Abstract
As a type of wetland ecosystem with off-season 30 m water level fluctuation, the huge changes in the ecological environment, plant species, and vegetation dynamics in the hydro-fluctuation zone of the Three Gorges Reservoir (TGR) area have attracted a wide range of attention. In this present study, six typical locations in the water level fluctuating zone were used as the research objects, and the effects of different water surface elevations on the stoichiometric characteristics and homeostasis of leaf nitrogen and phosphorus were studied through a sample survey method. Results revealed that leaf nitrogen content was linearly correlated with leaf phosphorus content along water surface elevation. And water surface elevation significantly affected the nitrogen and phosphorus content of dominant plants. Four dominant species [Cynodon dactylon (Linn.) Pers, Xanthium sibiricum Partin ex Wider, Abutilon theophrasti Medik, and Bidens pilosa Linn] exhibited specific differences in the phosphorus steady state index (Hp) and nitrogen steady state index (HN). Although belonging to different categories, both HP and HN of four dominant species were in the same order: X. sibiricum > A. theophrasti > C. dactylon > B. Pilosa. The interspecific differences in HN and HP indicated that there were differences in the characteristics of nutrient utilization of dominant species and their adaption to water surface elevation. Furthermore, as the elevation increases, the community coverage increased and the community stability index also increased. This might indicate that in the fluctuating zone habitat, the plant’s nitrogen and phosphorus utilization strategy affects the distribution and composition of plant community along water surface elevation, and ultimately affects the stoichiometric homeostasis on the community levels.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
As a powerful tool in ecology and biology, ecostoichiometry has been deeply applied to the assessment and interpretation of different ecosystems and ecological processes, such as individuals, populations, communities, and ecosystems (Sterner and Elser 2002; Han et al. 2005). A lot of studies have focused on the balance of chemical elements (Elser et al. 2009; Karimi and Folt 2006; Yu et al. 2011). Among them, the N/P ratio of plants can be widely used to judge nitrogen and phosphorus nutrient limitation to study the adaptability and stability of organisms to ecosystem changes (Güsewell 2004; He et al. 2008; Elser et al. 2009; Hu et al. 2014; Yu 2010, 2015; Tian et al. 2018).
Stoichiometric homeostasis, as a vital concept in ecological stoichiometry (Sterner and Elser 2002), depends on resource constraints, growth rates and growth constraints (Sistla et al. 2015). The stoichiometric homeostasis can reflect the potential physiological and biochemical distribution of biological reactions in the ecological system, and also reflects the ability of plants to sustain their own stability in different ecological environments (Elser et al. 2010; Yu et al. 2010). For example, species with a high steady-state index (H) are highly competitive and stable in terms of nutrition, while species with a low steady-state index (H), although growing faster and able to make full use of nutrients for growth in the case of adequate nutrition, are less stable and have a weak adaptability to the surrounding environment (Frost et al. 2005). Leaf steady-state index H is the most important stoichiometric characteristic to predict the success of plant species’ competition and the diversity of resource supply (Yu et al. 2010; Sardans et al. 2012).
To date, a few studies have reported stoichiometric homoeostasis index (H) in terrestrial vascular plants (Güsewell 2004; Yu et al. 2010) and freshwater vascular plants (Li et al. 2013; Su et al. 2019). However, there are few studies on the ecological stoichiometric balance of plant species, the vegetation, and ecosystem in the hydro-fluctuation zone.
