Abstract
Freshwater organisms in the Okavango Delta and Lake Ngami (Botswana) provide direct and indirect benefits to people and the economy of the region. However, their existence could be potentially threatened by human activities (primarily, upstream water abstraction and planned hydropower structures) coupled with climate change. For their protection, it is essential to know their distribution, ecology, and status of the ecosystems that they inhabit. Publications that record taxa from the Delta at species level are scarce, particularly aquatic macroinvertebrates. Identifying organisms to species level can provide more accurate information for environmental monitoring and conservation programmes but requires significant training and expertise. Here, we present a comprehensive taxonomical review of 2204 freshwater species from the Okavango Delta and Lake Ngami, with additional 355 species found in other areas of Botswana that are likely to be present in the study region. We also compare the diversity of the Okavango Delta and Lake Ngami with two other tropical wetlands: the Pantanal (Brazil) and the Kakadu Region (Australia). We show that biodiversity in the Okavango Delta and Lake Ngami is higher than in previous estimates, with recorded species richness dominated by phytoplankton and macroinvertebrates. Most species are widespread across the system and southern Africa. The resulting database includes new records (Bryozoa, Porifera), information on species conservation status, habitat, ecology, distribution in continental Africa, site details and taxonomical notes. This will be an essential resource for researchers, conservation managers, policy makers and consultants investigating freshwater biodiversity in tropical wetlands in the region.
Similar content being viewed by others
Introduction
The Okavango Delta, located in northern Botswana, is one of Africa’s largest inland wetlands (Keddy et al. 2009) and offers a unique set of conditions to support aquatic biodiversity. For several millennia, the annual flood pulse together with the activity of ecosystem engineers (hippos, elephants, termites and plants) (Mosepele et al. 2009), have shaped a mosaic of habitats that is an oasis for biodiversity in an area that would otherwise have been a desert.
The freshwater organisms that inhabit the Delta and the intermittently connected Lake Ngami, provide many direct and indirect benefits to local people. These benefits include fisheries, water purification and, more recently, tourism revenue (Mbaiwa 2011). However, in this region, freshwater taxa have been (until relatively recently) understudied compared to the more familiar and charismatic terrestrial megafauna (personal observation). Without more detailed knowledge of the freshwater biodiversity of the Okavango Delta, particularly at species level, it would be difficult to accurately know the ecological conditions of its waters and the severity of human impacts. The latter are currently low and localised, e.g. water pollution by boats and in the proximity of tourist lodges and human settlements (Mosepele and Mosepele 2021; Mogobe et al. 2018, 2019), but might be of greater importance if agricultural and tourist activities increase along the catchment.
It was not until the second half of the twentieth century that researchers started to study freshwater diversity of the Okavango Delta in more depth, focusing mainly on fish (Merron 1991,1993; Merron and Bruton 1995), plants (Ellery and Ellery 1997) and certain macroinvertebrate groups, including beetles (Bilardo and Rocchi 1987), dragonflies (Pinhey 1976), molluscs (Appleton 1979) and nematodes (Heyns and Coomans 1991). In the present century, the knowledge of aquatic diversity in the region has improved substantially, with more details about the processes that determine the distribution of macroinvertebrates (Dallas and Mosepele 2007, 2020; Davidson et al. 2012), fish (Mosepele et al. 2009) birds (Francis et al. 2021) and algae (Marazzi 2014, 2023). At species level, the composition and dynamics have been described in a comprehensive assessment by Appleton et al. (2003) and the reviews by Ramberg et al. (2006) and Mosepele and Mosepele (2021). Further works also contributed to fill the gaps on understudied taxa, including aquatic mites (Vidrine et al. 2006, 2007; Viets 1980; Smit 2012), zooplankton (Hoberg et al. 2002; Lindholm and Hessen 2007a, b; Siziba et al. 2011), diatoms (Mackay et al. 2012) and fish parasites (Van As and Van As 2015).
Nevertheless, despite a considerable number of studies, information at species level continues to be limited and patchy, especially for bacteria (Cronberg et al. 1995, 1996), parasites (Ramberg et al. 2006) and macroinvertebrates. The latter have generally been identified to family or morphospecies level in several studies (Dallas and Mosepele 2007, 2020; Davidson et al. 2012) (with exceptions for particular taxa). Among macroinvertebrates, Chironomidae, Ostracoda, Ephemeroptera and Trichoptera are particularly understudied because of their taxonomic difficulty and the lack of experts in African fauna.
Freshwater biodiversity continues to decline globally and rapidly, and at a faster rate than in terrestrial ecosystems (Albert et al. 2020; Reid et al. 2019). Wetlands are among the most impacted systems, with less than one fifth of the world’s preindustrial total remaining (Albert et al. 2020). This decline can be exacerbated by a range of factors, especially climate change, which in the Okavango Delta is predicted to shorten the length of the rainy season and increase the frequency and duration of droughts (Akinyemi and Abiodun 2019). If this is coupled with an increase in water abstraction and the construction of hydropower structures upstream of the Delta, biodiversity in the Okavango Delta will be under considerable threat (Wolski and Murray-Hudson 2007). We argue that studies at species level in the region will help to disentangle the responses of aquatic organisms to these impacts, and the biotic and abiotic conditions determining their distribution, which can be very species-specific in some groups, e.g. Chironomidae. The combination of taxonomical and molecular data at species level could also be used in the identification of new vectors of human and cattle diseases (as shown in several studies of mosquito populations in Botswana: Buxton et al. 2019, 2021) and identification of new species by specialists (or existent species by non-specialists). Finally, this species-level information—if well applied—could contribute to a better protection of local diversity (Mace 2004) through its inclusion in management and conservation programmes. It can also complement the existing Okavango Assessment System (OKASS), modified from the South African Scoring System (SASS) by Dallas and Mosepele (2007) for the Okavango Delta, which is being implemented by the Okavango Research Institute on a regular basis (Kemosedile et al. 2020).
As rates of freshwater biodiversity loss in tropical wetlands are not known with certainty, because species inventories are incomplete (Dudgeon et al. 2009), we have compiled a comprehensive dataset with all the freshwater species recorded in the literature in the Okavango Delta and Lake Ngami. We have also considered appropriate to use this dataset to update the review by Junk et al. (2006a), by comparing the diversity of the Okavango Delta (and Lake Ngami) with two other tropical systems subject to wet-dry cycles: the Pantanal (in Brazil) and the Kakadu Region (northern Australia). We also look at common traits that freshwater organisms share in these three dynamic systems to contend with those dry and wet periods.
The aims of this study, based on a systematic review of a wide range of biodiversity resources, are to provide: (1) a comprehensive list of freshwater organisms inhabiting the Okavango Delta and Lake Ngami, with information about their conservation status, habitat, ecology, distribution in Africa, site details and taxonomical notes (available on Zenodo: https://doi.org/10.5281/zenodo.8290159); (2) an overview of the taxonomic composition in the Delta-Lake Ngami of the nine groups considered: amphibians, birds, fish, macroinvertebrates, macrophytes, mammals, phytoplankton, reptiles and zooplankton; (3) an updated comparison among the biodiversity of the Delta-Lake Ngami, the Pantanal and the Kakadu Region; (4) information on the conservation status of the freshwater species that have been recorded in the Delta and Lake Ngami.
Material and methods
Study region
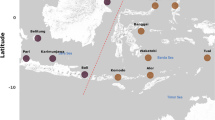
The Okavango Delta (Fig. 1) is the largest wetland in southern Africa, which fluctuates in size from 6000–8000 km2 during the dry season to > 15,000 km2 in the wet season (Alonso and Nordin 2003), with a total size, including islands and areas flooded historically (e.g. Lake Ngami, Mababe Depression) of 28,000 km2 (Junk et al. 2006a; Ramberg et al. 2006). It expands and recedes depending primarily on the pulses of water (flood pulses) received from the Angolan mountains and secondarily on the inputs from local rain. The hydrological gradient in the Delta creates high heterogeneity of habitats: channels (rivers), floodplains, lagoons, ponds and pools. These are inhabited by many species, including endangered and emblematic mammals (the African Wild Dog, Black Rhino and African Savanna Elephant) and birds, like the Wattled Crane. The Delta is protected under the UNESCO World Heritage (2014) and Ramsar Sites Information Service 2.0 (2021) programmes (Appleton et al. 2003; Ramberg et al. 2006).
Lake Ngami is an integral part of the Delta and an important breeding site for birds, with 69 freshwater bird species recorded in 2005 (Harebottle et al. 2006) and > 58,000 individuals when it flooded in 2004 (Hancock et al. 2007). It is intermittently connected to the Delta through the Kunyere and Nhabe Rivers and has an average fluctuating size of 327.6 km2 and depth of 3.5 m (Kurugundla et al. 2018).
In the present study, we compare the biodiversity of the Delta and Lake Ngami (in terms of number of taxa) with two other similar systems found in a similar latitude: the Pantanal (in Brazil) and Kakadu (in northern Australia). The Pantanal is well studied, and also subject to a predictable monomodal flood pulse (as the Okavango Delta), with areas that regularly flood and dry, albeit it is six times larger than the Okavango Delta, with a total area about 160,000 km2 (Junk et al. 2006b). The wetlands of the Kakadu Region are east of the city of Darwin in northern Australia. They include a higher variety of habitats, with intertidal mudflats, mangroves, hypersaline flats, freshwater floodplains and streams, covering an area of approximately 28,000 km2 (this comprises the Kakadu National Park and land within the western portion of Arnhem Land, part of the catchment of the East Alligator River). They are also subject to wet-dry cycles influenced by the monsoon (Finlayson et al. 2006).
Data compilation
Freshwater species recorded in the Okavango Delta and Lake Ngami
To establish which organisms should be included in this review, we followed the definition of freshwater species by Darwall et al. (2009): “those which depend upon freshwater habitats for any critical part of their life cycle” e.g. amphibians (egg and tadpole stage). We also included animals that spend a significant part of their lives in water or near it, for feeding, reproduction, etc., such as some freshwater birds, aquatic and semiaquatic mammals and reptiles. In total, we considered nine groups: amphibians, birds, fishes, macroinvertebrates, macrophytes, mammals, phytoplankton, reptiles and zooplankton.
