Senecio L. Taxon 56:1077–1104
Pelser PB, Kennedy AH, Tepe EJ, et al (2010) Patterns and causes of incongruence between plastid and nuclear Senecioneae (Asteraceae) phylogenies. Am J Botany 97:856–873. https://doi.org/10.3732/ajb.0900287
Peñas J, Cañadas E, Del Rio J (2019) Fitogeografìa de Sierra Nevada e implicaciones para la conservación. Biol la Conserv plantas en Sierra Nevada Principios y retos para su Preserv. Universidad de Granada, Granada, pp 81–116
Peredo EL, Revilla MÁ, Jiménez-Alfaro B et al (2009) Historical biogeography of a disjunctly distributed, Spanish alpine plant, Senecio boissieri (Asteraceae). Taxon 58:883–892
Pignatti S, Guarino R, La Rosa M (2017) Flora d’Italia, 2nd edn. Edagricole Calderini, Bologna
Rouy MG (1890) Diagnoses de plantes nouvelles pour la flore européenne. Bull La Société Bot Fr 37:162–168
Ren C, Hong Y, Wang L, Yang Q-E (2017) Generic recircumscription of Parasenecio (Asteraceae: Senecioneae) based on nuclear ribosomal and plastid DNA sequences, with descriptions of two new genera. Bot J Linn Soc 184:418–443. https://doi.org/10.1093/botlinnean/box034
Sánchez-Villegas RS, de la Peña BQ, Villegas MS et al (2022) Novedades corológicas y nomenclaturales para la flora vascular de la Sierra de Gredos (Sistema Central), III. Flora Montiberica 82:24–30
Sandel B, Arge L, Dalsgaard B et al (2011) The influence of late quaternary climate-change velocity on species endemism. Science (80-) 334:660–664
Schmitt T, Muster C, Schönswetter P (2010) Are disjunct alpine and arctic-alpine animal and plant species in the western Palearctic really “relics of a cold past”? Relict species: phylogeography and conservation biology. Springer, Berlin Heidelberg, pp 239–252
Schönswetter P, Suda J, Popp M et al (2007) Circumpolar phylogeography of Juncus biglumis (Juncaceae) inferred from AFLP fingerprints, cpDNA sequences, nuclear DNA content and chromosome numbers. Mol Phylogenet Evol 42:92–103
Skokanová K, Šingliarová B, Kochjarová J, Paule J (2019) Nuclear ITS and AFLPs provide surprising implications for the taxonomy of Tephroseris longifolia agg. and the endemic status of T. longifolia subsp. moravica. Plant Syst Evol 305:865–884
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. https://doi.org/10.1093/bioinformatics/btu033
StatSoft Incorporated (2013) Electronic Statistics Textbook. StatSoft, Tulsa. http://www.statsoft.com/textbook
Stucky BJ (2012) SeqTrace: a graphical tool for rapidly processing DNA sequencing chromatograms. J Biomol Tech JBT 23:90
Taberlet P, Fumagalli L, Wust-Saucy A-G, Cosson J-F (1998) Comparative phylogeography and postglacial colonization routes in Europe. Mol Ecol 7:453–464
Talavera G, Castresana J (2007) Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol 56:564–577
Thompson JD (1999) Population differentiation in Mediterranean plants: insights into colonization history and the evolution and conservation of endemic species. Heredity (edinb) 82:229–236. https://doi.org/10.1038/sj.hdy.6885040
Thuiller W, Georges D, Gueguen M, et al (2021) biomod2: ensemble platform for species distribution modeling
Tutin TG, Heywood VH, Burges NA et al (1980) Flora Europaea. Cambridge University Press, Cambridge
Vargas P (2003) Molecular evidence for multiple diversification patterns of alpine plants in Mediterranean Europe. Taxon 52:463–476
Wang L-Y, Pelser PB, Nordenstam B et al (2009) Strong incongruence between the ITS phylogeny and generic delimitation in the nemosenecio-sinosenecio-tephroseris assemblage (Asteraceae: Senecioneae). Bot Stud 50:435–442
Wasof S, Lenoir J, Aarrestad PA et al (2015) Disjunct populations European vascular plant species keep the same climatic niches. Glob Ecol Biogeogr 24:1401–1412
Watanabe S, Hajima T, Sudo K et al (2011) MIROC-ESM 2010: model description and basic results of CMIP5-20c3m experiments. Geosci Model Dev 4:845–872
White TJ, Bruns T, Lee S et al (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc Guid Methods Appl 18:315–322
Xu T, Shi Z, An Z (2018) Responses of ENSO and NAO to the external radiative forcing during the last millennium: results from CCSM4 and MPI-ESM-P simulations. Quat Int 487:99–111
Zizka A, Carvalho FA, Calvente A et al (2020) No one-size-fits-all solution to clean GBIF. PeerJ 8:e9916
Zuur AF, Ieno EN (2016) A protocol for conducting and presenting results of regression-type analyses. Methods Ecol Evol 7:636–645
Acknowledgements
We thank Lenka Martonfiová for chromosome counting. As well, we are in debt with Jesus del Río and José Algarra for field works on T. elodes. Seeds of T. coincyi were provided by Banco de Germoplasma Vegetal-Universidad Politécnica de Madrid “César Gómez Campo” (ESP003), Accession number: BGV-UPM 7610. We thank Izai Kikuchi for the English review of the manuscript. The authors acknowledge the support of NBFC to University of Torino (DBIOS), funded by the Italian Ministry of University and Research, PNRR, Missione 4 Componente 2, “Dalla ricerca all’impresa”, Investimento 1.4, Project CN00000033.
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adamo, M., Skokanová, K., Bobo-Pinilla, J. et al. Molecular evidence and environmental niche evolution at the origin of the disjunct distribution in three mountain endemic Tephroseris (Asteraceae) of the Mediterranean basin. Alp Botany 133, 117–133 (2023). https://doi.org/10.1007/s00035-023-00300-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00035-023-00300-w