Abstract
Road salt (NaCl) is commonly used as a deicer during winter to improve road safety, resulting in freshwater salinization. Such elevated chloride concentrations may have a strong effect on zooplankton, which are key elements in freshwater food webs. The aim of this study was to investigate the effect of chloride ion concentration and inter-pond environmental variability on zooplankton density in four urban ponds over 3 years differing in winter conditions. Analysis of variance showed significant differences in chloride ion concentration and zooplankton density regarding study year and ponds. Redundancy analysis of column water data showed that both the environmental variables (including chloride ion concentration) and the pond location significantly contributed to the model explaining the variability in zooplankton densities. However, the environment variable (referring to the environmental variables) had a smaller contribution (11%) than the POND variable (related to the spatial configuration of the ponds), which contributed 62%, indicating that the dynamics of zooplankton density depended primarily on ecosystem characteristics. Even so, although chloride content was not the main determinant of zooplankton density, their presence in aquatic ecosystems and potential interactions with other types of pollutants could have negative biological and ecological effects.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
To ensure road safety in regions that experience harsh winters, road salt, typically sodium chloride (NaCl), is commonly applied during cold periods. The growth in popularity of sodium chloride-based deicers can be attributed to their relatively low cost compared to other salts (such as calcium chloride or magnesium chloride) as well as their high effectiveness.
Although road salt is important to maintain road safety, there are drawbacks to its usage. Road salt can enter groundwater with runoff, leading to elevated chloride concentrations in drinking water wells (Bester et al., 2006) and surface waters. Elevated concentrations of chloride ions have been observed in lakes (Scott et al. 2019; Winter et al., 2011; Rogora et al., 2015), stormwater retention ponds (Gallagher et al., 2011; Tixier et al., 2012) and rivers (Hubbart et al., 2017; Corsi et al., 2015). Consequently, road salt application is one of the key causes of freshwater salinization syndrome (Cunillera-Montcusí et al., 2022; Kaushal et al., 2021), resulting in increasing salinization: an emerging threat to freshwater biodiversity (Reid et al., 2019).
In freshwater ecosystems, road salt-induced salinity has negative effects on zooplankton and various trophic interactions. On the species level, Martínez-Jerónimo (2007) observed that increasing concentrations of NaCl (between 2 and 8 g/l NaCl) negatively impacted the clutch size, total progeny, longevity, average lifespan and life expectancy, net reproduction rate (R0), population growth rate, as well as survivorship of Daphnia magna exposed to salinity. Similarly, survivorship, total progeny and R0 of Scapholeberis mucronata and Simocephalus vetulus were shown to decrease at NaCl concentrations between 500 to 625 mg/l (Gökçe and Özhan, 2014). Huber and co-workers (2023) found that chloride concentrations reaching the chronic (230 mg/l) and acute (860 mg/l) threshold levels reduced Daphnia mendotae vertical movement rate by 22–47%. Moreover, increased salinity has been shown to affect zooplankton communities and their structure. Elevated concentrations of chloride ions tested in mesocosm studies decreased the abundance of cladocerans and copeopods (Lind et al., 2018), triggered a trophic cascade when present together with fish predators (Hintz et al., 2017) and, at high Cl concentrations > 1000 mg/l, caused a shift towards rotifer-dominated communities (Van Meter and Swan, 2014). However, results from laboratory experiments suggest that Daphnia populations may develop adaptations to high concentrations of sodium chloride to increase their survival in high salinity habitats (Parlato and Kopp, 2020). It is crucial to assess whether, and to what extent, road salt affects zooplankton communities in their natural environment; it is also important to understand both the differential adaptive capacity of the organisms and the particularly significant regulatory role of zooplankton in freshwater ecosystems. Unfortunately, the effects of winter road salt application on zooplankton species are difficult to verify in the wild. This is particularly so in temperate regions where zooplankton rarely occurs outside the growing season as overwintering populations.
The present study attempts to fill this knowledge gap, also highlighted by Cunillera-Montcusí et al. (2022). Its main goal was to examine the effect of chloride concentrations on zooplankton density observed in four urban ponds of different environmental conditions located in the city of Lodz, Poland. The main focus is on chlorides because they are a component of the most commonly used de-icing salts, such as sodium chloride (NaCl), magnesium chloride (MgCl2) and calcium chloride (CaCl2), thus being the most representative signal of road salt contamination (Woodley et al., 2023). The research was conducted in 3 weather-differentiated years: 2019 being a typical winter in Poland, 2020 having an exceptionally mild winter without snow days and 2021 with a harsh, cold and snowy winter. We hypothesized that zooplankton density will be related to chloride ion concentrations in the water, as higher concentrations of chloride in harsher winters will reduce the density of zooplankton organisms, and that this effect may be modified by pond-specific environmental conditions. Furthermore, we postulate that, due to the different winter weather conditions in each year of the study, as well as the different characteristics of each pond, there will be a significant effect of pond and year and pond/year interaction on the concentration of analysed ions and zooplankton density.
The study was conducted over 3 full hydrological years to verify changes in road salt concentrations in aquatic ecosystems, during both the winter (resting) and growing season.
Materials and methods
Study area
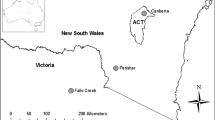
The study was performed in four urban ponds located in the city of Lodz, central Poland (51°45′ N, 19°28′ E). The first pond, Arturówek Górny (AG), on the Bzura River, is located in Arturówek—a recreational complex situated on the edge of Łagiewnicki Forest (Fig. 1a). In 2010–2015 the EH-REK project “Ecohydrological rehabilitation of recreational reservoirs in Arturówek” (Łódź) as a model approach to the rehabilitation of urban reservoirs” was responsible for the implementation of nature-based solutions in this area, improving water quality in the pond (Jurczak et al., 2019). Our sampling site was located at the pond inlet. The area around the pond receives a lower amount of road salt than the rest of the selected sites, as the use of road salt was prohibited on the roads in this area; the only likely source was surface transport by vehicles. Arturówek Górny pond also served as the reference ecosystem in our study.
