Abstract
A large proportion of studies assessing the impact of disturbances on the invertebrate community composition focus on a single life stage, assuming that those are an adequate indicator of environmental conditions. The effect of a specific disturbance may, however, depend on the life stage of the exposed organism. Therefore, we focused on the effect of spates on the caddisfly Agapetus fuscipes CURTIS (Trichoptera: Glossosomatidae) during different larval stages. A 2 year field study was performed in which we measured the discharge dynamics and population development of A. fuscipes in four lowland streams in The Netherlands. A stage-structured population model (i.e. StagePop) was used to test the impact of peak discharge on the different life stages, as larval instars 1–4 were not effectively sampled in the field. Four different mortality rates in response to spates were simulated, including a constant low, a constant high, a decreasing and an increasing impact per larval stage. This way, we were able to show a potential association between spates and population declines, where the stage-population model including decreasing impact by spates with increasing larval life stage most accurately described the population development of the larval instars 5–8. Focusing only on late instars could thus potentially result in underestimation of the effects of spates on this species. In conclusion, determination of responses of critical life stages to specific disturbances may help to identify the causes of the presence and absence of species, and thereby aid more effective management and restoration of degraded aquatic systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural flow variations, including spates and droughts, largely determine the spatial and temporal dynamics of invertebrate populations in running waters, as species have evolved traits that enable them to survive, exploit and even depend on these flow regimes (Resh et al. 1988; Poff et al. 1997; Lytle and Poff 2004). Disturbances outside the predictable flow regime to which stream organisms were originally adapted can, however, reduce population densities, and these adverse effects increase with the frequency, intensity and severity of the disturbance (Poff 1992; Lytle and Poff 2004). A specific disturbance may, however, lead to very different ecological responses depending on the (ontogenetic) life stage of the exposed organism, i.e. eggs, different larval stages, pupae and adults each have different sensitivities (Lancaster and Downes 2010). This was shown for chemical pollution to which early instars of different insect species were commonly more sensitive than later larval stages (e.g. McCahon and Pascoe 1988; Stuijfzand et al. 2000; Pineda et al. 2012). For hydrological disturbance it has been postulated that invertebrate responses depend on the timing of the event relative to the life history of the constituent invertebrate species (Boulton 2003; Lytle and Poff 2004; Nijboer 2004). Hence, the timing of harmful events in relation to the critical periods in the life cycle of the exposed species may be important in determining changes in the population structure after a disturbance (Lancaster and Downes 2010; Miller et al. 2012; Wesner 2019).
Studies assessing the impact of disturbances on invertebrate population dynamics that take the organism’s entire life cycle into consideration are, however, rare (but see Kohler and Hoiland 2001; Elliott 2006; Elliott 2013; Pandori and Sorte 2018). Most studies focus on a single life stage, generally late instars or aquatic adults (Lancaster and Downes 2010). This is partly due to the practical limitations involved in the sampling of early instars, since the mesh size of sample nets is commonly too large to retain these small individuals (Cummins and Wilzbach 1988). Alternatively, stage-structured population models (e.g. StagePop) may be used to simulate the impact of a disturbance on life stages that are difficult to sample in the field (Kettle and Nutter 2015). Hereby, the un-impacted population dynamics are modelled by using previously required information on reproduction, natural death rates and stage durations. Different scenarios can then be simulated in which the environmental variables or disturbances have a different effect on the death rates during each life stage, subsequently affecting the population dynamics during later stages (Kettle and Nutter 2015). Such stage-structured population models have previously been used to evaluate the effectiveness of pest control during different life stages of an invasive culicid mosquito species (Wieser et al. 2019). Stage-structured population models were further successfully applied to assess the effects of invasive species and drought on crayfish population dynamics during different life stages (Yarra and Magoulick 2019). Hence, these stage-population models may be a promising tool to simulate the effect of hydrologic disturbances on invertebrate population dynamics during different life stages, including those life stages that are difficult to collect.