Determining which plants are aquatic or semiaquatic in the Okavango Delta is difficult, as many are herbaceous that inhabit floodplains or grasslands that can be flooded at certain times of the year, being totally or partially submerged in water. We considered the following definition of macrophyte: “growing in or near water”. We extracted those species from Ellery (2003) that belonged to the following nine categories: aquatic tree, aquatic creeper, emergent aquatic, free-floating aquatic, floating-leaved aquatic, floating-stemmed aquatic, aquatic shrub, submerged aquatic and herbaceous wetland plant. We also included species from Ellery (2003) found in rainwater pans, margins of lakes, rivers and streams (excluding trees and shrubs with more terrestrial character) and confirmed their habitat and ecology based on Hyde et al. (2023), WFO (2023) and POWO (2023). All the ferns (Pteridophytes) described in Vega Hernández and Caudales (2001) were also included. We also added all the species in the section “aquatic plants” in Ellery and Ellery (1997) and those recorded in permanent water as well as their locations in the Delta.
All species names and location details were collected from four main sources: (1) scientific publications and reference books, either printed versions and/or uploaded on the following databases: Google Scholar, Scopus, Web of Science, ProQuest and Biodiversity Heritage Library; (2) reference lists from identified articles and books; (3) data from museum collections and research institutions uploaded to the Global Biodiversity Information Facility (GBIF 2021); (4) research-grade observations from iNaturalist (2022) and Naturgucker.de (2021) uploaded to GBIF (although we tried to prioritise data from research institutions and museum collections when possible).
The latest taxonomical classification of each group was checked and updated from a wide range of references (see Supplementary material 1). When specific sources could not be found, this information was retrieved from Encyclopaedia of Life (EOL 2021).
Comparison with similar systems
The number of freshwater taxa recorded in the Pantanal and Kakadu was extracted from: Alho et al. (2011a, Table 1); De-La-Monica-Freire and Heckman (1996); Finlayson et al. (1989, 2006); Junk et al. (2006a, b); Pott et al. (2011). References for each group are available in Table 9 in the Results section. We applied some modifications to certain groups (see next section: data analysis).
Within phytoplankton there are species of Cyanobacteria (Cyanobacteriota sensu Oren et al. 2022).
For macrophytes present in Kakadu, we extracted those species classified as aquatic (A) and semiaquatic (A/T) from the appendix by Finlayson et al. (1989) and calculated the number of species and percentage per family, checking the latest taxonomic classification on POWO (2023). Authority names were extracted from Cowie et al. (2000) as Finlayson et al. (1989) did not include them. It is important to note that, although the study by Finlayson et al. (1989) is one of the most comprehensive done in the Kakadu Region (an area of about 28,000 km2), it only covers the floodplain of Magela Creek (220 km2), a tributary of the East Alligator River.
We determined those aquatic or semiaquatic mammals (spending most of their lives in or near water) by searching their habitat and ecology on the IUCN website (IUCN 2023). We excluded bats (considered by Junk et al. 2006a and Finlayson et al. 2006); generalist animals that do not spend most of their time in floodplains or riparian habitats (on the ground close to water), but in a variety of them, like the Bush Dog (Speothos venaticus) or the Crab-eating Fox (Procyon cancrivorus); arboreal animals such as the opossums: Marmosa murina, M. constantiae, M. demerarae and Marmosops noctivagus (included as semi-aquatic by Alho et al. 2011a); animals that are mostly confined to margins of floodplains, such as dingoes, wallabies and the nabarlek in the Kakadu Region (Cowie et al. 2000). We did not include the following introduced animals: feral pig (Sus scrofa), horse (Equus caballus), donkey (Equus africanus) and cattle (Bos taurus), as they are terrestrial (although they may spend a considerable time in floodplains).
Finding criteria to classify animals and plants as aquatic or semiaquatic was challenging, and we suggest that the freshwater community needs to develop a more robust definition of what constitutes a ‘freshwater species’.
Conservation status, habitat, ecology and distribution in Africa, location, coordinates
Information about the conservation status, habitat, ecology and distribution in Africa was collected from the IUCN Red List of Threatened Species website (IUCN 2023) and complemented with reference books (Butchard, 2016; Griffiths et al. 2015 and others in the reference section). Distribution, habitat and ecology of macrophytes in Africa were collected from POWO (2023) and WFO (2023). Location names were checked and corrected using MapCarta (OpenStreetMap contributors 2022), Google Maps (2021) and specific maps from the sources of this review (including Appleton et al. 2003; Hart 1997; Mendelsohn et al. 2010; Merron, 1991 and others). All site coordinates were converted to degrees, minutes and seconds using the Decimal Degrees to Degrees, Minutes, Seconds conversion tool by RapidTables (2020).
Maps in Figs. 1 and 2 were made using ArcGIS 10.8 Esri Inc.
Data analysis
Freshwater species recorded in the Okavango Delta and Lake Ngami
In the calculations of the total number of species per group, the following were excluded: (1) those listed as “potentially present” in the dataset, which are found in other parts of Botswana but not in the Okavango Delta (see section on “potentially present” species further down); (2) those whose taxonomical name is dubious or unresolved, e.g. the diatom Eolimna minima (Grunow) Lange-Bertalot, nom. illeg., 1999; (3) species not identified further than genus by the authors (recorded as sp. or spp.). Single subspecies, formae and varieties were considered as species in the calculations (e.g. Sesbania bispinosa var. bispinosa included as S. bispinosa), or in the case of several present, grouped as a single species (e.g. Gomphocarpus fruticosus subsp. fruticosus and Gomphocarpus fruticosus subsp. rostratus grouped as a single G. fruticosus).
Comparison with similar systems
For a comparison of the number of aquatic invertebrates among wetlands Junk et al. (2006a) considered microcrustaceans (Copepoda, Cladocera), rotifers and molluscs (Gastropoda and Bivalvia). In this study we grouped Copepoda, Cladocera and Rotifera into the category “Zooplankton”, together with ciliates, amoebas, gastrotrichs and testaceans. For the macroinvertebrates, we grouped all organisms > 1 mm; this includes molluscs (Gastropoda, Bivalvia), Oligochaeta and Ostracoda. This criterion was also applied for data from the Kakadu Region extracted from Finlayson et al. (2006). Among amphibians, we only considered those species that breed in aquatic environments. For aquatic birds, we followed the definition by Junk et al. (2006b): “those birds, that feed almost exclusively by diving, swimming or wading, or that feed on shores or mudflats in the vicinity of water”.
Results
Species distribution in the Okavango Delta and Lake Ngami
Based on the sources used in this review, a total of 2204 freshwater species have been recorded in the Okavango Delta and Lake Ngami. The groups with the highest number of species are phytoplankton (753 species) and macroinvertebrates (482 species) (Fig. 3 below).
Most species are widespread across the Delta and southern Africa, but some have only been recorded in the Delta so far (e.g. several species of mites and parasites), and there are three confirmed endemics: the desmid Cosmarium pseudosulcatum var. okavangicum D.B. Williamson and L. Marazzi, 2013, the diatom Eunotia okawangoi Cholnoky, 1966 and the ostracod Sarscypridopsis harundineti Szwarc, Martens & Namiotko, 2021. Much further research is needed to map the distribution of many such species in the African continent and determine their degree of endemicity.
Species richness per group in the Okavango Delta and Lake Ngami
In this section we present the species richness and percentage of each family within each group considered in this review (Fig. 3). For some we only show the three families with the highest species richness. All the complete tables can be found in supplementary file 2.
Phytoplankton (Algae)
Dominated by green algae of the desmid families Desmidiaceae (247 species) and Closteriaceae (31 species), followed by diatoms of the family Eunotiaceae (28 species) (Table 1).
Zooplankton
Dominated by rotifers. The families with the highest richness are the rotifer families Lecanidae (46 species) and Lepadellidae (25 species), followed by water fleas of the family Chydoridae (23 species) (Table 2).
Macrophytes
Within aquatic plants, sedges (Cyperaceae) and grasses (Poaceae) dominate, with 93 and 71 species respectively, followed by sunflowers (Asteraceae) with 26 species. The most common growth forms are grasses, sedges and reeds (58.7%), followed by emergent, free-floating creepers (34.3%). Submerged species represent 7% of the total number of species (Table 3).
Macroinvertebrates
The macroinvertebrates are a very heterogeneous group comprised of species from very different phyla. In the study areas, richness is dominated by Odonata (96 species), Diptera (83 species) and Coleoptera (68 species) (Fig. 4 below).
Fish
Dominated by cichlids (Cichlidae) and minnows and carps (Cyprinidae), with 24 and 19 species respectively, followed by freshwater elephantfish (Mormyridae) with 10 species (Table 4).
Amphibians
Grassland frogs (Ptychadenidae) and true toads (Bufonidae) are the families with the highest number of species, with 9 and 8 species respectively (Table 5).
Reptiles
Dominated by freshwater snakes (Colubridae) and terrapins (Pelomedusidae), with 5 and 4 species respectively (Table 6).
Mammals
Aquatic mammal species are dominated by ruminants (Bovidae) and otters (Mustelidae), with 4 and 2 species respectively (Table 7).
Birds
Dominated by herons (Ardeidae) and sandpipers (Scolopacidae), with 18 and 15 species respectively (Table 8).
Comparison with similar systems: the Pantanal and the Kakadu Region
As seen in Table 9 below, the number of freshwater species recorded in the Okavango Delta is considerable higher than in the Pantanal and the Kakadu Region, although this comparison must be taken with caution (see discussion).
Conservation status
The conservation status of many species in the Delta and Lake Ngami has not been assessed (see discussion). Of those assessed, the majority are of least concern, with the exceptions in the table below. There are 10 near threatened species, 9 vulnerable, 2 endangered and 1 critically endangered, the Yellow-belly bream (Serranochromis robustus) (Table 10).