The second urban pond, Zbiornik Wasiaka (ZW), on the Sokołówka River, is located in a residential area consisting of single-family houses (Fig. 1b). The third urban pond, Julianów Górny (JG), again on the Sokołówka River, is located in Adam Mickiewicz Park, close to two paved alleys (Fig. 1c). The fourth urban pond, Zbiornik Zgierska (ZZ), on the Sokołówka River, is located downstream from the Adam Mickiewicz Park area; it is separated from the park by a two-lane street with moderate traffic that runs near to the pond (Fig. 1d). The surface area, mean depth and capacity of each urban pond are presented in Table S1, Supplementary Material.
In Poland, the city municipalities are not required to collect information about the amounts of road salt used in winter. However, we were able to acquire this information for part of the city where, during the winter season 2020/2021, approximately 6.5 tonnes of NaCl was used per kilometer of traffic lane. Data for other seasons were not available.
Maps of the studied ponds and their area. a. AG = Arturówek Górny (2017), b. ZW = Zbiornik Wasiaka, c. JG = Julianów Górny, d. ZZ = Zbiornik Zgierska. Coordinates of the Łódź city as well as all of the sampling points are presented in the decimal degrees format. Source: https://geoportal.lodzkie.pl/imap
Meteorological data source and analyses
Daily meteorological data (precipitation and temperature) were taken from national monitoring networks held by the Institute of Meteorology and Water Management-National Research Institute. The nearest station was Lodz-Lublinek (ID 351190465). To determine the long-term averages and compare them with the state of the weather during the study period, the data for the period 1951–2020 were taken from the website https://meteomodel.pl/, which presents meteorological data from national monitoring programs.
Surface and pore water sampling and analyses
Field monitoring was conducted for three hydrological years: 2018/2019, 2019/2020 and 2020/2021. According to the definition formulated by the Polish Institute of Meteorology and Water Management-National Research Institute, the hydrological year, by convention, begins on 1 November and lasts until 31 October, which serves as the basis for calculating the country's water balance. The end of October/November usually marks the end of rainwater run-off from summer-autumn heavy rainfall and storms, and thus the beginning of the autumn-winter period, when a drop in temperature promotes the development of ice phenomena, and the likelihood of snowfall increases. Column water and pore water samples were taken twice a month for the whole year; samples were taken more often during melting periods to determine maximum chloride concentrations and the period of time for which they remained elevated. Column water samples were taken 20 cm below the surface. Sediment samples were taken from the top 20 cm of sediments by sediment coring device, and then pore water was extracted by centrifugation (3000 rpm for 10 min) in the laboratory. Column and pore water were analysed in a Dionex® ion chromatograph with a cation column (CG18, IonPac CS18, CSRS-ULTRA II) and an anion column (AG22, IonPac AS22, ASRS e ULTRA II) to determine the concentrations of chlorides, sodium, potassium, magnesium, calcium, nitrates, sulphates, ammonium and phosphates.
Zooplankton sampling and analyses
The zooplankton samples were taken every 2 weeks, from the start of the calendar spring until the end of calendar autumn each year. Samples were collected in the limnetic zone at the inflow part of each pond. Water was taken from the 1-m layer below the surface of from each urban pond (sample size 20 l) and then filtered through a plankton net with 50-µm mesh size. In the laboratory, the samples were concentrated to 10 ml and fixed using Lugol’s solution. The zooplankton were subjected to qualitative and quantitative analyses using a glass Sedgewick-Rafter chamber with a 1-ml capacity and a Nikon microscope (magnification 40−100×). When the abundance of individuals of a given taxonomic group was high (> 150 individuals per subsample), all zooplankton animals in the chamber were counted as standard (the contents of a single chamber were counted). When the abundance of zooplankton in one subsample was lower, a second subsample was taken and individuals from the next chamber were counted. The density of each found species (for cladocerans and rotifers) or orders (for copepods) was calculated using the equation: N = XVz / Vk Vp, where X = number of counted individuals in the chamber; Vz = volume of the density from which the sample for microscopic determination was taken (ml); Vk = volume of the chamber (ml); Vp = volume of the compacted sample (l).
For copepods, the organisms were only grouped based on their order (Calanoida or Cyclopoida). Copepod nauplii were counted separately and were not included in the statistical analyses. Other copepod life stages were not differentiated and counted. Morphological analyses of the collected individuals were performed according to Amoros (1984), Benzie (2005) and Ejsmont-Karabin et al. (2004).
Statistical analyses
Statistical analyses were performed using both Statistica version 13.3 software (StatSoft) (for ANOVA) and CANOCO v. 5.15. (Microcomputer Power, Ihhaca, NY, USA) (for RDA). The Shapiro-Wilk normality test was used to determine the data distribution. Parametric tests were used for further analyses (one-way ANOVA and two-way ANOVA, with Tukey’s range test as a post hoc test). One-way ANOVA was applied to test the effect of year on the concentration of ions in each individual pond. To test for the effect of years and ponds and their interactions on differences in ion concentration and zooplankton density, we used two-way ANOVA with the years and ponds as categorical variables and ion concentration or zooplankton densities (total and for selected zooplankton groups) as dependent variables. Due to the large differences between years and ponds, the data used for the ANOVA tests were transformed according to the ln(x + 1) equation. Results were considered significant if the p-value was < 0.05.
Redundancy analysis (RDA) method was applied to examine the relationships between zooplankton density and environmental variables. RDAs were selected because they enabled the compilation of all selected taxa and environmental parameters (ter Braak i Šmilauer, 2012).