The Three Gorges Dam is mainly used to store water in summer with a water level of about 145 m, and in winter with a water level of about 175 m. In the continuous storage and release of water in the Three Gorges Reservoir (TGR) area, the water has formed a large water level difference and formed a significant hydro-fluctuation zone (Ye et al. 2011). This artificial water storage mechanism has led to significant changes in the ecological environment of the TGR area (Zhang et al. 2011). Under the fluctuation and disturbance of water level, these unstable factors led to further serious degradation of riparian ecosystem, leading to significant changes in plant community composition and structure (Wang et al. 2018). Under the influence of the fluctuation of water level, plants gradually degenerate after the flood disaster, but the vegetation exposed to the air grows and flourishes (Jian et al. 2018). Therefore, the life activities of the ecosystem in this region are very active, with biological diversity, frequent human activities and vulnerability of the ecological environment (Yin et al. 2019). Kong et al. (2020) reported the spatial variability of nutrient and stoichiometric characteristics among different plants of the littoral zone of TGR. However, as the dominant factor determining plant distribution (Wu et al. 2009), the effects of water level elevation on leaf nitrogen and phosphorus stoichiometry and stoichiometric homeostasis are not clear. In the study, we choose the section from Zhongxian County of Chongqing to Zigui County of Yichang City to carry out a field investigation. This aim of the study was to investigate the effects of water surface elevation on stoichiometric characteristics and homeostasis of leaf nitrogen and phosphorus in hydro-fluctuation zone of the TGR. Furthermore, we tried to explain the distribution and composition of the plant community by plant nitrogen and phosphorus utilization strategy to adapt to changed water surface elevation in the hydro-fluctuating habitat.
Materials and methods
Study sites and field sampling
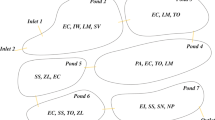
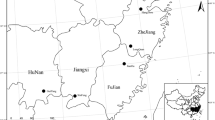
The section from Zhongxian County of Chongqing (N30°03′–30°35′, E107°32′– E108°14′) to Zigui County of Yichang City (N30°38′–31°11′, E114°18′– E111°0′) is located in the middle and downstream of the TGR from central Chongqing to the west of Hubei Province, with a total length of 419.5 km. The whole investigated area is located in subtropical monsoon humid climate region. In June 2018, a field investigation was carried out regarding nitrogen and phosphorus content of plant leaves and soil located in hydro-fluctuation zone of the section from Zhongxian County of Chongqing to Zigui County of Yichang City, which included six sampling sites. The six sampling sites are Shibao Zhai (N30°23′48″ E108°8′43″), Hanfeng Pool (N31°8′40″ E108°30′36″), Qukou Town (N31°7′26″E108°28′13″), Shuitian Dam (N31°2′52″ E110°41′53″), Wan’gu Temple (N30°1′3″ E110°44′51″), and Xiangxi River (N30°57′60″ E110°45′35″), respectively (Fig. 1). Field habits and work pictures at six sampling sites was shown as Fig. S1 in supplementary materials.
At each survey site, we selected a sampling section with less human interference, similar habitat characteristics, and obvious dominant species. Each section was divided into three water surface elevations (L:145–155 m, M: 155–165 m, H:165–175 m). Three 1 m × 1 m quadrats were set up at each elevation, with a total of 54 quadrats for six sampling sites. In each quadrat, 30–50 fresh, full, and complete leaves of each dominant species were randomly selected. The aboveground biomass was sampled by clipping all plants on the ground within a 1 m × 1 m quadrat, and all the plants were sorted to species and record. Relative biomass of one species was calculated as the ratio of biomass of single species to all species in a quadrat. Community coverage was obtained by visual grid method. At the same time, considering the distribution of plant communities, plant sampling was carried out in 30 m transects on both sides of the quadrat. All plant leaves with good growth in the transect were selected and brought back to the laboratory in dry envelope bags for testing.
For soil sampling, we also divided each sampling sites into three elevations: 145 m–155 m, 155 m–165 m, and 165 m–175 m above the sea level, and took three depths of each quadrat, 0–10 cm, 10 cm–20 cm, and 20 cm–30 cm, respectively. For each depth, we collected 0.2 g soil sample with the soil drilling machine and brought back to the laboratory for measurements.
Laboratory analysis
After being desiccated at 105 °C for 1 h, plants leaves were dried in a 60 °C oven for 48 h. Then, the leaves were ground with a grass grinder. The semimicro-Kjeldahl method, as described by Bremner (1996), was used to measure the total nitrogen concentration in leaves, using the digestion methods described by Moreno-Alvarado et al. (2017). After digestion according to He et al (2008), we measure the total P concentrations in leaves by the molybdate/stannous chloride method, as described by Kuo (1996).