Discussion
In the present study we found that freshwater biodiversity in the Okavango Delta is higher than previously thought, with some 2204 freshwater species recorded in the literature. Junk et al. (2006a) and Ramberg et al. (2006) estimated less than a thousand species, although, as stated by them, available data for accurate comparisons were insufficient at the time of both publications. Some groups are very likely to be grossly underestimated, and there is a considerable difference in size between the systems (the Kakadu Region: ̴ 28,000 km2 sensu Finlayson et al. 2006; the Pantanal: ̴ 160,000 km2 according to Junk et al. 2006b; the Okavango Delta: ̴ 28,000 km2 according to Ramberg et al. 2006). In addition, many studies have focused only on specific areas, such as the Magela floodplain in the Kakadu Region (Finlayson et al. 1989, 2006) or the Pantanal of Poconé (De-Lamonica-Freire and Heckman 1996; Do Prado et al. 1994; Hardoim and Heckman 1996; Heckman, 1998). Finally, the criteria to determine what species can be considered aquatic and semiaquatic vary between studies.
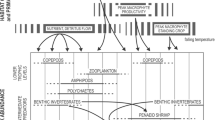
Freshwater organisms in the Okavango Delta and connected Lake Ngami are adapted to constant change, i.e. floods and droughts, due to the seasonality of the local precipitation and the flood pulse, that is ultimately the main force shaping their composition and dynamics (Davidson et al. 2012; Mosepele and Mosepele 2021). In such a dynamic environment, we found that (recorded) richness is dominated by phytoplankton, macroinvertebrates and, to a lesser extent, macrophytes. In the Kakadu, phytoplankton dominates, followed by macroinvertebrates and zooplankton (Table 9, above). The Pantanal has a high richness of zooplankton and phytoplankton species, followed by macrophytes. It is remarkable that most amphibians in the Pantanal are terrestrial or arboreal, and only two can be considered aquatic or semiaquatic (Lysapsus limellus and Pseudis paradoxa) (Junk et al. 2006b). Phytoplankton, macroinvertebrates and zooplankton thrive when nutrients accumulated on the floodplains are mobilised by the flood pulse, thereby supporting fish populations and higher trophic levels in a cascade effect (Davidson et al. 2012; Mackay et al. 2012; Marazzi 2014; Siziba et al. 2011). They also benefit from the presence of macrophytes, which offer surface (where periphyton can grow) and nutrients (when they decay) (Mazebedi 2019).
Species composition and dynamics per group in the Okavango Delta and Lake Ngami
Bacteria is a group that is rarely studied in tropical wetlands. The only information available in the Delta other than Cyanobacteria is a list of 34 species of public health interest, included in two publications by Cronberg et al. (1995, 1996), who sampled in the central section (Jao/Boro River) and the Kwando/Linyanti/Chobe region. Many species on that list belong to the family Enterobacteriaceae and are pathogenic or opportunistic pathogens, affecting fish (e.g. Listonella anguillarum), plants (e.g. Dickeya chrysanthemi, Pseudomonas asplenii), humans and cattle (e.g. Brucella melitensis, Escherichia coli, Enterobacter spp.). Since the focus of those studies was not on recording species diversity, we decided not to include the results in our evaluations. Moreover, the actual number of species in the Delta is likely to be of the order of tens of thousands and include many undescribed taxa.
Most of the 753 phytoplankton species known to occur in the Okavango Delta and Lake Ngami are benthic or periphytic, and the highest diversity occurs in seasonally flooded areas, where there is higher heterogeneity of habitats and water depth, in contrast to the permanently flooded area known as Panhandle (Fig. 1). There is also a zonation by size, with larger species (e.g. Eunotia pectinalis, Synedra spp.) being more common in the Panhandle, and smaller species in distal regions (e.g. Boro and Santandibe). This might be explained by nutrient concentration and flood disturbance, which interrupts algal succession in lower reaches and favours smaller algae with faster nutrient uptake (e.g. Cosmarium spp., Monoraphidium arcuatum, Scenedesmus spp.), while in the Panhandle, bigger diatoms might compete better for the lack of nutrients (Marazzi 2014). Taxon richness, diversity and biomass are lower during the flooding phase, as there is a dilution effect (Marazzi 2014). The family Desmidiaceae is the most species rich (247 species, 32.8%), followed by the Closteriaceae (31 species, 4.1%). Some species of desmids (Cosmarium spp., Staurastrum spp.) have developed morphological adaptations that allow them to withstand drought for several months, such as small surface/volume ratio, thick cell walls and mucilage secretion (see Štastný 2008).
Zooplankton richness in the Delta is dominated by rotifers (families Lecanidae and Lepadellidae) and water fleas (Chydoridae), but at different stages of the flood. Rotifers (especially Brachionus calyciflorus and Asplanchna sieboldii) can survive in waters with high temperature (as high as 40 ºC) and high turbidity during drought, as long as oxygen and salinity are not extreme (Crome and Carpenter 1988). These are the dominant taxa across the Delta (Cronberg et al. 1996). Water fleas usually appear in higher numbers during the filling phase (Lake 2011) or high-water phase (peak) (Crome and Carpenter 1988; Siziba et al. 2011). They avoid fish predation by feeding on epiphytic algae accumulated among macrophytes, which also offer refuge (Siziba et al. 2011). Zooplankton species in the Okavango Delta can withstand droughts for years in the sediments by remaining dormant or producing egg banks, and increase in population sizes in very short time when the flood arrives. These booms are facilitated by very short generation times and the availability of nutrients derived from animal dung (Siziba et al. 2013). Rare species (e.g. Asplanchnopus multiceps, Epiphanes senta, Sinantherina sp.) may appear when dry areas are inundated very occasionally (once in decades) (West 2016). Several authors have found that the highest diversity and biomass of zooplankton occurs in seasonal floodplains (Lindholm et al. 2009; Ramberg et al. 2006; Siziba et al. 2013), while channels (rivers) have low species richness and abundance, which can be attributed to fish predation (Cronberg et al. 1996). As a defence mechanism against fish, some cladocerans (e.g. Daphnia laevis) may undergo cyclomorphosis, with changes such an increase in tail length (Lindholm 2002).
Most of the known 402 macrophyte species in the Delta belong to the families Cyperaceae (sedges) and Poaceae (grasses). There is an increase in local species richness (alpha diversity) from the Upper Panhandle to the lower reaches of the delta, with the greatest number recorded in the southwest of the Moremi Game Reserve, where there is high habitat heterogeneity and unique species in isolated pools (Ellery and Tacheba 2003). The distribution is determined by six factors: (1) the hydrological regime, (2) nutrient and sediment supply, (3) sediment deposition, (4) type of substratum, (5) salinity and (6) activity of ecosystem engineers (Ellery and Tacheba, 2003; Murray-Hudson et al., 2014). Again, the flood pulse is the main force shaping plant diversity, and, as the length of the hydroperiod increases, plant communities change from open woodland to aquatic communities and vice versa (Murray-Hudson et al. 2014).
Within the macroinvertebrates, the orders Odonata, Diptera and Coleoptera have the highest number of species (96, 83 and 68 respectively). These three groups have multiple adaptations to drought, and their adult stages can fly out of drying pools or pans in the search of persisting waterbodies (Lake 2011). In the case of Libellulidae, the richest Odonata family in the Delta, some species can produce a new generation in temporary pools and fly very long distances, such as Pantala flavescens (Suhling and Martens 2007). Beetles of the genera Rhantus and Eretes have been seen migrating from drying ponds and might even have the ability to communicate the signal among individuals (Lytle et al. 2008). Other adaptations in Diptera (e.g. Aedes) and Odonata (e.g. most Lestes and some Sympetrum) are egg diapause and having several generations per year, e.g. Trithemis monardi (Kipping 2010; Pachka et al. 2016; Suhling and Martens 2007). Macroinvertebrate species richness is relatively uniform across the Delta, probably because of low oxygen conditions in some sites and predation pressure by opportunistic fishes (Alonso and Nordin 2003). Richness (at species level) is higher in isolated and ephemeral pools in Moremi Game Reserve and Chief’s Island areas (Alonso and Nordin 2003). The actual number of macroinvertebrate species in the Delta might be in excess of 800, with around 100 species of Chironomidae (non-biting midges) alone (personal observation).
The number of fish species is difficult to estimate because of the unresolved taxonomic status of some taxa (e.g. Micropanchax spp.) but may well be over 100. Furthermore, in recent publications by Bragança et al. (2020, 2021), southern African species of the genus Lacustricola have been regrouped, using morphometric, meristic and osteological characters. These revisions, combined with DNA-based studies, are urgently needed to complete the taxonomy of freshwater fishes in Southern Africa (Chakona et al. 2022), which was estimated to be about 90% resolved by Skelton (1996). In the Delta and Lake Ngami Cichlidae (Cichlids) and Cyprinidae (Minnows and Carps) are the families with the highest number of species. Catfish (Order Siluriformes) are also very abundant. The cichlids are midwater slow swimmers with laterally compressed bodies and broad fins, which makes them agile predators, both in open waters and among vegetation. They have a flexible diet, from being mainly piscivorous, to insectivorous or detritivores, which is clearly an advantage in a dynamic system (Ramberg et al. 2006). Minnows and carp have a fusiform body with short dorsal and anal fins that allow them to manoeuvre at high speeds; they are all predators on the upper layer of open water (Ramberg et al. 2006). Overall, there is a low biomass of fish in the Delta, perhaps due to the general low concentration of nutrients in the water (Högberg et al. 2002). Their distribution is influenced by the permanence of water and the flow rate (Merron 1991; Ramberg et al. 2006).