The present study applied this approach to the caddisfly Agapetus fuscipes CURTIS (Trichoptera: Glossosomatidae), as there is adequate information available on the population dynamics of A. fuscipes in unimpacted upper courses of European streams where the species can locally reach high densities (e.g. Nielsen 1942; Castro 1975; Becker 1990; Sangpradub et al. 1999; Becker 2005). A. fuscipes is a case-building species with a univoltine life cycle consisting of an egg stage, eight larval instars, a pupae stage and a terrestrial flying adult (Castro 1975). Several life stages of A. fuscipes are generally simultaneously present in the stream (Becker 2005). Some knowledge on the stress responses for different life stages of A. fuscipes is already available, showing that unpredictable drops in stream water levels may result in the desiccation and subsequent loss of pupae above the water line (Nielsen 1942; Marchant and Hehir 1999). A laboratory experiment showed that late instar larvae endure more respiratory difficulties than early instar larvae when material is deposited upon them (Majecki et al. 1997). Moreover, terrestrial adults may be affected by disturbance of the riparian vegetation, with attendant impacts on larval population densities (Harrison et al. 2000). The literature is, however, ambiguous concerning the sensitivity of A. fuscipes to spates. Some studies reported that A. fuscipes populations were very susceptible to spates (Jones et al. 1977), while others reported that larvae were relatively unaffected (Giller et al. 1991). The discrepancy between these studies may be related to the timing of the peak discharge in relation to its life cycle, as argued above, but this explanation lacks verification.
Therefore, we aimed to gain a better understanding of the effect of spates on the population dynamics of A. fuscipes during different larval stages. We hypothesized that first instar larvae are more sensitive to spates than final instar larvae, i.e. high currents may cause dislodgment of first instar larvae as they attach themselves poorly to the gravel (Jones et al. 1977; Nijboer 2004). To test this hypothesis, we performed a 2 year field study in which we measured discharge dynamics and the population development of A. fuscipes in four lowland streams in The Netherlands and tested the larval instar specific mortality rates in response to spates using a stage‐structured population model. Normally, these four streams have a relatively stable discharge pattern. However, in the first year of the field study several severe spates occurred, providing a ‘natural experiment’ to evaluate the effect of these spates on the population development of A. fuscipes compared to the more stable second year.
Materials and methods
Study area
The field study was performed in two regions in The Netherlands, one region in Zuid-Limburg (region I: 50°54′ N; 5°48′ E), and one region in the Veluwe (region II: 52°04′ N; 5°52′ E) (Fig. 1a). In each region, two headwater streams were selected; region I Bunderbosbeek (BU) and Strabekervloedgraaf (ST: Fig. 1b), region II Seelbeek (SE) and Oude beek (OB). The water chemistry differed between the two regions, as the soil in Zuid-Limburg is more calcareous than in the Veluwe, leading to a higher mean pH (BU = 7.2 ± 0.1 and ST = 7.3 ± 0.2 vs SE = 6.9 ± 0.2 and OB = 7.0 ± 0.1), higher mean electrical conductivity (BU = 702 ± 108 and ST = 558 ± 90 vs SE = 342 ± 48 and OB = 193 ± 11 µS/cm) and higher concentration of some micro-ions in the stream water (Supplementary material A, Table S1). In spring and autumn, mean daily water temperatures were similar in the four streams (Supplementary material A, Table S1 and Fig. S1a). In summer, the water temperature was higher in the ST stream than in the other streams, while in winter the water temperature was lower in the ST and SE streams than in the BU and OB streams. In each stream, two sections of 5 m length were selected, which were up to 10 cm deep and up to 2 m wide (Fig. 1c). Coarse gravel was the most frequently observed substrate category in each section, i.e. 59% coverage in the SE stream, 61% in the OB stream, 74% in the BU stream and 83% in the ST stream (Supplementary material A, Fig. S2). Larvae generally inhabit these gravel beds, feeding on biofilms growing on hard substrates (Fig. 1d; Castro 1975; Becker 1990).