Soil samples collected are dried until they lose moisture and ground through a 1-mm sieve to remove debris. Total N concentrations of the soil were also determined by the semimicro-Kjeldahl method (Bremner 1996). While the total phosphorus concentration in soil were determined by anti-spectrophotometry with sodium hydroxide and molybdenum antimony (Kuo 1996).
Calculation and analysis
According to Su et al. (2019), the stoichiometric steady-state H was calculated by the equation:
where y is the N (or P) content of plants, the x is the average N (or P) content of three different soil depths (0–10 cm, 10 cm–20 cm, and 20 cm–30 cm, respectively) and c is a constant.
To explore the effect of water-level elevation on plant leaf nutrients, we defined y as the average N (or P) content of plants from different sampling locations with the same water surface elevation, and defined x as the average N (or P) content of the soil from different sampling locations with the same water surface elevation.
We use SPSS software to complete data processing, analysis, and rendering. Community H (ComH) was calculated by the species relative biomass (ratio of biomass of single species to all species) multiplied by species H in a quadrat, as follows (Yu et al. 2010; Su et al. 2019):
where n is the total number of plant species in the quadrat, and i varies from 1 to n. Then, linear regression method was carried out to study the relationship between community coverage and community H.
Results
Nitrogen and phosphorus content of the plants with water surface elevation
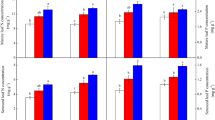
We took 120 plant samples in the ebb and fall zone of TGR, with 50 plant species included. The nitrogen content of these 120 plant samples ranged from 3.22 mg·g−1 to 68.37 mg·g−1, with an average value of 23.71 mg·g−1. The phosphorus content ranged from 0.22 mg·g−1 to 13.97 mg·g−1, with an average value of 3.29 mg·g−1. In the water surface elevation 145 m–155 m (L), 155 m–165 m (M), and 165 m–175 m (H), plant tissue N was significantly correlated with plant tissue P, and R2 values were 0.25 (p = 0.04), 0.37 (p < 0.001) and 0.28 (p < 0.001), respectively. Higher means of N content of plant tissue was found at H section (Fig. 2).
The number of plant species increased with increasing water surface elevation (12 species from 145 m to 155 m, 17 species from 155 m to 165 m, 45 species from 165 m to 175 m). For non-dominant species, the nitrogen content ranged from 8.40 mg·g−1 to 68.37 mg·g−1, with the average value of 30.07 mg·g−1. The phosphorus content ranges from 0.22 mg·g−1 to 13.97 mg·g−1, with the average value of 4.28 mg·g−1. Cynodon dactylon (Linn.) Pers, Abutilon theophrasti Medik, Xanthium sibiricum Partin ex Wider, and Bidens pilosa Linn were all found in three water surface elevations.
For four dominant species, the nitrogen content ranged from 5.22 mg·g−1 to 43.18 mg·g−1, with the average value of 19.43 mg·g−1. The phosphorus content ranges from 1.23 mg·g−1 to 9.29 mg·g−1, with the average value of 4.29 mg·g−1. The nitrogen and phosphorus content of X. sibiricum and A. theophrasti slightly increased with the water surface elevation increasing (Fig. 3). For C. dactylon, lower phosphorus and higher nitrogen content was found at the water surface elevation 155 m–165 m. For B. Pilosa, no significant difference of nitrogen and phosphorus content existed between different elevations.
Homeostasis index of nitrogen and phosphorus for dominant species in the hydro-fluctuation zone
The stoichiometric steady-state coefficient of phosphorus (HP) and nitrogen (HN) of four dominant species were showed in Figs. 4 and 5.