Most of the amphibians require both aquatic and terrestrial habitats for their life cycle, and in the Delta, only three are fully aquatic (Xenopus laevis, X. muelleri and X. petersii). Their diversity is influenced by habitat availability, which is maximum in the periphery of the Delta (Le Roux 2010). Bufonidae (True toads) and Ptychadenidae (Grassland frogs) are the families with the highest number of species; many are well adapted to changeable environments and can breed in temporary waters, such as pans and rain pools (e.g. Poyntonophrynus kavangensis, Sclerophrys garmani, Sclerophrys poweri, Ptychadena mossambica, Ptychadena anchietae) (Butchart 2016). There are two types of breeding strategies: continuous and explosive. In the former, the flood pulse triggers a second breeding peak, while inhibiting reproduction in the latter, for which rainfall acts as a cue (Le Roux 2010). Both types of breeders have widespread distribution across the Delta.
The reptiles are all able to exploit both freshwater and terrestrial habitats. Snakes of the family Colubridae and terrapins (Pelomedusidae) have the highest number of species. With the exception of Crotaphopeltis barotseensis, a largely aquatic snake (Rasmussen 1997), the rest of species are semiaquatic, feeding mainly on frogs, although some also on fish (e.g. Limnophis bangweolicus and Natriciteres olivacea). Terrapins are well adapted to changing environments, such as temporary pans, as they can bury themselves and enter into a torpid state. Their diet is varied, feeding generally on frogs, aquatic insects and molluscs, but Pelomedusa subrufa and Pelusios rhodesianus feed on aquatic plants as well (Butchart 2016).
Within aquatic or semiaquatic mammals (those that spend most of their lives in or near water), ruminants (Bovidae) and otters (Mustelidae) have the highest number of species. Bovids in the Delta have modified hooves that allow them to move through mud; Tragelaphus spekii and Kobus leche are also good swimmers, being able to exploit different habitats. They are selective grazers on grasses, being common on floodplains (Butchart 2016). Cattle (Bos taurus) and donkeys (Equus africanus) can spend a considerable time grazing in floodplains and it is common to see cows wading in water feeding on aquatic plants (personal observation), but we have not included them, as they are predominantly terrestrial. The two species of otters (Aonyx capensis and Hydrictis maculicollis) are predominantly aquatic, and thus more common in the permanently flooded Panhandle and permanent lagoons and channels across the Delta. Their diet is mainly piscivorous, but they can also feed on frogs and crabs (Butchart 2016; Griffiths et al. 2015).
Densities of birds are generally low in the Delta, except in areas where nutrients or fish accumulate, as in the Thamalakane River, lagoons (e.g. Xakanaxa, Gadikwe), drying pools and the Lake Ngami when it floods (Hancock et al. 2007). Breeding colonies are associated with high frequency of inundation (once every 5 years at least) and the presence of riparian woodland, such as Water figs (Ficus verruculosus) and reeds (Phragmites australis), which favour roosting (Francis et al. 2021; Hancock et al. 2007). Ardeidae (herons) and Scolopacidae (sandpipers) are the families with the highest number of species. They generally have an omnivorous and opportunistic diet, feeding on aquatic and terrestrial prey and even seeds (e.g. Ardea spp., Calidris spp., Numenius spp.). The Okavango Delta supports the largest concentration of Wattled cranes (Grus carunculata) in the world, 75–80% of the world’s estimated Slaty egrets (Egretta vinaceigula) and the largest colonies of Marabou storks (Leptoptilos crumenifer) and Yellow-billed storks (Mycteria ibis) in southern Africa. The endangered Grey-crowned crane (Balearica regulorum) is occasionally present (Hancock et al. 2007).
Conservation status and endemic species in the Okavango Delta and Lake Ngami
According to the report by Darwall et al. (2009), the levels of threat to the freshwater fauna in southern Africa are low, with 7% of the species classified as endangered. However, this needs to be updated, since the data to assess the conservation status of many taxa are insufficient. For instance, in the Delta and Lake Ngami, none of the phytoplankton species, only a single species of zooplankton (out of 261 recorded), 123 species of aquatic macroinvertebrates (out of 477) and 108 macrophytes (out of 178) have been assessed globally. The conservation status of fish and amphibians is better known, with 85 fish (out of 100) and 37 amphibians (out of 38) being assessed.
The Delta is a crucial refuge and breeding site for the cichlid Serranochromis robustus, a piscivorous fish that is critically endangered because of heavy fishing and habitat destruction (IUCN 2023), the endangered Grey-crowned crane (Balearica regulorum) and the Waterwheel plant (Aldrovanda vesiculosa). Van Rensburg (2001) found specimens of the endangered snail Lobogenes michaelis in Guma Lagoon channel, but we think that they should be re-examined by experts because of its taxonomical difficulty. Therefore, we included the species as “potentially present” in the dataset.
Although previous reviews reported no endemics (Ramberg et al. 2006), we found two confirmed endemic species of algae from the Delta in the literature: Cosmarium pseudosulcatum var. okavangicum D.B. Williamson and L. Marazzi, 2013, and Eunotia okawangoi Cholnoky, 1966. This number will certainly increase in the future as the knowledge on the taxonomy and distribution of understudied groups of algae and other organisms improves. For instance, several mites of the genus Arrenurus described by Viets (1980) and Smit (2012) and Unionicola botswaniana by Vidrine et al. (2006) have only been recorded in the Delta, but they might be present in other African countries as well.
“Potentially present” species: connections between the Delta and other systems in Botswana
Most of the species listed as “potentially present” on the database (available on Zenodo) have been found in the Chobe-Kwando-Linyanti system and the Makgadikgadi Pans. Due to dispersal traits mentioned above (e.g. flying insects, etc.), wind and animals acting as dispersal agents, and similarity of conditions between the Delta and the Chobe-Kwando-Linyanti system, it seems very likely that many of those species might be found in the Delta as well. In fact, Cronberg et al. (1995) observed that plankton assemblages in the Okavango Delta were similar to the Kwando-Linyanti-Chobe system.
There are three key reasons why species observed in the Kwando-Linyanti-Chobe area and Makgadikgadi Pans are likely to be found in the Delta. First, the Delta is connected to the Kwando-Linyanti system (although very occasionally) through the Selinda Spillway in the northeast, ultimately reaching the Zambezi basin (Van As and Van As 2015). This channel was dry for 3 decades, but it has been flowing again since 2009. Second, dust, which is very likely originated in the Makgadikgadi Pans, can be transported by the predominant easterly winds and deposited on tree-covered islands in the seasonal floodplains of the Delta (Humphries et al. 2020). This dust may contain resistant forms of aquatic organisms (e.g. spores, bryozoan statoblasts, cladoceran resting eggs), and diatoms (Kristiansen 1996). Third, animals migrating from the Makgadikgadi Pans to the Okavango Delta might act as dispersal vectors of many aquatic species, such as waterfowl (as seen in other regions: Coesel and Duque 1988; Reynolds and Cumming 2015) and zebras, which have recently been tracked following an ancient migratory route between the two systems (Bartlam-Brooks et al. 2011). Elephants, warthogs and rhinos might also be involved (Vanschoenwinkel et al. 2011) but future studies should address this question.
Comparison with similar systems: the Pantanal and the Kakadu Region
In their review, Junk et al. (2006a) compared the overall species diversity of seven wetlands, including freshwater, terrestrial and sometimes even marine species, e.g. fishes in the Everglades. Because species data were insufficient at the time of that publication, numbers of some groups were grossly underestimated, and new studies have been published since then. In this section we try to give an updated comparison, limited to aquatic (freshwater) and semiaquatic species in three tropical wetlands of the southern hemisphere: the Pantanal, the Kakadu Region, and the Okavango Delta and Lake Ngami.
The information about bacteria (other than Cyanobacteria) present in the three tropical wetlands considered in this review is scarce. They were not mentioned by Junk et al. (2006a), Ramberg et al. (2006) or Finlayson et al. (2006). In the Pantanal, Silva Nunes et al. (2001) carried out a preliminary analysis of the abundance of planktonic bacteria on the main rivers of the Upper Paraguay River Basin (plateau and Pantanal plains), finding an average of 278 × 104 ml−1, although the number of species was not determined. In Kakadu National Park, Nelson et al. (2016) found 16691 OTUs in floodplain soils subject to inundation, which therefore could be considered semi-aquatic, although not planktonic and not strictly freshwater, as there was salt intrusion in the system. As previously mentioned, from the Okavango Delta there is only one list of 34 species published by Cronberg et al. (1995, 1996). From the reduced number of studies, we can conclude that there is a big gap in the knowledge of bacteria populations in tropical wetlands.
In the Pantanal, 337 species of phytoplankton have been identified (Junk et al. 2006b) compared to the Kakadu Region (690) (Finlayson et al. 2006) and the Delta (753); this seems an underestimation. The family Desmidiaceae (Phylum Charophyta) is the most species rich of the three systems (Do Prado et al. 1994; Finlayson et al. 2006; Junk et al. 2006b; Marazzi 2014), especially during high water, as they are capable of surviving in this nutrient-depleted phase (Do Prado et al. 1994). Marazzi (2014) estimated that 66% of the algal species (of a total of 496) and 72% of the genera thought to exist in the Okavango Delta were found in his study, thus the number of 755 species found in this review might be very close to the actual number of extant species.
There is a high diversity of zooplankton in the Pantanal, with 736 species recorded and a dominance of testate amoebae and rotifers (Junk et al. 2006b). This high number may be an indication that the group might be understudied in both the Kakadu Region (with 289 species) (Finlayson et al. 2006) and the Delta (where 265 species have been recorded). Interestingly, rotifers represent a high percentage in the three systems (Finlayson et al. 2006; Junk et al. 2006b), while sarcodines and ciliates are less numerous, particularly ciliates, which are outnumbered by testaceans (Hardoim et al. 1996; Junk et al. 2006). This could be due to water temperature tolerance, as rotifers are able to survive in habitats with temperatures that can reach or even exceed 40 ºC (such as shallow pools during the dry season), while ciliates are not well adapted. Many taxa can withstand periodic dryness as cysts below the sediment surface and reappear quickly after the first rains or floods (Hardoim et al. 1996).