a Occurrence of A. fuscipes (dots) and location of study regions (squares) in The Netherlands: I Zuid-Limburg and II Veluwe, b picture of the Strabekervloedgraaf (ST stream), c schematic overview of the field set-up, dA. fuscipes larvae on gravel bed, e shovel used for sampling, f measured head capsule width of A. fuscipes
Data collection
Discharge dynamics The water level (m) of each stream was measured every 15 min for 2 years from April 2002 until April 2004 with a mini-Troll model ssp-100 (In-Situ inc, Ft. Collins, CO, USA) installed in a monitoring well (Fig. 1c). To be able to translate the water level measurements into discharge, a cross-section profile of the stream was measured every 2 weeks at 10 cm intervals across the channel. Discharge (Q) was calculated in m3/s from the corrected water level and cross-section data using the slope-area method (Boiten 2000; details in Nijboer et al. 2003). Discharge data were normalized to the median flow (Q50 or base flow) to enable comparison of the streams with different flow magnitude (Riis et al. 2008).
Agapetus fuscipes Population density and head capsule width were measured every 2 weeks during the first year. In the second year sampling was continued every 3 months, which was considered sufficient to follow general trends in population dynamics based on the results from the first year. In each 5 m stream section, three random samples were taken from the gravel beds (Fig. 1c; N = 6 per stream). For each sample, the top gravel layer was collected with a shovel from a surface area of 45 cm2 and placed into plastic buckets (Fig. 1e). A total area of 270 cm2 was sampled per stream. This sampling surface was considered sufficient as the density of A. fuscipes was very high on these gravel beds, and could reach up to 388 larvae/270 cm2 (25th percentile = 9; median = 64; 75th percentile = 103 larvae/270 cm2). The samples were stored for one night aerated at 5 °C, sieved through a 0.16 mm mesh sieve and sorted. All larvae and pupae of A. fuscipes were preserved in 70% ethanol. The head capsule width was measured under 50 × magnification with a microscope equipped with a horizontal micrometer scale, to the nearest 0.025 mm (Fig. 1f). The A. fuscipes larvae were assigned to eight larval instars, based on the head width classes defined by Castro (1975). Based on the entire sampling collection over 2 years, the number of specimens increased from the first larval instar to the fifth (Fig. 2), suggesting that early instars 1–4 were not collected effectively from the field.
Stage-structured population model
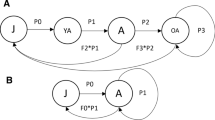
As larval instars 1–4 were not collected effectively from the field, we used a stage-structured population model to analyze the effect of peak discharges on the development of the A. fuscipes population density. The natural life cycle of the population was modeled using the delay-differential equation formulation by Nisbet and Gurney (1983). This formulation assumes that once an individual is born it passes through different life stages, unless it dies. The stage durations and background or natural death rates were based on parameters measured previously in unimpacted streams (see “Model parameters”). Discharge peaks were then used as input to the model to alter the mortality rates of each individual in the population based on its life stage and the intensity of the spate. This way we could assess the effect of discharge during all stages, i.e. also those that were not sampled effectively, on the population development during later stages. We simulated four scenarios with different sensitivity (mortality rates) to spates and tested which scenario corresponded best with the measured A. fuscipes population density of larval instars 5–8 (see “Model testing”).
Model parameters
Parameter values were obtained from Castro (1975), who extensively monitored A. fuscipes in the Breitenbach, an unimpacted headwater stream in Central Germany with dimensions and temperature regimes similar to our studied streams (Supplementary material A, Fig. S1b). The stage-structured population model comprised the egg stage, the eight larval instars, the pupal and adult stage. The time for one larval instar to develop into the next instar depends on the cohort and water temperature (Castro 1975). After each moult, the larvae leave their old case and build a new one from sand grains (Hanna 1961). The mean durations of these stages over all cohorts were 33, 25, 34, 44, 42, 45, 50, 40, 27, 29, and 4 days, respectively (Castro 1975). The sex-ratio was 2:1 (♂:♀), with a female laying 200 eggs on average during her 4 day life as an adult, so we simplified the reproduction rate to 200/4 = 50 eggs per female per day (Castro 1975). Background mortality rates were estimated from the data on population density of larval instars 4–8 of Castro (1975), by dividing the area under the density curve by the duration of each larval instar (Southwood 1978). The other stages were either not sampled or not sampled effectively, so we extrapolated the results assuming a linear trend. This resulted in slightly higher background mortality rates of the early life stages than those of the later life stages, decreasing from 0.016 to 0.006 day−1.