Along water level elevation, the nitrogen steady-state index (HN) and the phosphorus steady-state index (HP) of four dominance species ranged from 1.13 to 5.75 (Fig. 4) and from 1. 40 to 2.24 (Fig. 5), respectively. X. sibiricum has the highest value, while B. pilosa has the lowest value of HN and HP. Persson et al. (2010) pointed out that the HN (HP) of a species can vary from below 1.33 to above 4, including four categories. Therefore, B. pilosa was plastic for N element (0 < HN < 1.33) and weakly plastic for P element (1.33 < HP < 2), both C. dactylon and A. theophrasti were weakly homeostatic for N element (2 < HN < 4) and weakly plastic for P element (1.33 < HP < 2), while X. sibiricum was homeostatic for N (HN > 4) and weakly homeostatic (2 < HP < 4) for P element.
Relationship between community steady-state index and coverage
The community coverage from six sampling region increased with the elevation of water level. Linear regression revealed the community HN and HP were positively correlated with the community coverage (Fig. 6).
Discussion
The effect of water surface elevation on leaf nitrogen and phosphorus stoichiometry
A number of studies have demonstrated that plant N and P concentrations are significantly affected by temperature and latitude (Reich and Oleksyn 2004; Tang et al. 2018), habitat (Koerselman and Meuleman 1996; Yu et al. 2011), and plant functional type (Yu et al. 2011; Xia et al. 2014). In the present study, the phosphorus content of 120 plant samples ranged from 0.22 mg·g−1 to 12.97 mg·g−1, with an average of 3.29 mg·g−1, and the nitrogen content ranged from 3.22 mg·g−1 to 68.37 mg·g−1, with an average of 23.71 mg·g−1. Obviously, the mean nitrogen and phosphorus content of the plant species in the falling zone was higher than that of the grassland ecosystem (Han et al. 2005) and the global leaf metering model (Reich and Oleksyn 2004), while close to wetland plant (Hu et al. 2014) and aquatic plants of eastern China (Xia et al. 2014). This indicates that plants of hydro-fluctuation zone of TGR (a special wetland type) gradually forms stoichiometric characteristics similar to wetland vegetation and aquatic vegetation in the process of vegetation evolution (Kong et al. 2020). As a kind of wetland ecosystem, plants in the falling zone always face a variety of stress environments (Xu et al 2009), and water level elevation is the dominant factor determining plant distribution (Wu et al 2009) and vegetation restoration (Zhu et al. 2020). And after the impoundment, the number of plant species in the hydro-fluctuation belt of TGR area decreased significantly, with more single genus and single species and the community composition was simplified (Lei et al 2014; Ke et al. 2020). Under the anti-seasonal periodic fluctuation of water level, the life forms of herbaceous plants (including annual and perennial plants) have replaced the life forms of trees, shrubs, and vines as the dominant life forms of plants in the L and M elevations (145 m–165 m) of hydro-fluctuation belt of TGR (Guo et al. 2019; Ke et al. 2020). In the study, the variation of species number and species composition with the elevation (Fig. 2) confirmed it. In an in situ experiment, Li et al. reported that N and P stoichiometry of submerged plants was not directly affected by water level, but by the species itself (Li et al. 2013). Therefore, plant species composition is an important factor affecting stoichiometric characteristics at species level and community level. In the study, the means of N content of four dominant species is lower than that of non-dominant species, and the variation of leaf nitrogen and phosphorus content with elevation existed with specific differences (Fig. 3). As mentioned above, different life forms and species composition existed at different elevations, which resulted in the different stoichiometric characteristics. This could partly explain the higher mean of nitrogen content of the plant species found at the elevation of 165–175 m.