The number of macrophytes in the Kakadu Region is difficult to estimate because of its vast area. As explained in the methodology, most studies have focused on the Magela floodplain (Finlayson et al. 1989, 2006). We extracted a total of 83 aquatic and semi-aquatic species from Finlayson et al. (1989), but numbers vary considerably between studies; for instance, Junk et al. (2006a) reported 75 species. Poaceae (grasses) is the family with the highest number of species in the Magela floodplain, followed by Cyperaceae; this is also the case in the Pantanal (Do Prado et al. 1994; Junk et al. 2006b; Pott et al. 2011). Many plants in this family are rhizomatous and therefore can reproduce both sexually and vegetatively, which is advantageous in environments with water-level fluctuations (Cowie et al. 2000). In the Okavango Delta, Junk et al. (2006a) estimated around 350 species; following our criteria, we found 402 aquatic and semiaquatic species, with Cyperaceae (sedges) being the most species-rich family, followed by Poaceae. Some taxa in Cyperaceae have underground storage organs that allow them to persist during the dry season and a well-developed aerenchyma that facilitates gas exchange and provides buoyancy (Cowie et al. 2000).
Eighty species of macroinvertebrates (excluding zooplankton, see methodology) have been described in the Pantanal, which compared with the Kakadu Region (512) and the Delta (482) seems grossly underrepresented. Therefore, this number is expected to increase as more taxonomic studies are being undertaken. Among insects, chironomids (Diptera: Chironomidae) are the most diverse in the Pantanal and the Kakadu Region (122 species) (Finlayson et al. 2006; Junk et al. 2006). This might be also the case in the Okavango Delta, pending the publication of a preliminary checklist of chironomids by the correspondent author, which might have around 100 species (personal observation). For now, dragonflies are the most species-rich in the Okavango Delta, with 96 species recorded. Generally, the invertebrate taxa found in the three systems have two types of life cycle: they either produce a single generation per year (e.g. Porifera) or multiple generations interrupted by resting stages (e.g. several beetle species within the genus Berosus). Some species in the families Belostomatidae, Hydrophilidae and Dytiscidae spend the dry season as adults within the sediment (aestivation), or migrating to waterbodies that have not dried up, mating with the onset of the first rains (Heckman 1998). Many others produce drought-resistant forms, such as statoblasts (Bryozoa), gemmulae (Porifera) or persistent eggs (some species of Chironomidae).
Regarding fish, in the Pantanal, the most species-rich families are Characidae (Order Characiformes, 76 species, 28.9%) and Loricariidae (Order Siluriformes, 36 species, 13.7%). According to Finlayson et al. (2006), 62 species of fish have been recorded in freshwaters in the Kakadu Region, of which 44 are entirely freshwater, 4 reproduce in estuarine or marine waters and 14 are marine or estuarine that enter non-tidal freshwaters. The families Eleotrididae (8 species, 12.9%) and Terapontidae (6 species, 9.7%) have the highest number of species, both within the Order Perciformes (Finlayson et al., 2006). In the Delta, Cichlidae (Order Perciformes, 24 species, 24%) and Cyprinidae (Order Cypriniformes, 19 species, 19%) are the richest. Many fishes in the Pantanal and the Delta are omnivorous and r-strategists (Junk et al. 2006b), while in the Kakadu Region, there is a broad range of feeding strategies depending on the location within the catchment, from herbivores/detritivores to omnivores and predators. Many species also exhibit some form of parental care (Finlayson et al. 2006). In all systems, fishes actively move between habitats (nurseries, corridors, and floodplains) when these are re-connected by the flood pulse during the wet season. They have also adapted their life cycle to the flood pulse, with many species spawning at the beginning of the wet season. High natural fish mortalities (“fish kills”) are common in the three systems when the first flows bring high concentrations of organic matter and stir the sediments, depleting the oxygen in the water, although some species have developed specific adaptations to anoxia (Finlayson et al. 2006; Junk et al. 2006b; Ramberg et al. 2006).
There is a poor diversity of aquatic or semiaquatic amphibian species in the Pantanal, with only two species (Lysapsus limellus and Pseudis paradoxa), as the majority are terrestrial or arboreal (Junk et al. 2006b). By contrast, in the Kakadu wetlands there are 26 species of amphibians in 4 families, including the invasive Cane toad (Finlayson et al. 2006). In the Okavango Delta, there are 38 freshwater species distributed in 9 families, and no invasive species have been recorded.
Junk et al. (2006b) reported 12 aquatic or semiaquatic reptiles from the Pantanal, the same number as in the Okavango Delta, but in the former, there is a higher variety of snakes (65 species). The Kakadu wetlands have a higher diversity of reptiles, with 19 aquatic and semi-aquatic species recorded. Some inhabit estuarine habitats, but can also migrate long distances upstream, e.g. Crocodylus porosus (IUCN 2023). There is one fully aquatic snake (Acrochordus arafurae), six species of freshwater turtles and two species of crocodile: the Estuarine or Saltwater crocodile (Crocodylus porosus) and the Freshwater crocodile (Crocodylus johnstoni) (Finlayson et al. 2006; Press et al. 1995).
The most updated study of mammals in the Pantanal was published by Alho et al. (2011a), who recorded 174 species, of which we considered 12 as predominantly aquatic or semiaquatic (Table S14 in Supplementary material 1). These include three species of rats, two species of water opossum, two species of otter, the Capybara, the Marsh deer, the Crab-eating racoon, the South American tapir and the introduced Water buffalo (Bubalus bubalis) (Alho et al. 2011b). The lowest number of aquatic or semiaquatic mammals is found in Kakadu, with seven species: four rodents, two species of dasyiurids (Finlayson et al. 2006) and the Water Buffalo, which is also introduced to this system (Woodward et al. 2011). We did not consider bats. In the Okavango Delta, there are 11 species, of which only the hippopotamus and 2 species of otter spend most of their lives in water, while others, such as the Water mongoose, Water and Cane rats and Swamp musk shrew, live predominantly in riparian habitats (Butchart 2016; Junk et al. 2006a). In addition, several domestic stock species that may use the floodplains but are predominantly terrestrial have been introduced to the three systems (and therefore not considered in the comparison). Cattle (Bos taurus) and horses (Equs caballus) are present in the three systems, while the Wild boar (Sus scrofa) can be found in the Pantanal and the Kakadu Region (Alho et al. 2011b; Finlayson et al. 2006; Woodward et al. 2011). Donkeys are common in the Okavango Delta (personal observation) and the Kakadu Region (Woodward et al. 2011). In the latter, camels (Camelus dromedarius) and goats (Capra hircus) have also been introduced (Woodward et al. 2011).
The total number of waterbirds in the Pantanal (“aquatic species” sensu Junk et al. 2006b) is 64, but unfortunately, the detailed list with the most abundant families is not available, as calculations by Junk et al. (2006b) were based on an unpublished study by Petermann. They reported the order Ciconiiformes as the most species rich. In the Kakadu Region, Finlayson et al. (2006) recorded 99 species of waterbirds (107 species in Kakadu National Park according to Junk et al. 2006a and 92 according to BMT WMB 2010). The family Scolopacidae (order Charadriiformes) is the richest, which in the Delta is the second richest. In the Delta we found 141 waterbird species, with the family Ardeidae (Order Pelecaniformes) being the richest, followed by Scolopacidae (Charadriiformes). As mentioned above, members of this family have an omnivorous and opportunistic diet.
Regarding endemic species, the Kakadu Region has the highest number, since there is a unique family of shrimps (Kakaducarididae) with one genus (Leptopalaemon: 5 species) and a genus of isopod (Eophreatoicus: 28 species) only found in Kakadu National Park and western Arnhem land, as well as one species of fish (Pingalla midgleyi) and two species of amphibians (Litoria personata and Uperoleia arenicola). Furthermore, a few species are only found in northern Australia (Finlayson et al. 2006; IUCN, 2023). In the Pantanal, very few endemics occur, and according to Pott et al. (2011) only the peanut Arachis vallsii Krapov. and W.C.Greg. can be considered aquatic, although there are a few very rare plant species or with very restricted distribution as well. In the Okavango Delta only two species of algae are confirmed endemics (Cosmarium pseudosulcatum var. okavangicum and Eunotia okawangoi).
Conclusions and future research
This study compiled the current knowledge at species level in the Okavango Delta and Lake Ngami; future research should focus on understudied groups: bacteria, parasites, Chironomidae, Ephemeroptera, Nematoda, Oligochaeta, Trichoptera and specific taxa within Crustacea (Anostraca, Notostraca, Ostracoda and the former Conchostraca). The flood pulse is the main force shaping the composition of all groups of freshwater organisms in the three wetland systems compared in this review, which have developed a broad array of adaptations to live in a challenging environment with constant water level fluctuations and physicochemical changes. Common traits to deal with water changes found across taxa are the following: omnivorous diet (feeding on aquatic or terrestrial prey), resistance forms (spores, cysts, etc.), short generation times and/or several generations per year. Despite the geographical distance, the three systems share a considerable number of taxa within the main groups considered, as many species have cosmopolitan or circumtropical distribution (De-La-Monica-Freire and Heckman 1996; Hardoim and Heckman 1996; Heckman 1998). However, there are also some endangered and rare or restricted-distribution species, which contribute to the value of these systems for biodiversity and conservation. Maintaining the natural flow regime that creates the heterogeneity of habitats in the Okavango Delta is key for many groups of plants and animals and should be a priority in the management of the system. Any future developments within or upstream of the Delta must be considered with caution to preserve its delicate balance and pristine nature.