For pore water samples, 12 quantitative variables and one nominal variable (POND) were analysed. Quantitative variables were: average temperature on the day of sampling [temp (d)], average temperature of 7 days before sampling [temp (7)], total precipitation on the day of sampling [precip (1)], total precipitation within 7 days before sampling [precip (7)], chloride [chlorid], phosphates [phospha], sulphates [sulph], sodium [Na], ammonia [amo], potassium [K], magnesium [Mg] and calcium [Ca]. For column water samples, ten quantitative variables and one nominal variable (POND) were analysed. Quantitative variables were: average temperature on the day of sampling [temp (d)], average temperature of 7 days before sampling [temp (7)], total precipitation on the day of sampling [precip (1)], total precipitation within 7 days before sampling [precip (7)], chloride [chlorid], sulphates [sulph], sodium [Na], potassium [K], magnesium [Mg] and calcium [Ca]. To avoid multicollinearity of variables, the most strongly correlated variables were eliminated based on the Pearson correlation matrix (Pearson's correlation coefficient r > 0.8). For both groups of samples, i.e., samples from pore water and samples taken from the column water, the Na variable was strongly correlated with the concentration of Cl ions (chloride), and the temp (7) variable was correlated with the temp (d); therefore, the Na and temp (7) variables were omitted from further analysis. In both cases, the Pearson correlation coefficients were > 0.9.
Further analysis of the collected material was initiated through gradient length calculations via detrended canonical correspondence analysis (DCCA). The length of the longest gradient in the DCCA was equal to 1.0. Therefore, to assess the dependence of zooplankton density on environmental variables, the redundancy analysis (RDA) method was applied. The significance of the RDA axes in explaining the density of zooplankton taxonomic groups was determined, as was the significance of the effects of particular variables on the model of ordering, which was based on the Monte Carlo test with 999 random permutations. The RDA biplot displays the zooplankton and environmental variables as vectors, and dummy environmental variables are displayed as points. The data were log(x + 1)-transformed, and, in the RDA, centering of the zooplankton data was applied.
To isolate and determine the influence of the location of a given pond on the community of zooplankton in the model, the POND variable was considered a covariate. RDA was first conducted using the entire data set. Subsequently, to isolate the amount of variation explained by pond location effects, we used a partial RDA (ter Braak and Šmilauer, 2012), in which the variable POND served as a covariate, and the ordination was only constrained by environmental variables.
As a complement to the above analysis, variation partitioning for the two groups (fractions) was used (Lepš and Šmilauer, 2012). The first group was the POND (i.e., zooplankton density ~ POND), and the second group was the other variables describing the environmental condition (i.e., zooplankton density ~ the environment variable). The additional fraction was the shared part (i.e., zooplankton density ~ POND + environment).
Biodiversity indexes, including Dominance Index (D), Simpson’s Index of Diversity (D’) and Shannon-Wiener Diversity Index (H’), were calculated using the Past 4.03. software. Indexes were calculated for total zooplankton as well as two zooplankton groups: cladocerans and rotifers.
Results
Meteorological data
Meteorological data for the city of Lodz, the site of the present research, has been collected since 1951 by the national monitoring service. Some base meteorological statistics were obtained daily (temperature, precipitation and snowcover) for the investigated hydrological years. These are compared to multiyear average values (1951–2020) in Table S2 (Supplementary Material).
All 3 hydrological years had lower year sum of precipitation, shorter non-vegetation period (daily mean temperature < 5 °C—range in Poland climate zone), shorter winter (daily mean temperature < 0 °C) and higher daily mean temperature than the 1951–2020 multiyear average. A cooler period was noted in hydrological year 2019/2020, which was also characterized by the lowest number of days with meteorological winter (daily mean temperature < 0 °C), days of non-vegetation period, total precipitation in non-vegetation period, total precipitation in meteorological winter and days with snow cover.
Water quality of the analysed urban ponds
Changes in chloride concentrations during the hydrological years
Chloride ion concentrations measured in water column and pore water during 3 hydrological years in the four urban ponds are depicted in Figs. 2 and 3 with the values given after the ln(x + 1) transformation.
Chloride concentrations exceeded the 640 mg/l acute toxicity threshold and persisted for the longest time in the pore water in the ZW pond during the year 2021. These high chloride concentrations were maintained for 124 days, 62 of which were winter days, and the rest were spring days. Chloride concentrations exceeding the 120 mg/l chronic toxicity threshold persisted for the longest time in pore water in both JG and ZZ ponds during the year 2020. In both ponds they were maintained for 348 days, 91 of which were winter days.
Mean chloride and sodium ion concentrations in different years and urban ponds
Over the 3-year study period, the highest mean concentrations of chloride and sodium in column water (as an average for all ponds) were measured in 2021, the year with the coldest winter (mean: 424.26 mg/l and 119.83 mg/l, respectively). Also, high concentrations were measured in 2019 (mean: 335.39 mg/l Cl− and 155.78 mg/l Na+), with the lowest mean concentrations observed in 2020 (61.76 mg/l Cl− and 25.95 mg/l Na+). Two-way ANOVA analyses confirmed significant differences in chloride (p < 0.001) (Fig. 4a, Fig. S1a, Supplementary Material) and sodium (p < 0.001) (Fig. 4c, Fig. S2a, Supplementary Material) concentrations in column water between years.
The mean chloride and sodium concentrations measured in pore water were the highest in 2021 (mean: 1259.97 mg/l and 355.36 mg/l respectively), followed by 2019 (mean: 303.21 mg/l Cl− and 142.66 mg/l Na+) and 2020 (mean: 141.86 mg/l Cl− and 53.53 mg/l Na+). Two-way ANOVA analyses showed significant differences for chloride (p < 0.001) (Fig. 4b, Fig. S1c, Supplementary Material) and sodium (Fig. 4d, Fig. S2c, Supplementary Material) (p < 0.001) concentrations measured in pore water between the years.
The highest mean chloride and sodium ion concentrations in column water were observed in the ZW pond (mean: 650.54 mg/l and 238.42 mg/l respectively), followed by the JG pond (mean: 416.98 mg/l Cl− and 129.91 mg/l Na+), ZZ pond (average: 207.6 mg/l Cl− and 72.84 mg/l Na+) and lastly the reference pond AG (mean: 59.49 mg/l Cl− and 21.89 mg/l Na+); no distinction was found between years. Two-way ANOVA analyses confirmed significant differences in chloride (p < 0.001) (Fig. 4a, Fig. S1b, Supplementary Material) and sodium (p < 0.001) (Fig. 4c, Fig. S2b, Supplementary Material) ion concentrations in column water between the urban ponds.