The effect of water surface elevation on stoichiometric homeostasis
The stoichiometric homeostasis H could reflect the ability of plants regulating their element contents in response to nutrient environments changes (Sterner and Elser 2002; Persson et al. 2010; Yu et al. 2010; Golz et al. 2015; Li et al. 2013, 2018). Stoichiometric homeostasis is often element (Li et al. 2013) and species specific (Li et al. 2018). Although there were significant differences in plant nitrogen and phosphorus between sampling sites, the same as reported by Kong et al (2020), and soil nitrogen and phosphorus at six sites also had significant spatial changes, the four dominant plants showed obvious homeostasis and interspecific differences. As showed in Figs. 4 and 5, four dominant species exhibited specific-differences in the phosphorus steady state index (HP) and nitrogen steady state index (HN). Although belonging to different categories, both HP and HN of four dominant species was in the same order: X. sibiricum > A. theophrasti > C. dactylon > B. pilosa. Species with steady-state stoichiometry generally predominate in less nutritional and stable environments (Yu et al. 2010; Li et al. 2018; Su et al. 2019). So, they can be used as an important stoichiometric index to predict the succession of large plant communities and ecosystems responding to varied environmental conditions (Elmqvist et al. 2003; Schefferet et al. 2015; Yu et al 2015; Su et al. 2019). In the present study, the HP and HN of X. sibiricum were higher among the four dominant species, indicating that the X. sibiricum were more adaptable, and had higher utilization of nitrogen and phosphorus. However, the first-dominant species in the hydro-fluctuation zone of the section from Zhongxian County of Chongqing to Zigui County of Yichang City of TGR was C. dactylon other than X. sibiricum (Ke et al. 2020). A possible reason is that C. dactylon is a kind of perennial herb with clonal habit, which is vegetatively reproduced by its stolon. And its root system is developed and can adapt to the periodic water level change of TGR better than other species. Community stability positively depended on root biomass (Tilman et al. 2006). Obviously, plants in the falling zone always face periodic water level change. While varied periodic water level change would induce soil stoichiometric imbalance, predominance of high steady state index species would be replaced by low-steady state index species (Su et al. 2019). As reported by Ke et al. (2020), the relative density, relative frequency, and relative coverage of C. dactylon in plant community was higher than X. sibiricum at three water surface elevations. This can explain the higher dominance of C. dactylon compared with X. sibiricum.. Furthermore, the water level in the hydro-fluctuation belt of TRG had fluctuated nine times by 2018, but the succession of plant communities needs a long time to occur. The current vegetation is still in the initial stage of renewal and adaptation, and has not reached stability. This may also be one of the reasons for the inconsistency between plant dominance and stoichiometric homeostasis of C. dactylon and X. sibiricum. For B. pilosa, low HN and HP indicated low stability but strong plasticity to adapt to the varying external environment. This result was consistent with the reports by Yu et al. (2010, 2011). NO3−, as the main soil nitrogen nutrient, is prone to leaching, thus frequent water level fluctuation in the water-fluctuation zone can easily lead to soil nitrogen deficiency. This is why most wetland ecosystems, including the littoral zone of TGR, exhibit nitrogen limitation (Hu et al. 2014; Kong et al. 2020). According to stable leaf nutrient concentration hypothesis (Han et al. 2011), nitrogen is much more stable. This could explain the different categories between HN and HP of the same dominant species, for example, both C. dactylon and A. theophrasti was weakly homeostatic for Nitrogen element and weakly plastic for phosphorus element (Figs. 4, 5).
Relationship between community steady-state index and coverage
As discussed above, the periodic water level variation of TGR changed the plant composition of the hydro-fluctuation belt, and the plant diversity decreased significantly compared with the pre-water storage period (Wang et al. 2011). However, compared with the early flooding period, some plants adapted to the anti-seasonal water level fluctuations and formed new plant communities. And species diversity and coverage have increased (Ke et al. 2020). Due to the different flooding depth and time at different elevations in the hydro-fluctuation belt, there are significant differences in the species composition of plant community at different elevation section (Lei et al 2014). On the one hand, it explains the increase of species number with elevation in this study (Fig. 2), on the other hand, it also explains the change of species stoichiometric characteristics with elevation. As a result, elevation would also affect the coverage and species diversity of plant communities. In the study, increased community coverage with elevation confirmed it (Fig. 6). Species diversity could enhance the resilience of ecosystem states and their ability to stabilize themselves (Elmqvist et al. 2003). It is generally believed that the higher the species diversity index, the more stable the plant community. Investigation on plant community located in the same section reported that species diversity index increased along elevation gradient (Ke et al. 2020). Since community H was calculated as species relative biomass (ratio of biomass of single species to all species) multiplied by species H in a quadrat, the higher community H was found in higher elevation (Fig. 6). In detail, although X. sibiricum and C. dactylon are dominant species at three elevation, C. dactylon and B. Pilosa with low H was found more at L and M section, while X. sibiricum and A. theophrasti with high H were found more at M and H section. This could partly explain the higher community H found at high elevation. Stoichiometric equilibrium had been demonstrated to be associated with species dominance and ecosystem stability (Yu et al. 2010, 2015; Su et al. 2019). In our study, the positive relationship between community steady-state index and community coverage (Fig. 6) partly confirmed it. It maybe suggested that the variation of plant community composition, influenced by anti-seasonal periodic fluctuation of water level, was the determinant factor of stoichiometric homeostasis based on plant community.