Data availability
The dataset is available in the Zenodo repository following this link: https://doi.org/10.5281/zenodo.8290159
References
Akinyemi FO, Abiodun BJ (2019) Potential impacts of global warming levels 1.5 °C and above on climate extremes in Botswana. Clim Change 154:387–400. https://doi.org/10.1007/s10584-019-02446-1
Albert JS, Destouni G, Duke-Sylvester SM, Magurran AE, Oberdorff T, Reis RE, Winemiller KO, Ripple WJ (2020) Scientists warning to humanity on the freshwater biodiversity crisis. Ambio 50:85–94. https://doi.org/10.1007/s13280-020-01318-8
Alho CJR, Camargo G, Fischer E (2011a) Terrestrial and aquatic mammals of the Pantanal. Braz J Biol 71:297–310. https://doi.org/10.1590/S1519-69842011000200009
Alho CJR, Mamede S, Bitencourt K, Benites M (2011) Introduced species in the Pantanal: implications for conservation. Braz J Biol 71:321–325. https://doi.org/10.1590/S1519-69842011000200011
Appleton CC (1979) The Unionacea (Mollusca, Lamellibranchiata) of South-Central Africa. Ann South Afr Mus 77:151–174
Appleton CC, Curtis BA, Alonso LE, Kipping J (2003) Chap. 4: Freshwater Invertebrates of the Okavango Delta, Botswana. In: Alonso LE, Nordin L (eds) A rapid biological assessment of the aquatic ecosystems of the Okavango Delta, Botswana: High Water Survey. RAP Bulletin of Biological Assessment, 27. Conservation International, Washington DC, pp 58–68. https://www.conservation.org/docs/default-source/publication-pdfs/rap27_okavango_delta_botswana_jun-2000.pdf?Status=Master&sfvrsn=fcff068e_3
Bartlam-Brooks HLA, Bonyongo MC, Harris S (2011) Will reconnecting ecosystems allow long-distance mammal migrations to resume? A case study of a zebra Equus burchelli migration in Botswana. Oryx 45:210–216. https://doi.org/10.1017/S0030605310000414
Bilardo A, Rocchi S (1987) Contributo alla conoscenza degli Haliplidae e dei Dytiscidae del Botswana. Atti Societa Italiana di Scienze Naturali. Museo Civico di Storia Naturale di Milano 128:15–106
BMT WBM (2010) Ecological Character Description for Kakadu National Park Ramsar Site. Australian Government. Department of Sustainability, Environment, Water, Population and Communities. https://rsis.ramsar.org/RISapp/files/29354130/documents/AU204_ECD1510.pdf
Bragança PHN, Van Zeeventer RM, Bills R, Tweddle D, Chakona A (2020) Diversity of the southern Africa Lacustricola Myers, 1924 and redescription of Lacustricola johnstoni (Günther, 1894) and Lacustricola myaposae (Boulenger, 1908) (Cyprinodontiformes, Procatopodidae). ZooKeys 923:91–113. https://doi.org/10.3897/zookeys.923.48420
Bragança PHN, Skelton PH, Bills R, Tweddle D, Chakona A (2021) Revalidation and Redescription of Lacustricola chobensis (Fowler, 1935) and Description of a New Miniature Species of Lacustricola from Southern Africa (Cyprinodontiformes: Procatopodidae). Ichthyol Herpetol 109:123–137. https://doi.org/10.1643/i2020046
Butchart D (2016) Wildlife of the Okavango. Penguin Random House South Africa Ltd., Midrand, South AFrica
Buxton M, Lebani K, Nyamukondiwa C, Wasserman RJ (2019) First record of Aedes (Stegomyia) aegypti (Linnaeus, 1762) (Diptera: Culicidae) in Botswana. BioInvasions Rec 8:551–557. https://doi.org/10.3391/bir.2019.8.3.10
Buxton M, Nyamukondiwa C, Wasserman RJ, Othenin-Girard V, Pigeault R, Christe P, Glaizot O (2021) Surveillance Studies Reveal Diverse and Potentially Pathogenic-Incriminated Vector Mosquito Species across Major Botswana Touristic Hotspots. Insects 12:913. https://doi.org/10.3390/insects12100913
Chakona A, Jordaan MS, Raimondo DC, Bills RI, Skelton PH, Van Der Colff D (2022) Diversity, distribution and extinction risk of native freshwater fishes of South Africa. J Fish Biol 100:1044–1061. https://doi.org/10.1111/jfb.15011
Cholnoky BJ (1966) Die diatomeen in unterlaufe des okawango-flussen. In: Cholnoky BJ (ed) Diatomeaceae I. Beihefte zur Nova Hedwigia Suppl. 21, pp 1–102
Coesel P, Duque SR (1988) Distributional patterns in some neotropical desmid species (Algae, Chlorophyta) in relation to migratory bird routes. Revue d'hydrobiologie tropicale 21:197–205. https://horizon.documentation.ird.fr/exl-doc/pleins_textes/cahiers/hydrob-trop/26434.pdf
Crome FHJ, Carpenter SM (1988) Plankton community cycling and recovery after drought - dynamics in a basin on a flood plain. Hydrobiologia 164:193–211. https://doi.org/10.1007/BF00005940
Cronberg G, Gieske A, Martins E, Prince Nengu J, Stenström I-M (1995) Hydrobiological Studies of the Okavango Delta and Kwando/Linyanti/Chobe River, Botswana I Surface Water Quality Analysis. Botsw Notes Records 27:151–226. http://www.jstor.org/stable/40980045
Cronberg G, Gieske A, Martins E, Prince Nengu J, Stenström I-M (1996) Major ion chemistry, plankton, and bacterial assemblages of the Jao/Boro River, Okavango Delta, Botswana: the swamps and flood plains. Archiv für Hydrobiologie 107:335–407
Cowie ID, Short PS, Osterkamp P, Madsen M (eds) (2000) Floodplain Flora: A Flora of the Coastal Floodplains of the Northern Territory, Australia. ABRS, Canberra, Australia. https://doi.org/10.2307/4119447
Dallas HF, Mosepele B (2007) A preliminary survey and analysis of the spatial distribution of aquatic invertebrates in the Okavango Delta, Botswana. Afr J Aquat Sci 32:1–11. https://doi.org/10.2989/AJAS.2007.32.1.1.138
Dallas HF, Mosepele B (2020) Spatial variability of aquatic macroinvertebrate assemblages in the Okavango Delta, Botswana: considerations for developing a rapid bioassessment tool. Afr J Aquat Sci 45:350–363. https://doi.org/10.2989/16085914.2019.1704215
Darwall WRT, Tweddle D, Skelton PH, Smith KS (2009) Chap. 1: Background. In: Darwall WRT, Smith KG, Tweddle D, Skelton P (eds) The Status and Distribution of Freshwater Biodiversity in Southern Africa. IUCN and Grahamstown, Gland, Switzerland SAIAB, South Africa, pp 1–15
Davidson TH, Mackay AW, Wolski P, Mazebedi R, Murray-Hudson M, Todd M (2012) Seasonal and spatial hydrological variability drives aquatic biodiversity in a flood-pulsed, sub-tropical wetland. Freshw Biol 57:1253–1265. https://doi.org/10.1111/j.1365-2427.2012.02795.x
De-Lamonica-Freire E, Heckman CW (1996) The Seasonal Succession of Biotic communities in wetlands of the tropical Wet-and-dry climatic Zone III: the Algal communities in the Pantanal of Mato Grosso, Brazil, with a Comprehensive List of the known species and revision of two desmid taxa. Int Revue ges Hydrobiol 81:253–280. https://doi.org/10.1002/iroh.19960810209
Do Prado AL, Heckman CW, Martins FR (1994) The Seasonal Succession of Biotic Communities in Wetlands of the Tropical Wet-and-Dry Climatic Zone: 11. The Aquatic Macrophyte Vegetation in the Pantanal of Mato Grosso, Brazil. Int Revue ges Hydrobiol 79:569–589. https://doi.org/10.1002/iroh.19940790407
Dudgeon D, Arthington AH, Gessner MO, Kawabata Z-I, Knowler DJ, Lévêque C, Naiman RJ, Prieur-Richard AH, Soto D, Stiassny MLJ, Sullivan CA (2009) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163–182. https://doi.org/10.1017/S1464793105006950
Ellery K, Ellery W (1997) Plants of the Okavango Delta: A field guide. Tsaro Publisher, Durban
Ellery WN (2003) Appendix 7 Plant species recorded from the Okavango Delta. In: Alonso LE, Nordin L (eds) A rapid biological assessment of the aquatic ecosystems of the Okavango Delta, Botswana: High Water Survey RAP Bulletin of Biological Assessment 27. Conservation International, Washington DC, pp 148–181
Encyclopedia of Life (EOL) (2021). https://eol.org Accessed 6 October 2021
Finlayson CM, Bailey BJ, Cowie ID (1989) Macrophyte Vegetation of the Magela Creek flood plain, Alligator River Region, Northern Territory. Research Report No. 5. Australian Government Publishing Service, Canberra. https://www.dcceew.gov.au/sites/default/files/documents/rr5.pdf Accessed 15 April 2023
Finlayson CM, Lowry J, Bellio MG, Nou S, Pidgeon R, Walden D, Humphrey C, Fox G (2006) Biodiversity of the wetlands of the Kakadu Region, northern Australia. Aquat Sci 69:374–399. https://doi.org/10.1007/s00027-006-0852-3
Francis R, Bino G, Inman V, Brandis K, Kingsford RT (2021) The Okavango Delta’s waterbirds – Trends and threatening processes. Global Ecol Conserv 30:e01763. https://doi.org/10.1016/j.gecco.2021.e01763
GBIF.org (2021) GBIF Home Page. https://www.gbif.org. Accessed 15 August 2021
Google M (2022) Okavango Delta. Satellite image. https://www.google.co.uk/maps/place/Okavango+Delta/@19.3186955,22.4455928,9z/data=!3m1!4b1!4m5!3m4!1s0x19566cd6a10d2727:0x415c0ceb3cb2bae!8m2!3d-19.6510095!4d22.9058802. Accessed 1 June 2021
Griffiths C, Picker M, Day J (2015) Freshwater Life. A Field Guide to the Plants and Animals of Southern Africa. Penguin Random House, Cape Town. (South Africa)