The highest mean pore water chloride and sodium concentrations were found in the ZW (1757.69 mg/l Cl− and 544.31 mg/l Na+), followed by JG (619.63 mg/l Cl− and 150.41 mg/l Na+), ZZ (406.22 mg/l Cl− and 140.69 mg/l Na+), with the lowest concentrations measured in AG (89.61 mg/l Cl− and 34.9 mg/l Na+). Two-way ANOVA analyses confirmed significant differences in chloride (p < 0.001) (Fig. 4b, Fig. S1d, Supplementary Material) and sodium (p < 0.001) (Fig. 4d, Fig. S2d, Supplementary Material) ion concentrations in pore water between the urban ponds.
Significant year*pond interactions were observed for both chloride (Fig. 4a, b) and sodium (Fig. 4c, d) ion concentrations measured in both column and pore water (p = 0.01 for Cl− and p = 0.02 for Na+ in column water; p < 0.001 for Cl− and p < 0.001 for Na+ in pore water).
The mean, minimum, maximum and standard deviation values of the concentrations of chloride, sodium and other ions (nitrate, phosphate, sulphate, ammonium, potassium, magnesium and calcium) observed during 3 hydrological years, as well as in the four ponds, are shown in Tables S3, S4 and S5 in the Supplementary Material.
Changes in chloride ion concentrations measured in the column water in the studied urban ponds (a, b, c, d) during the 3 hydrological years: 2019 (solid line), 2020 (broken line), 2021 (dotted line). Horizontal lines indicate the limit values for chronic and acute toxicities of chlorides in surface water, as established in Canada (CEQG, 2011) and the USA (US EPA, 1988). The grey area indicates the astronomical winter period. All of the data had been transformed according to the ln(x + 1) equation
Changes in chloride ion concentrations measured in the pore water in the studied urban ponds (a, b, c, d) during the 3 hydrological years: 2019 (solid line), 2020 (broken line), 2021 (dotted line). Horizontal lines indicate the limit values for chronic and acute toxicities of chlorides in surface water as established in Canada (CEQG, 2011) and the USA (US EPA, 1988). The grey area indicates astronomical winter period. All of the data had been transformed according to the ln(x + 1) equation
Chloride (a, b) and sodium (c, d) concentration (ln(x + 1) mg/l) measured in the column and pore water of each pond and each year. Vertical bars represent 0.95 confidence intervals. Uppercase letters indicate significant differences between years; lowercase letters indicate significant differences between ponds
Zooplankton structure and density
In general, the highest mean total zooplankton densities were observed in ZZ (2742.83 specimens/l in 2019, 1989.92 specimens/l in 2020 and 1527.61 specimens/l in 2021).
The mean values in the reference AG were 254.36 specimens/l in 2019, 353.5 specimens/l in 2020 and 190.29 specimens/l in 2021. The lowest mean densities were observed in JG (42.33 specimens/l in 2019, 17.92 specimens/l in 2020 and 10.36 specimens/l in 2021) as well as in ZW (135.36 specimens/l in 2019, 7.38 specimens/l in 2020 and 2.75 specimens/l in 2021).
Two-way ANOVA for the total zooplankton density showed significant differences between years (p < 0.01) and urban ponds (p < 0.001). In 2019, total zooplankton density was significantly higher than in 2020 or 2021, with no significant difference between 2020 and 2021 (Fig. 5a). Total zooplankton density was significantly higher in ZZ than in AG, ZW and JG; in addition, total zooplankton density was also significantly higher in AG than in JG and ZW (Fig. 5b).
Mean density of total zooplankton, copepods, cladocerans and rotifers observed during the 3 hydrological years (a) and in four studied urban ponds (b). All of the data had been transformed according to the ln(x + 1) equation. Vertical bars represent 0.95 confidence intervals. Different capital letters indicate significant differences between years and urban ponds (p < 0.05)
Two-way ANOVA analysis confirmed the presence of significantly more copepods and cladocerans in 2019 than in 2020 (p = 0.01), with no significant differences between 2019 and 2021, or between 2020 and 2021. For the rotifers, no significant difference in density was observed between years (Fig. 5a). All three groups of zooplankton were the most abundant in ZZ (copepods p < 0.001, cladocerans p < 0.001, rotifers p < 0.001). There were also significantly more rotifers in AG compared with JG (p = 0.01) and ZW (p < 0.001). AG demonstrated higher density of cladocerans and copepods than ZW (p = 0.02 and p = 0.02, respectively) (Fig. 5b). For total zooplankton and rotifer densities, there was no interaction between ponds and years (p = 0.745 and p = 0.265, respectively). For copepod and cladoceran density, interactions were significant (p < 0.05).
In terms of zooplankton community structure, the urban ponds studied were mainly dominated by rotifers, with the exception of ZZ during the years 2019 and 2020, when the distribution of rotifers and cladocerans was nearly even. Species that dominated most frequently were Keratella quadrata and K. cochlearis (as well as K. cochlearis in its tecta form, albeit to a lesser extent).
Additional information on the characteristics of the zooplankton communities of the studied ecosystems, including the list of all observed species and selected biodiversity indexes, is presented in the supplementary material (Table S6, Table S7) together with graphs showing changes in zooplankton densities during the study period (Fig. S3).
Relationship between zooplankton and environmental parameters
For the RDA data collected for the column water, the first ordination axis explained 52.8% of the variability in zooplankton community density. Pond ZZ was most strongly positively correlated with the first axis. The second axis described 2.4% of the observed variability and was related to the Ca variable (Fig. S4a, Supplementary Material). Pond ZZ, Pond AG, K ion concentration and Cl ion concentration had a statistically significant effect (Monte Carlo test) on the model examining the relationships between zooplankton density and environmental variables (Table S8, Supplementary Material). In the partial RDA, the variables K ion concentration, Cl ion concentration and average temperature on the day of sampling [temp (d)] had statistically significant impacts on the model (Fig. 6a). The variables K ion concentration, Cl ion concentration and temp (d) had statistically significant impacts on the model.