Conclusion
In our study, leaf nitrogen content was linearly correlated with leaf phosphorus content along the water surface elevation in the hydro-fluctuating zone of TGR. As the water surface elevation increased, the number of plant species in the fluctuating zone increased. The difference in plant nitrogen and phosphorus steady-state index (HN and HP) indicates that there are interspecific differences of nutrient utilization of dominant species and their adaption to water surface elevation. Furthermore, the community coverage increased and the community stability index also increased with water surface elevation. This might indicate that in the fluctuating zone habitat, the plant’s nitrogen and phosphorus utilization strategy to adapt to changed water surface elevation affects the distribution and composition of plant community, and ultimately affects the stoichiometric homeostasis on the community levels.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bremner JM (1996) “Nitrogen-total,” In: Sparks DL, Page AL, Helmke PA, Loeppert RH (Ed) Methods of soil analysis. Part 3 chemical methods. Soil science society of america, American Society of Agronomy, Madison, WI. pp 1085–1121
Elmqvist T, Folke C, Nystrom M, Peterson G, Bengtsson J, Walker B (2003) Response diversity, ecosystem change, and resilience. Front Ecol Environ 1:488–494
Elser JJ, Andersen T, Baron JS, Bergstrom AK, Jansson M, Kyle M et al (2009) Shifts in lake N: P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 326:835–837. https://doi.org/10.1126/science.1176199
Elser JJ, Fagan WF, Kerhoffa J et al (2010) Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol 186:593–608
Frost PC, Evans-White MA, Finkel ZV, Jensen TC, Matzek V (2005) Are you what you eat? Physiological constraints on organismal stoichiometry in an elementally imbalanced world. Oikos 109(1):18–28. https://doi.org/10.1111/j.0030-1299.2005.14049.x
Golz A-L, Burian A, Winder M (2015) Stoichiometric regulation in micro-and mesozooplankton. J Plankton Res 37:293–305. https://doi.org/10.1093/plankt/fbu109
Guo Y, Yang S, Shen YF et al (2019) Study on the natural distribution characteristics and community species diversity of existing plants in the Three gorges Reservoir. Acta Ecol sinica 39(12):4255–4265
Güsewell S (2004) N: P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266. https://doi.org/10.1111/j.1469-8137.2004.01192.x
Han W, Fang JY, Guo DL, Zhang Y (2005) Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol 168:377–385. https://doi.org/10.1111/j.1469-8137.2005.01530.x
Han WX, Fang JY, Reich PB, Ian Woodward F, Wang ZH (2011) Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol Lett 14: 788–796 10.1 1 1 1/j.1461–0248.201 1.01641.x
He JS, Wang L, Flynn DFB, Wang XP, Ma WH, Fang JY (2008) Leaf nitrogen: phosphorus stoichiometry across Chinese grassland biomes. Oecologia 155:301–310. https://doi.org/10.1007/s00442-007-0912-y
Hu WF, Zhang WL, Zhang LH, Chen XY, Lin W, Zeng GS, Tong C (2014) Stochiometric characteristics of nitrogen and phosphorus in major wetland vegetation of China. Chin J Plant Ecol 38(10):1041–1052
Jian Z, Ma F, Guo Q, Qin A, Xiao W (2018) Long-term responses of riparian plants’ composition to water level fluctuation in China’s Three Gorges Reservoir. Plos One 13(11):e0207689. https://doi.org/10.1371/journal.pone.0207689