Hancock P, Muller M, Tyler S (2007) Inventory of Birds of the Okavango Delta Ramsar Site. Babbler 49:3–29
Hardoim EL, Heckman CW (1996) The Seasonal Succession of Biotic Communities in Wetlands of the Tropical Wet-and-Dry Climatic Zone: IV. The Free-Living Sarcodines and Ciliates of the Pantanal of Mato Grosso, Brazil. Int Revue ges Hydrobiol 81:367–384. https://doi.org/10.1002/iroh.19960810307
Harebottle DM, D’Arcy P, Hancock P, Hearn RD, Wheeler M, Brewster C (2006) Report on a waterbird ringing study at Lake Ngami, Botswana. African Avian Demography Unit Research Report no 70. Department of Statistical Sciences, University of Cape Town
Hart RC (1997) A Limnological profile of the Upper Okavango Delta at low water level. South Afr J Aquat Sci 23:21–33. https://doi.org/10.1080/10183469.1997.9631398
Heckman CW (1998) The Seasonal Succession of Biotic Communities in Wetlands of the Tropical Wet-and-Dry Climatic Zone: V. Aquatic Invertebrate Communities in the Pantanal of Mato Grosso, Brazil. Int Revue ges Hydrobiol 83:31–63. https://doi.org/10.1002/iroh.19980830105
Heyns J, Coomans A (1991) Longidoridae from Botswana (Nematoda). Phytophylactica 23:29–37. https://journals.co.za/doi/pdf/10.10520/AJA03701263_1436
Högberg P, Lindholm M, Ramberg L, Hessen DO (2002) Aquatic food web dynamics on a floodplain in the Okavango delta. Botsw Hydrobiologia 470:23–30. https://doi.org/10.1023/A:1015693520169
Humphries MS, Benitez-Nelson CR, Bizimis M, Ralph TJ, Larkin ZT, McCarthy TS (2020) Dust provenance and its role as a potential fertilizing agent for the Okavango Delta, Botswana. Earth Surf Proc Land 45:1705–1716. https://doi.org/10.1002/esp.4840
Hyde MA, Wursten BT, Ballings P, Coates MP (2023) Flora of Botswana https://www.botswanaflora.com. Accessed 27 Februrary 2023
iNaturalist contributors iNaturalist (2022) iNaturalist Research-grade Observations. iNaturalist.org. Occurrence dataset. https://doi.org/10.15468/ab3s5x. Accessed via GBIF.org 4 September 2021
IUCN (2023) The IUCN Red List of Threatened Species. Version 2023-2. https://www.iucnredlist.org. Accessed 15 April 2023
Junk WJ, Brown M, Campbell IC, Finlayson M, Gopal B, Ramberg L, Warner BG (2006a) The comparative biodiversity of seven globally important wetlands: a synthesis. Aquat Sci 68:400–414. https://doi.org/10.1007/s00027-006-0856-z
Junk WJ, da Cunha CN, Wantzen KN, Petermann P, Strüssmann C, Marques MI, Adis J (2006b) Biodiversity and its conservation in the Pantanal of Mato Grosso, Brazil. Aquat Sci 68:278–309. https://doi.org/10.1007/s00027-006-0851-4
Keddy PA, Fraser LH, Solomeshch AI, Junk WJ, Campbell DR, Arroyo MT, Alho CJ (2009) Wet and wonderful: the world's largest wetlands are conservation priorities. Bioscience 59:39–51. https://doi.org/10.1525/bio.2009.59.1.8
Kemosedile T, Khaneguba W, Mothobi R, Mogojwa B, Mosie I, Makati K, Murray-Hudson M (2020) Long Term Time-Series Data on Aquatic Macro-invertebrates Monitoring by Okavango Research Institute, Botswana. Version 1.2. Okavango Research Institute. Sampling event dataset. https://doi.org/10.15468/kcjznw Accessed 1 December 2022
Kipping J (2010) The dragonflies and damselflies of Botswana –an annotated checklist with notes on distribution, phenology, habitats and Red List status of the species (Insecta: Odonata). Mauritiana (Altenburg) 21:126–204
Kristiansen J (1996) Dispersal of freshwater algae–a review. Hydrobiologia 336:151–157. https://doi.org/10.1007/BF00010829
Kurugundla CN, Parida BP, Buru JC (2018) Revisiting Hydrology of Lake Ngami in Botswana. Hydrol: Curr Res. https://doi.org/10.4172/2157-7587.1000301
Lake PS (2011) Drought and Aquatic Ecosystems: Effects and Responses. Wiley-Blackwell, Hoboken, New Jersey. https://doi.org/10.1002/9781444341812
Le Roux M (2010) Amphibian diversity and breeding behaviour in the Okavango Delta. Dissertation, North West University, Potchefstroom (South Africa). https://repository.nwu.ac.za/handle/10394/4467. Accessed 3 Mar 2023
Lindholm M (2002) Predator-induced cyclomorphosis of Daphnia laevis (Branchiopoda, Cladocera) in a tropical floodplain (Okavango Delta, Botswana). Crustaceana 75:803–814. https://doi.org/10.1163/156854002760289773
Lindholm M, Hessen DO (2007a) Zooplankton succession on seasonal floodplains: surfing on a wave of food. Hydrobiologia 592:95–104. https://doi.org/10.1007/s10750-007-0713-7
Lindholm M, Hessen DO (2007b) Competition and niche partitioning in a floodplain ecosystem: a cladoceran community squeezed between fish and invertebrate predation. Afr Zool 42:158–164. https://doi.org/10.1080/15627020.2007.11407392
Lindholm M, Hessen DO, Ramberg L (2009) Diversity, dispersal and disturbance: cladoceran species composition in the Okavango Delta. Afr Zool 44:24–35. https://doi.org/10.1080/15627020.2009.11407436
Lytle DA, Olden JD, McMullen LE (2008) Drought-escape behaviors of aquatic insects may be adaptations to highly variable flow regimes characteristic of desert rivers. Southwest Nat 53:399–402. http://www.jstor.org/stable/20424945
Mace GM (2004) The role of taxonomy in species conservation. Philos Trans Royal Soc 359:711–719. https://doi.org/10.1098/rstb.2003.1454
Marazzi L (2014) Biodiversity and Biomass of Algae in the Okavango Delta (Botswana), a Subtropical Flood-Pulsed Wetland. Dissertation, University College of London. https://discovery.ucl.ac.uk/id/eprint/1459199
Marazzi L, Mackay AW, Mazebedi R, Jones VJ (2023) Assemblage Patterns of Microalgae along the Upstream to Downstream Gradient of the Okavango Delta: Abundance, Taxonomic Diversity, and Functional Diversity. Water 15:2692. https://doi.org/10.3390/w15152692
Mazebedi R (2019) Trophic characteristics of aquatic habitats with different flooding regimes in the Okavango Delta, Botswana. Dissertation, University College of London. https://discovery.ucl.ac.uk/id/eprint/10073384
Mbaiwa JE (2011) Changes on traditional livelihood activities and lifestyles caused by tourism development in the Okavango Delta. Botsw Tourism Manage 32:1050–1060. https://doi.org/10.1016/j.tourman.2010.09.002
Mendelsohn JM, vanderPost C, Ramberg L, Murray-Hudson M, Wolski P, Mosepele K (2010) Okavango Delta: Floods of Life. RAISON, Windhoek, Namibia
Merron GS (1991) The ecology and management of the fishes of the Okavango Delta, Botswana, with particular reference to the role of the seasonal floods. Dissertation, Rhodes University. http://hdl.handle.net/10962/d1005112
Merron GS (1993) The diversity, distribution and abundance of the fishes in the Moremi Wildlife Reserve, Okavango Delta, Botswana. South Afr J Wildl Res 23:115–122.https://doi.org/10.10520/EJC116934
Merron GS, Bruton MN (1995) Community ecology and conservation of the fishes of the Okavango Delta. Botsw Environ Biology Fishes 43:109–119. https://doi.org/10.1007/BF00002478
Mogobe O, Mosimanyana E, Masamba WRL, Mosepele K (2018) Monitoring water quality of the Upper Okavango Delta. In: Revermann R, Krewenka KM, Schmiedel U, Olwoch JM, Helmschrot J, Jürgens N (eds) Climate change and adaptive land management in southern Africa - assessments, changes, challenges, and solutions. Biodiversity and Ecology, vol 6. Klaus Hess Publishers, Göttingen and Windhoek, pp 106–111. https://doi.org/10.7809/b-e.00311
Mogobe O, Masamba WRL, Mosepele K (2019) Trace metal concentrations in a pristine Ramsar site: the Okavango Delta. SN Appl Sci. https://doi.org/10.1007/s42452-019-1602-1
Mosepele K, Peter BM, Glenn SM, David RP, Mosepele B (2009) Fish, Floods, and Ecosystem Engineers: Aquatic Conservation in the Okavango Delta. Botsw BioScience 59:53–64. https://doi.org/10.1525/bio.2009.59.1.9
Mosepele B, Mosepele K (2021) Chap. 11: Review of Aquatic Biodiversity Dynamics in the Okavango Delta: Resilience in a Highly Fluctuating Environment. In: Devlin A, Pan J, Manjur Shah M (eds) Inland Waters-Dynamics and Ecology. IntechOpen, London, p 1
Murray-Hudson M, Wolski P, Murray-Hudson F, Brown MT, Kashe K (2014) Disaggregating Hydroperiod: Components of the Seasonal Flood Pulse as Drivers of Plant Species Distribution in Floodplains of a Tropical Wetland. Wetlands 34:927–942. https://doi.org/10.1007/s13157-014-0554-x
Naturgucker.de (2021) Occurrence dataset. https://www.gbif.org/dataset/6ac3f774-d9fb-4796-b3e9-92bf6c81c084 Accessed via GBIF.org 3 October 2021. https://doi.org/10.15468/uc1apo
Nelson TM, Streten C, Gibb KS, Chariton AA (2016) Bacteria in tropical floodplain soils are sensitive to changes in saltwater. Mar Freshw Res 69:1110–1123
OpenStreetMap contributors (2022) MapCarta. https://mapcarta.com. Accessed 5 June 2020
Oren A, Mares J, Rippka R (2022) Validation of the names Cyanobacterium and Cyanobacterium stanieri, and proposal of Cyanobacteriota phyl. nov. Int J Syst Evol MicroBiol 72:5528. https://doi.org/10.1099/ijsem.0.005528