In the RDA model for column water samples, covering all variables, cladocerans, rotifers and zooplankton were strongly positively correlated with the Pond ZZ variable. In addition, rotifers and zooplankton were negatively correlated with nitrate (Nitra) and sulphate (Sulph) variables (Fig. S4a, Supplementary Material). When the variable POND was included as a covariate, no significant correlation was found between zooplankton density and environmental variables (Fig. 6a), although the density of all zooplankton groups demonstrated clear negative correlation with chloride and a positive correlation with potassium. The variation partitioning analysis showed that both the environment variable and the pond factor significantly contributed to explaining the variability in zooplankton density. The environment variable had a smaller contribution to the explained variation (11%) than did the POND variable (62%). The mean contribution (mean square) of the POND variable was > 10 times higher than the environment variable (Table S9, Supplementary Material).
For pore water sample data, the first axis explained 47.3% of the variability in density. Pond ZZ was most strongly positively correlated with the first axis. The second axis described 2.4% of the observed variability and was related to the temp (d) variable (Fig. S4b, Supplementary Material). Pond ZZ, Pond AG and temp (d) had a statistically significant effect (Monte Carlo test) on the model that examined the relationships between zooplankton density and environmental variables (Table S10, Supplementary Material). In the partial RDA, when using the POND variable as a covariate, the first axis explained 4.9% of the variation, and the second axis described 1.6% of the variation (Fig. 6b). In the RDA model for samples from pore water, including all variables, rotifers and zooplankton were strongly positively correlated with the POND ZZ variable. In contrast, cladocerans were moderately correlated with this variable (Fig. S4b, Supplementary Material). When the POND variable was included as a covariate, no significant correlation was found between zooplankton groups and environmental variables (Fig. 6b). The variation partitioning analysis showed that only POND factors significantly contributed to explaining the variability in zooplankton density. The environment variable made a smaller contribution to the explained variation (3%) compared to the POND variable (75%) (Table S11, Supplementary Material).
Discussion
Climate change and freshwater salinization are emerging threats for freshwater biodiversity, as are the cumulative impacts of these and other stressors (Reid et al., 2019). Climate change results in not only an increase in the average temperature but also changes in global and regional water cycles (Douville et al., 2021). Studies have confirmed an ongoing increase in temperature and decrease in snow cover duration during winter in Poland (Tomczyk et al., 2021) as well as increasing precipitation during the winter season (Szwed, 2018). Our results confirm this trend, with higher daily mean temperature and lower numbers of days with snow cover (Table S2; Supplementary Material).
However, there remains a need to ensure road safety in winter by road salt application. Chloride-based deicer use represents one source of anthropogenic salinization of freshwater ecosystems. Considering its prevalence, as well as possible long-term consequences of road salt usage, the topic of road salt pollution has gained more attention in recent years (Corsi et al., 2015; Hintz and Relyea, 2019; Szklarek et al., 2022). Notably, the maximum Cl− concentrations in reservoirs after winter and their persistence are influenced by various meteorological (temperature and precipitation) and hydrological (average flow, retention time, deep) conditions. If a wet winter characterized by salt application is followed by a dry spring, the length of the period with elevated Cl− concentration may persist, thus generating greater ecotoxicity pressure on freshwater communities. The winter-spring characteristics of Cl− concentration might play a crucial role in the functioning of zooplankton and other organisms, but understanding this relationship needs long-term research. In the present study, Cl− concentrations above acute toxicity were noted until March in column water (Wasiaka [ZW] pond and Julianów [JG] pond, Fig. 2) and until June in pore water (Wasiaka [ZW] pond, Fig. 3), but longer Cl− chronic toxicity was observed in all three ponds supplied by road salt runoff.
As salinisation of water due to road salt is not a seasonal problem and can persist throughout the growing season, various studies have examined the effects of road salt pollution on individual species of zooplankton (Hintz et al., 2019; Venancio et al., 2018; Coldsnow et al., 2017) as well as freshwater food webs (Hintz and Relyea, 2017; Lind et al., 2018; Van Meter and Swan, 2011). For example, Van Meter and Swan (2014) observed that the rotifer family Brachionidae dominates zooplankton communities exposed to elevated chloride concentrations (93%), whereas the same family composed only 27% of the zooplankton community in a low chloride treatment. Van Meter and co-workers (2011) found that elevated chloride concentrations caused a decrease in copepod and cladoceran densities while not affecting the C. nauplii or rotifer densities.
The existing studies in the literature play a key role in understanding the complex effect that elevated chloride concentrations may have on zooplankton species and the structure of freshwater foodwebs. However, the majority of the studies concerning the effects of road salt on zooplankton are performed using mesocosm studies or controlled conditions (e.g., toxicity biotests). There are far fewer studies examining the effects of road salt pollution on zooplankton inhabiting natural environments. One valuable study is that of Bielańska-Grajner and Cudak (2014), who examined the effects of salinity on species diversity of rotifers inhabiting 14 anthropogenic water bodies located in Silesian Upland, Poland; however, it focuses on salinization originating from the mining industry and not road salt pollution.
This study is one of the first works to examine the effect of road salt pollution on zooplankton inhabiting the natural environment. We expected that the use of large amounts of road salt, especially during cold winters, would cause a significant increase in chloride concentrations in the studied ecosystems and affect the dynamics of zooplankton density. One of the most important results was obtained by RDA analysis, which confirmed, as we correctly assumed, that chloride concentrations may be relevant to their impact on zooplankton. However, although chlorides were the most important among all factors related to water chemistry, the dynamics of zooplankton density depended primarily on pond-specific ecosystem characteristics. RDA showed that Zgierska (ZZ) pond, Arturówek (AG) pond, potassium (K) and chloride (Cl) had a statistically significant effect on the model examining the relationships between zooplankton density and environmental variables. Potassium and chloride ion content also had a statistically significant impact on the model after using the POND variable as a covariate. Although these ions were not significantly correlated with zooplankton density in this case, a clear negative correlation was observed for all zooplankton groups with chloride. The RDA clearly showed that in the urban ponds studied, the effects of salt and other environmental factors on zooplankton density were masked by significant differences in pond characteristics.