Karimi R, Folt CL (2006) Beyond macronutrients: element variability and multielement stoichiometry in freshwater invertebrates. Ecol Lett 9:1273–1283. https://doi.org/10.1111/j.1461-0248.2006.00979.x
Ke ZY, Wang Q, Shen QY, Xie MT, Xiao HL, Liu Y (2020) Characteristics of plant community in the hydro-fluctuation belt of the three Gorges Reservoir at the Zhong to Zigui section. Res Environ Yangtze Basin 29(9):1975–1985
Koerselman W, Meuleman AFM (1996) The vegetation N: P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450. https://doi.org/10.2307/2404783
Kong WW, Wang XF, Hf Lu, Liu TT, Gong XJ, Liu H, Yuan XZ (2020) Ecological storichiometry of four typical herbaceous species in the littoral zone of Three gorge Reservoir. Acta Ecol Sin 40(13):4493–4506
Kuo S (1996) Phosphorus. In: Bigham JM (Ed) Methods of soil analysis. Part 3. Chemical methods. Soil Science Society of America, American Society of Agronomy, Madison, Wis., pp 869–919.
Lei B, Wang YC, You YF, Zhang S, Yang CH (2014) Diversity and structure of herbaceous plant community in typical water-level fluctuation zone with different spacing elevations in Three Gorges Reservior. J Lake Sci 26(4):600–606
Li W, Cao T, Ni LY et al (2013) Effects of water depth on carbon, nitrogen and phosphorus stoichiometry of five submersed macrophytes in an in situ experiment. Ecol Eng 61:358–365
Li W, Li Y, Zhong J, Fu H, Tu J, Fan H (2018) Submerged macrophytes exhibit different phosphorus stoichiometric homeostasis. Front Plant Sci 9:1207. https://doi.org/10.3389/fpls.2018.01207
Moreno-Alvarado M, GarcÍa-Morales S, Trejo-Téllez L, Hidalgo-Contreras J, Gómez-Merino F (2017) Aluminum enhances growth and sugar concentration, alter macronutrient status and regulates the expression of NAC transcription factors in rice. Front Plant Sci 8:1–16
Persson J, Fink P, Goto A, et al (2010) To be or not to be what you eat: regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 119: 741–751. 10.1 11/j.1600–0706.2009.18545.x
Reich PB, Oleksyn J (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. PNAS 101(30):11001–11006
Sardans J, Rivas-Ubach AJ, Peñuelas J (2012) The C: N: P stoichiometry of organisms and ecosystems in a changing world: a review and perspectives. Perspect Plant Ecol 14:33–47
Scheffer M, Carpenter SR, Dakos V, Van Nes E (2015) Generic indicators of ecological resilience: inferring the chance of a critical transition. Annu Rev Ecol Evol S 46:145–167. https://doi.org/10.1146/annurev-ecolsys-112414-054242
Sistla SA, Appling AP, Lewandowska AM, Yaylor BN, Wolf AA (2015) Stoichiometric flexibility in response to fertilization along gradients of environmental and organismal nutrient richness. Oikos 124(7):949–959
Sterner RW, Elser JJ (2002) Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton University Press, Princeton
Su H, Wu Y, Xia W, Yang L, Chen J, Han W, Fang J, Xie P (2019) Stoichiometric mechanisms of regime shifts in freshwater ecosystem. Water Res 149:302–310
Tang ZY, Xu WT, Zhou GY, Bai YF, Li JX, Tang XL, Chen DM, Liu Q, Ma WH, Xiong GM, He HL, He NP, Guo YP, Guo Q, Zhu JL, Han WX, Hu HF, Fang JY, Xie ZQ (2018) Patterns of plant carbon, nitrogen, and phosphorus concentration in relation to productivity in China’s terrestrial ecosystems. Proc Natl Acad Sci USA 115:4033–4038
Tian D, Yan ZB, Niklas KJ, Han WX, Kattge J, Reich PB, Luo YK, Chen YH, Tang ZY, Hu HF, Wright IJ, Schmid B, Fang JY (2018) Global leaf nitrogen and phosphorus stoichiometry and their scaling exponent. Natl Sci Rev 5:723–739