Pachka H, Annelise T, Alan K, Power T, Patrick K, Véronique C, Janusz P, Ferran J (2016) Rift Valley fever vector diversity and impact of meteorological and environmental factors on Culex pipiens dynamics in the Okavango Delta, Botswana. Parasites and Vectors 9:434. https://doi.org/10.1186/s13071-016-1712-1
Pinhey E (1976) Dragonflies (Odonata) of Botswana, with ecological notes. Occasional Papers of the National Museums and Monuments of Rhodesia 5:524–610
Pott VJ, Pott A, Lima LCP, Moreira SN, Oliveira AKM (2011) Aquatic macrophyte diversity of the Pantanal wetland and upper basin. Braz J Biol 71:255–263
POWO (2023) Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. http://www.plantsoftheworldonline.org/. Accessed 23 February 2023
Press T, Brock J, Anderson A (1995) Fauna. In: Press T, Lea D, Webb A, Graham A (eds) Kakadu: Natural and Cultural Heritage and Management. Australian Nature Conservation Agency, North Australia Research Unit, The Australian National University, Darwin, Australia, pp. 167–216. https://openresearch-repository.anu.edu.au/bitstream/1885/46993/3/KakaduNaturalandCulturalHeritageandManagement2.pdf
Ramberg L, Hancock P, Lindholm M, Meyer T, Ringrose S, Sliva J, Van As J, VanderPost C (2006) Species diversity of the Okavango Delta, Botswana. Aquat Sci 68:310–337. https://doi.org/10.1007/s00027-006-0857-y
Ramsar Sites Information Service 2.0 (2021). https://rsis.ramsar.org/ris/879. Accessed 4 Oct 2023
Rasmussen JD (1997) On two little known African water snakes (Crotaphopeltis degeni and C. barotseensis). Amphibia-Reptilia 18:191–206. https://doi.org/10.1163/156853897X00062
RapidTables.com (2020) Decimal Degrees to Degrees, Minutes, Seconds conversion tool. https://www.rapidtables.com/convert/number/degrees-to-degrees-minutes-seconds.html Accessed 1
Reid AJ, Carlson AK, Creed IF, Eliason EJ, Gell PA, Johnson PTJ, Kidd KA, MacCormack TJ, Olden JD, Ormerod SJ, Smol JP, Taylor WW, Tockner K, Vermaire JC, Dudgeon D, Cooke SJ (2019) Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol Rev 94:849–873. https://doi.org/10.1111/brv.12480
Reynolds C, Cumming GS (2015) The role of waterbirds in the dispersal of freshwater cladocera and bryozoa in southern Africa. Afr Zool 50:307–311. https://doi.org/10.1080/15627020.2015.1108164
Shine R (1986) Diets and abundances of aquatic and semi-aquatic reptiles in Alligator Rivers Region. Technical Memorandum 16, Supervising Scientist for the Alligator Rivers Region. Australian Government Publishing Service, Canberra, Australia. https://www.dcceew.gov.au/sites/default/files/documents/tm16.pdf
Silva Nunes VD, Calheiros DF, Oliveira MD, Duran NL (2001) Preliminary Assessment of Bacteria Abundance in Rivers of the Pantanal Wetlands – Brazil. In: Phelps D, Shelke G (eds) Bridging the Gap: Meeting the World's Water and Environmental Resources Challenges. American Society of Civil Engineers. https://doi.org/10.1061/9780784405697
Siziba N, Chimbari MJ, Masundire J, Mosepele K (2011) Spatial variations of microinvertebrates across different microhabitats of temporary floodplains of lower Okavango Delta, Botswana. Afr J Ecol 50:43–52. https://doi.org/10.1111/j.1365-2028.2011.01289.x
Siziba N, Chimbari MJ, Mosepele K, Masundire H, Ramberg L (2013) Inundation frequency and viability of microcrustacean propagules in soils of temporary aquatic habitats of lower Okavango Delta. Botsw Ecohydrology 6:722–730. https://doi.org/10.1002/eco.1293
Skelton PH (1996) A historical review of the taxonomy and biogeography of freshwater fishes in South Africa - the past 50 years. Trans Royal Soc South Afr 51:91–114. https://doi.org/10.1080/00359199609520602
Smit H (2012) New records of the water mite family Arrenuridae from the Afrotropical region, with the description of 11 new species and two new subspecies (Acari: Hydrachnidia). Zootaxa 3187:1–31. https://doi.org/10.11646/zootaxa.3187.1.1
Štastný J (2008) Desmids from ephemeral pools and aerophytic habitats from the Czech Republic. Biologia 63/6:888–894. https://doi.org/10.2478/s11756-008-0138-4
Suhling F, Martens A (2007) Dragonflies and damselflies of Namibia. Gamsberg McMillan Publishers, Windhoek
Szwarc A, Martens K, Namiotko T (2021) Two new Cypridopsinae Kaufmann, 1900 (Crustacea, Ostracoda) from southern Africa. ZooKeys 1076:83–107. https://doi.org/10.3897/zookeys.1076.76123
UNESCO World Heritage Centre (2014) World Heritage List reaches 1000 sites with inscription of Okavango Delta in Botswana. https://whc.unesco.org/en/news/1159. Accessed 4 Oct 2023
Van As LL, Van As JG (2015) Branchiuran parasites (Crustacea: Branchiura) from fishes in the Okavango (Botswana) and Zambezi (Namibia) systems. Afr J Aquat Sci 40:9–20. https://doi.org/10.2989/16085914.2015.1014995
Van Rensburg CJ (2001) Snail borne larval trematodes of the Okavango Delta, Botswana. Dissertation, University of the Free State. http://hdl.handle.net/11660/7458
Vanschoenwinkel B, Waterkeyn A, Nhiwatiwa T, Pinceel T, Spooren E, Geerts A, Clegg B, Brendonck L (2011) Passive external transport of freshwater invertebrates by elephant and other mud-wallowing mammals in an African savannah habitat. Freshw Biol 56:1606–1619. https://doi.org/10.1111/j.1365-2427.2011.02600.x
Vega Hernández E, Caudales R (2001) Pteridophytes of the Okavango Delta (Botswana), Southern Africa. Anales Jardín Botánico de Madrid 58:311–323
Vidrine MF, Lötter Z, Van As JG, Bastian-Stanford M, Hazelton-Robichaux SR (2006) Unionicola (Iridinicola) botswaniana n. subgen., n. sp. (Acari: Unionicolidae) from freshwater mussels in Botswana. Int J Acarol 32:297–300. https://doi.org/10.1080/01647950608684472
Vidrine MF, Lötter Z, Van As JG, Bogan AE, Bastian-Stanford M (2007) Unionicola (Coelaturicola) gledhilli n. subgen., n. sp. (Acari: Unionicolidae) from freshwater mussels in Botswana and East Africa. Int J Acarol 33:167–171. https://doi.org/10.1080/01647950708684519
Viets KO (1980) New Arrenurus Species (Acari, Hydrachnellae, Arrenuridae) from Southern Africa. J Limnological Soc South Afr 6:12–18. https://doi.org/10.1080/03779688.1980.9633201
West DT (2016) Zooplankton of the Okavango Delta and associated basins in Botswana. Dissertation, University of the Free State. https://scholar.ufs.ac.za/handle/11660/5399
WFO (2023) World Flora Online. http://www.worldfloraonline.org. Accessed 03 February 2023
Williamson DB, Marazzi L (2013) A new Cosmarium (Chlorophyta, Desmidiaceae) variety from the Okavango Delta, Botswana. Quekett J Microsc 42:35–37
Wolski P, Murray-Hudson M (2007) Managing water abstractions for preserving the Okavango wetlands, Botswana. In: Okruszko T, Maltby E, Szatylowicz J, Miroslaw-Swiatek D (eds) Wetlands: monitoring, modelling and management. Taylor and Francis Group, London. https://doi.org/10.1201/9781482288476
Woodward E, Bayliss P, Dray A, Perez P (2011) Participatory Modelling Workshops for Feral Animal Management in Kakadu National Park: May 2010. CSIRO Publishing, Clayton, Australia. https://publications.csiro.au/rpr/download?pid=csiro:EP158283&dsid=DS2
Acknowledgments
We thank Dr Hammami Pachka for sharing her dataset, Rafaël Govaerts (Kew Gardens) for giving advice on the taxonomy and distribution of some species of macrophytes, Dr Timothy S. Wood (Bryo Technologies) and Dr Beth Okamura (NHM) for confirming the identification of Bryozoan specimens. We also thank Dr Michael Geiser (NHM) and Keita Matsumoto (NHM) for helping with the identification and taxonomy of Coleoptera. We thank the Okavango Research Institute (ORI) for giving advice and providing boats and sampling equipment. We are grateful to Billy Mogojwa and Ineelo Mosie for their assistance in the field. We thank two anonymous reviewers for their kind and insightful comments, which helped to improve the manuscript. Finally, we thank all the scientists cited in this work and supplementary material, whose dedication and determination made possible the advance of taxonomy in Southern Africa during the last century, many times working in challenging environments.
Funding
This work was supported by the Natural Environment Research Council (grant no. NE/S007229/1).
Author information
Authors and Affiliations
Contributions
K.M. led field sampling. L.M.C. led the writing of the manuscript, with M.A.C., J.A.C., B.W.P. and A.W.M. All authors contributed to editing and read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moliner Cachazo, L., Makati, K., Chadwick, M.A. et al. A review of the freshwater diversity in the Okavango Delta and Lake Ngami (Botswana): taxonomic composition, ecology, comparison with similar systems and conservation status. Aquat Sci 85, 115 (2023). https://doi.org/10.1007/s00027-023-01008-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-023-01008-z