For the Arturówek (AG) pond, characterised by the lowest road salt concentrations, the chloride and sodium ion content in column water or pore water did not appear to have any negative effect on zooplankton. RDA showed that the key factors relevant to this ecosystem were weather conditions. Thus, our results confirmed that the nature-based solutions used in our reference pond (sedimentation-biofiltration systems; Jurczak et al., 2019) effectively reduce the influx of road pollutants, including road salt, contributing to improved environmental quality.
The situation was different in the other three urban ponds, which were exposed to high road salt inputs. The RDA showed that for these water samples, the density of total zooplankton or the density of individual zooplankton groups was negatively correlated with the concentration of chloride ions, although the strength of this correlation was weakened by the significant differences in the environmental characteristics of the ponds. Notably, the correlations relate to the growing season; wintertime was excluded because of the absence of zooplankton. During winter, the chloride concentrations in the column water samples were above the acute toxicity level in all tested urban ponds in 2021, i.e., the year with high road salt use, and in Julianów (JG) pond and Wasiaka (ZW) pond in 2019, i.e., the year with the typical winter.
Although we predicted a decrease in road salt concentrations during the growing season, chronic chloride toxicity persisted in the urban ponds long after the end of winter and was still observed in May, June and even July in Zgierska (ZZ) pond (Fig. 2). This means that elevated chloride concentrations persisted in the environment during the period of zooplankton hatching and development. Disturbingly high chloride concentrations, exceeding acute and chronic toxicity levels, persisted in the pore water throughout hydrological years 2019, 2020 and 2021 in all urban ponds except the reference Arturówek (AG) pond (Fig. 3). In Zgierska (ZZ) pond, chloride concentrations were elevated, even in the year with a mild winter. The increase in concentrations observed during periods without de-icing suggests that chlorides may have been stored over winter in hydrological reservoirs, such as a shallow groundwater system, and slowly released throughout the year. A similar phenomenon has been observed in streams in the northern US (Corsi et al., 2015).
Although water temperature is an important factor that regulates the abundance and distribution of rotifers (Yin et al. 2018), many studies indicate that variability in rotifer abundance is driven by “bottom-up” forces related to food supply (e.g., Yoshida et al., 2003; Ejsmont-Karabin, 2012). The waters of the studied reservoirs were rich in suspended organic matter (unpublished data), which was especially true for Julianów (JG) pond and Zgierska (ZZ) pond sites. In the former, the source of the organic matter could be, among other, fallen dead leaves which accumulate at the bottom of the pond. During summer, Zgierska (ZZ) pond becomes a habitat of the plants from the Potamogeton genus, which heavily cover the water surface and begin to decay during late summer and autumn. It is also possible that organic matter may have had an anthropogenic source, e.g., from pollutants entering the urban ponds via faulty sewer systems of the nearby residential areas. Rotifers from the Keratella genus are known to consume detritus, organic aggregates and bacteria (Gilbert, 2022), which means that two species that dominated in our study, K. cochlearis and K. quadrata, had favorable feeding conditions. The relationship between the amount of organic matter as a habitat for bacteria and protozoa, a common part of the food of most rotifer species, and rotifer density has been confirmed in field studies, e.g., by Wilk-Woźniak and co-authors (2014) who showed a significant positive correlation between rotifer abundance and the amount of allochthonous organic matter input to the flowing lake Dobczyce (Poland). Moreover, both K. cochlearis and K. quadrata are eurytopic species; therefore, their dominance is not surprising.
The presence of road salt-derived chlorides in freshwaters should not be underestimated. Depending on the characteristics and degree of pollution/eutrophication of the water bodies, the substances present may interact additively, synergistically or antagonistically with road salt (Woodley et al., 2023). This can pose unforeseen risks to organisms and disrupt the functioning of freshwater ecosystems.
Conclusions
Few studies of the relationship between anthropogenic pollutants, such as road salt, and zooplankton in freshwater bodies have been performed under natural conditions. This may be due to the high variability and dynamics of natural ecosystems, diversity of interacting factors influencing them and complexity of the relationships between organisms and the environment, making it difficult to interpret the results obtained. Furthermore, the problem of water salinity is often downplayed and/or not fully recognized. The results of our 3-year study lead to conclusions that can broaden knowledge of the effects of salinity on aquatic organisms.
Our research shows that the impact of road salt on urban freshwater ecosystems is not a seasonal problem, i.e., it does not only affect the winter period. Elevated concentrations of chloride ions persist in both sediment and water throughout the year and can negatively affect zooplankton during the growing season.
Moreover, our findings are the first from an environmental study to show that zooplankton density is negatively correlated with chloride ion concentrations exceeding chronic toxicity levels in water. This phenomenon was found to be a consequence of cold and snowy winters. However, pond-specific factors (e.g., biotic interactions), which are key to the dynamics of zooplankton density, may have modified the response of zooplankton to the presence of road salt.