Tilman D, Reich PB, Knops JMH (2006) Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 44:629–632
Wang JC, Zhu B, Wang T (2011) Characteristics of restoration of natural herbaceous vegetation of typical water-level fluctuation zone after flooding in the Three Gorges Reservoir area. Res Environ Yangtze Basin 20(5):603–610
Wang CY, Xie YZ, Ren QS, Li CX (2018) Leaf decomposition and nutrient release of three tree species in the hydro-fluctuation zone of the Three Gorges Dam Reservoir, China. Environ Sci Pollut R 25:23261–23275. https://doi.org/10.1007/s11356-018-2357-8
Wu QX, Han GL, Tang Y (2009) Effects of water level fluctuations on ecological environment of lake/ reservoir riparian zone: a review. Earth Environ 37(4):446–453
Xia CX, Yu D, Wang Z et al (2014) Stoichiometry patterns of leaf carbon, nitrogen and phosphorous in aquatic macrophytes in eastern China. Ecol Eng 70:406–413
Xu Y, Liu WZ, Liu GH (2009) Plant distribution in freshwater lakeshore: relative importance of species pool limitation vs. niche limitation. Chin J Plant Ecol 33(3):546–554
Ye C, Li S, Zhang Y, Zhang Q (2011) Assessing soil heavy metal pollution in the water-level-fluctuation zone of the Three Gorges Reservoir, China. J Hazard Mater 191:366–372. https://doi.org/10.1016/j.jhazmat.2011.04.090
Yin DL, Wang YM, Xiang YP, Xu QQ, Xie Q, Zhang C, Liu J, Wang DY (2019) Production and migration of methylmercury in water-level-fluctuating zone of the Three Gorges Reservoir, China: Dual roles of flooding-tolerant perennial herb. J Hazard Mater 381:1–9. https://doi.org/10.1016/j.jhazmat.2019.120962
Yu Q, Chen Q, Elser JJ, He N, Wu H, Zhang G et al (2010) Linking stoichiometric homoeostasis with ecosystem structure, functioning and stability. Ecol Lett 13:1390–1399. https://doi.org/10.1111/j.1461-0248.2010.01532.x
Yu Q, Elser JJ, He N, Wu H, Chen Q, Zhang G (2011) Stoichiometric homeostasis of vascular plants in the Inner Mongolia grassland. Oecologia 166:1–10. https://doi.org/10.1007/s00442-010-1902-z
Yu Q, Wilcox K, Pierre KL, Knapp AK, Han X, Smith MD (2015) Stoichiometric homeostasis predicts plant species dominance, temporal stability, and responses to global change. Ecology 96:2328–2335
Zhang Q, Lou Z (2011) The environmental changes and mitigation actions in the Three Gorges Reservoir region, China. Environ Sci Policy 14:1132–1138. https://doi.org/10.1016/j.envsci.2011.07.008
Zhu KW, Chen YC, Zhang S, Lei B, Yang ZM, Huang L (2020) Vegetation of the water-level fluctuation zone in Three Gorges Reservoir at the initial impoundment state. Glob Ecol Conserv 21:e00866
Acknowledgements
The research was supported by the National Natural Science Foundation of China (32170383), National Key R and D Program of China (2016YFC0502208), and the International Collaborative Research Fund for Young Scholars in the Innovation Demonstration Base of Ecological Environment Geotechnical and Ecological Restoration of River and Lakes.
Author information
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, H., Sun, T., Liu, Y. et al. Stoichiometric characteristics and homeostasis of leaf nitrogen and phosphorus responding to different water surface elevations in hydro-fluctuation zone of the Three Gorges Reservoir. Aquat Sci 85, 80 (2023). https://doi.org/10.1007/s00027-023-00977-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-023-00977-5