Data availability
The raw dataset generated and analysed during the study are currently available in the Zenodo repository, https://doi.org/10.5281/zenodo.7924181
References
Amoros C (1984) Introduction Pratique a la Systematique des Organisms des Eaux Continentals Françaises: Crustacés Cladocéres. Extrait du Bulletin Mensuel de la Société. Linnéenne de Lyon 53:3–4 (in French)
Benzie JAH (2005) The genus Daphnia (including Daphniopsis) (Anomopoda: Daphniidae). In: Dumont HJF (ed) Guides to the Identification of the Macroinvertebrates of the Continental Waters of the World. Kenobi Productions, Leiden: Backhuys Publishers, Ghent, pp 1–374
Bester ML, Frind EO, Molson JW, Rudolph DL (2006) Numerical investigation of road salt impact on an urban wellfield. Ground Water 44:165–175. https://doi.org/10.1111/j.1745-6584.2005.00126.x
Bielańska-Grajner I, Cudak A (2014) Effects of Salinity on Species Diversity of Rotifers in Anthropogenic Water Bodies. Pol J Environ Stud 23(1):27–34
CEQG (2011) Canadian water quality guidelines for the protection of aquatic life. Can Environ Qual Guidel 65:1–9
Coldsnow KD, Mattes BM, Hintz WD, Relyea RA (2017) Rapid evolution of tolerance to road salt in zooplankton. Environ Pollut. https://doi.org/10.1016/j.envpol.2016.12.024
Corsi SR, De Cicco LA, Lutz MA, Hirsch RM (2015) River chloride trends in snowaffected urban watersheds: increasing concentrations outpace urban growth rate and are common among all seasons. Sci Total Environ 508:488–497. https://doi.org/10.1016/j.scitotenv.2014.12.012
Cunillera-Montcusí D, Beklioğlu M, Cañedo-Argüelles M, Jeppesen E, Ptacnik R, Amorim CA, Arnott SE, Berger SA, Brucet S, Dugan HA, Gerhard M, Horváth Z, Langenheder S, Nejstgaard JC, Reinikainen M, Striebel M, Urrutia-Cordero P, Vad CF, Zadereev E, Matias M (2022) Freshwater salinisation: a research agenda for a saltier world. Trends Ecol Evol 37:440–453. https://doi.org/10.1016/j.tree.2021.12.005
Douville H, Raghavan K, Renwick J, Allan RP, Arias PA, Barlow M, Cerezo-Mota R, Cherchi A, Gan TY, Gergis J, Jiang D, Khan A, Pokam Mba W, Rosenfeld D, Tierney J, Zolina O (2021) Water Cycle Changes. In: Zhai VP, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI, Huang M, Leitzell K, Lonnoy E, Matthews JBR, Maycock TK, Waterfield T, Yelekçi O, Yu R, Zhou B (eds) Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp 1055–1210
Ejsmont-Karabin J, Radwan S, Bielańska-Grajner I (2004) Monogononta – atlas of species 32B. In: Radwan S (ed) Rotifers (Rotifera), The freshwater Fauna of Poland. Polskie Towarzystwo Hydrobiologiczne, Uniwersytet Łódzki. Oficyna Wydawnicza Tercja, Łódź, pp 147–448 (in Polish)
Ejsmont-Karabin J (2012) The usefulness of zooplankton as lake ecosystem indicators: Rotifer trophic state index. Pol J Ecol 60(2):339–350
Gallagher MT, Snodgrass JW, Ownby DR, Brand AB, Casey RE, Lev S (2011) Watershed-scale analysis of pollutant distributions in stormwater management ponds. Urban Ecosyst 14:469–484. https://doi.org/10.1007/s11252-011-0162-y
Gilbert JJ (2022) Food niches of planktonic rotifers: diversification and implications. Limnol Oceanogr 67:2218–2251. https://doi.org/10.1002/lno.12199
Gökçe D, Özhan DO (2014) Effects of salinity tolerances on survival and life history of 2 cladocerans. Turkish J Zool. https://doi.org/10.3906/zoo-1304-21
Hintz WD, Relyea RA (2017) A salty landscape of fear: responses of fish and zooplankton to freshwater salinization and predatory stress. Oecologia 185:147–156. https://doi.org/10.1007/s00442-017-3925-1
Hintz WD, Relyea RA (2019) A review of the species, community, and ecosystem impacts of road salt salinisation in fresh waters. Freshw Biol 64:1081–1097. https://doi.org/10.1111/fwb.13286
Hintz WD, Jones DK, Relyea RA (2019) Evolved tolerance to freshwater salinization in zooplankton: life-history trade-offs, cross-tolerance and reducing cascading effects. Philos Trans R Soc B Biol Sci 374:20180012. https://doi.org/10.1098/rstb.2018.0012
Hubbart JA, Kellner E, Hooper LW, Zeiger S (2017) Quantifying loading, toxic concentrations, and systemic persistence of chloride in a contemporarymixed-land-use watershed using an experimental watershed approach. Sci Total Environ 581–582:822–832. https://doi.org/10.1016/j.scitotenv.2017.01.019
Huber ED, Wilmoth B, Hintz LL, Horvath AD, McKenna JR, Hintz WD (2023) Freshwater salinization reduces vertical movement rate and abundance of Daphnia: interactions with predatory stress. Environ Pollut 330:121767. https://doi.org/10.1016/j.envpol.2023.121767
Jurczak T, Wagner I, Wojtal-Frankiewicz A, Frankiewicz P, Bednarek A, Łapińska M, Kaczkowski Z, Zalewski M (2019) Comprehensive approach to restoring urban recreational reservoirs. Part 1 – Reduction of nutrient loading through low-cost and highly effective ecohydrological measures. Eco Eng 131:81–98. https://doi.org/10.1016/j.ecoleng.2019.03.006
Kaushal SS, Likens GE, Pace ML, Reimer JE, Maas CM, Galella JG, Utz RM, Duan S, Kryger JR, Yaculak AM, Boger WL, Bailey NW, Haq S, Wood KL, Wessel BM, Park CE, Collison DC, Aisin BY, ’aaqob I, Gedeon TM, Chaudhary SK, Widmer J, Blackwood CR, Bolster CM, Devilbiss ML, Garrison DL, Halevi S, Kese GQ, Quach EK, Rogelio CMP, Tan ML, Wald HJS, Woglo SA (2021) Freshwater salinization syndrome: from emerging global problem to managing risks. Biogeochemistry 154:255–292. https://doi.org/10.1007/s10533-021-00784-w
Lepš J, Šmilauer P (2012) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge
Lind L, Schuler MS, Hintz WD, Stoler AB, Jones DK, Mattes BM, Relyea RA (2018) Salty fertile lakes: how salinization and eutrophication alter the structure of freshwater communities. Ecosphere. https://doi.org/10.1002/ecs2.2383
Martínez-Jerónimo F, Martínez-Jerónimo L (2007) Chronic effect of NaCl salinity on a freshwater strain of Daphnia magna Straus (Crustacea: Cladocera): a demographic study. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2006.08.009
Parlato BP, Kopp R (2020) Adaptive Tolerance to Sodium Chloride in Daphnia magna. Kent Journ Undergrad Schol 4(1):2
Reid AJ, Carlson AK, Creed IF, Eliason EJ, Gell PA, Johnson PTJ, Kidd KA, MacCormack TJ, Olden JD, Ormerod SJ, Smol JP, Taylor WW, Tockner K, Vermaire JC, Dudgeon D, Cooke SJ (2019) Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol Rev 94:849–873. https://doi.org/10.1111/brv.12480
Rogora M, Mosello R, Kamburska L, Salmaso N, Cerasino L, Leoni B, Garibaldi L, Soler V, Lepori F, Colombo L, Buzzi F (2015) Recent trends in chloride and sodium concentrations in the deep subalpine lakes (Northern Italy). Environ Sci Pollut Res 22:19013–19026. https://doi.org/10.1007/s11356-015-5090-6
Scott R, Goulden T, Letman M, Hayward J, Jamieson R (2019) Long-term evaluation of the impact of urbanization on chloride levels in lakes in a temperate region. J Environ Manage 244:285–293. https://doi.org/10.1016/j.jenvman.2019.05.029
Szklarek S, Górecka A, Wojtal-Frankiewicz A (2022) The effects of road salt on freshwater ecosystems and solutions for mitigating chloride pollution – a review. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.150289
Szwed M (2018) Variability of precipitation in Poland under climate change. Theor Appl Climatol 135:1003–1015. https://doi.org/10.1007/s00704-018-2408-6
ter Braak CJF, Šmilauer P (2012) Canoco reference manual and users guide: software for ordination (version 5.0). Microcomputer Power, Ithaca
Tixier G, Rochfort Q, Grapentine L, Marsalek J, Lafont M (2012) Spatial and seasonal toxicity in a stormwater management facility: evidence obtained by adapting an integrated sediment quality assessment approach. Water Res 46:6671–6682. https://doi.org/10.1016/j.watres.2011.12.031
Tomczyk AM, Bednorz E, Szyga-Pluta K (2021) Changes in air temperature and snow cover in winter in Poland. Atmosphere 12:68. https://doi.org/10.3390/atmos12010068
US EPA (1988) Ambient aquatic life water quality criteria for chloride. United States Environ Prot Agency 44:1–24
Van Meter RJ, Swan CM (2014) Road salts as environmental constraints in urban pond food webs. PLoS One. https://doi.org/10.1371/journal.pone.0090168
Van Meter RJ, Swan CM, Leips J, Snodgrass JW (2011) Road salt stress induces novel food web structure and interactions. Wetlands 31:843–851. https://doi.org/10.1007/s13157-011-0199-y
Venâncio C, Ribeiro R, Soares AMVM, Lopes I (2018) Multigenerational effects of salinity in six clonal lineages of Daphnia longispina. Sci Total Environ 619–620:194–202. https://doi.org/10.1016/j.scitotenv.2017.11.094
Warsza R, Lipińska D, Tomczak A, Wysmyk-Lamprecht B, Kwiatkowska N, Pielużek K, Lipińska A, Stobińska A, Jach K, Miłosz M (2017) Studium uwarunkowań i kierunków zagospodarowania miasta Łodzi, Opracowanie ekofizjograficzne [Study of Conditions and Directions of Spatial Development of the City of Łódź. Ecophysiographic Study]. (in Polish)
Wilk-Woźniak E, Pociecga A, Amirowicz A, Gasiorowski M, Gadzinowska J (2014) Do planktonic rotifers rely on terrestrial organic matter as a food source in resevoir ecosystems? Intern Rev of Hydrobiol 99:157–160. https://doi.org/10.1002/iroh.201301717
Winter JG, Landre A, Lembcke D, O’Connor EM, Young JD (2011) Increasing chloride concentrations in Lake Simcoe and its tributaries. Water Qual Res J Canada 46:157–165. https://doi.org/10.2166/wqrjc.2011.124
Woodley A, Hintz LL, Wilmoth B, Hintz WD (2023) Impacts of water hardness and road deicing salt on zooplankton survival and reproduction. Sci Rep 13:2975. https://doi.org/10.1038/s41598-023-30116-x
Yin L, Ji Y, Zhang Y, Chong L, Chen L (2018) Rotifer community structure and its response to environmental factors in the Backshore Wetland of Expo Garden. Shanghai Aquac and Fishe 3(2):90–97. https://doi.org/10.1016/j.aaf.2017.11.001
Yoshida T, Urabe J, Elser JJ (2003) Assessment of ‘top–down’ and ‘bottom–up’ forces as determinants of rotifer distribution among lakes in Ontario, Canada. Ecol Res 18:639–650. https://doi.org/10.1111/j.1440-1703.2003.00596.x
Acknowledgments
This study was supported by grant no. 2018/28/C/NZ8/00235, “Impact of road salt pollution in winter on zooplankton hatching success from resting eggs.” Funded by the National Science Centre (Poland). We thank Tomasz Bedyk for collecting and sharing daily meteorological data from Łódź-Lublinek weather station (ID 351190465).
Author information
Authors and Affiliations
Contributions
AG: Data curation, Formal analysis, Investigation, Visualization, Writing—original draft, Writing—review & editing. SS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Validation, Writing—original draft, Writing—review & editing, Resources. PF: Formal analysis, Software, Resources, Visualization. KK: Formal analysis, Software, Visualization. AW-F: Conceptualization, Formal analysis, Methodology, Supervision, Validation, Writing—original draft, Writing—review & editing
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Górecka, A., Szklarek, S., Frankiewicz, P. et al. Does winter application of road salt affect zooplankton communities in urban ponds?. Aquat Sci 85, 111 (2023). https://doi.org/10.1007/s00027-023-01009-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-023-01009-y