Model testing
The simulation was initiated with the immigration of 1 adult per day over 120 days, as most adults emerge over a 4 month period, resulting in the presence of several developmental stages present at the same time (Becker 2005; Nijboer 2004). For each measurement year the model was run separately, as A. fuscipes populations may recover quickly after stress (Nijboer 2004). A ‘spin up’ time was applied to get all stages established, with 394 days for the BU stream, SE stream and OB stream and 424 days for the ST stream to match the respective pupation period in each stream. To determine the effect of spates we selected discharge peaks exceeding thresholds relevant for invertebrate communities in lowland streams, including small spates of 2–4 times the Q50, medium spates of 4–8 times the Q50 and high spates of > 8 times the Q50 (Verdonschot and van den Hoorn 2010). Mortality rates were set to test four different scenarios (i.e. responses) to spates: (1) constant low, no impact by spates, (2) constant high, all larval stages are highly sensitive to spates, (3) decrease with instar stage, the sensitivity of the larvae to spates decreases exponentially with increasing larval stage, and (4) increase with instar stage, the sensitivity of larvae to spates increases exponentially with increasing larval stage (Fig. 3). We assumed a density loss of 80% for high spates, 40% for medium spates, and 20% for small spates (Bond and Downes 2003; Death 2008; Supplementary material B). The root mean square error (RMSE) was then used to test which scenario corresponded best with the measured A. fuscipes population density development of larval instars 5–8. The RMSE estimates the standard deviation of the model, so smaller values indicate a better fit. The unit is the same as the unit of the dependent variable, i.e. number of specimen/270 cm2. The analysis was performed in R version 3.4.1, using r package ‘StagePop’ to construct the stage–structured population models (Kettle and Nutter 2015), ‘PBSddesolve’ to solve the delay- differential equations (Schnute et al. 2013) and ‘Metrics’ to calculate the RMSE values (Hamner et al. 2018).
Four potential scenarios for the effects of spates on A. fuscipes population density losses per larval instar: 1) constant low, no impact by spates, 2) constant high, all larval stages are highly sensitive to spates, 3) decrease with instar stage, the sensitivity of the larvae to spates decreases with increasing larval stage, and 4) increase with instar stage, the sensitivity of larvae to spates increases with increasing larval stage (corresponding mortality rates for population density loss in each model in Supplementary material B)
Results
Discharge dynamics The base flow (Q50) was higher in the OB stream (0.014 m3/s) than in the other three streams (0.004 m3/s). The flow duration curves showed that in the BU stream more peak discharges occurred in the first measurement year than in the second year (Supplementary material C). All peak discharges took place from May to October 2002 with one high spate (> 8 times the Q50) on 13 July 2002 (Fig. 4a). Discharge peaks occurred during both years in the ST stream, although they were higher in the first year. High spates took place on 13 July, 20 and 21 August, and 3 November 2002 (Fig. 4b). Discharge in the SE stream was rather stable during both years, as only some small spates occurred between April and May 2002 and one in November 2002 (Fig. 4c). In the OB stream, more peak discharges occurred in the first year than in the second year, with one high spate on 27 October 2002 (Fig. 4d).
Impact of discharge dynamics (Q) on A. fuscipes population densities during different life stages from April 2002–April 2004 in four streams a Bunderbosbeek (BU stream), b Strabekervloedgraaf (ST stream), c Seelbeek (SE stream), d Oude beek (OB stream). Measurements of population densities of larval instars 5–8 and pupae from year 1 are shown in red bars (sampled monthly) and from year 2 in blue bars (sampled every 3 months). The best fitting stage-structured population model, which assumes that the sensitivity of larvae to spates decreases with increasing larval stage, is shown for each aquatic life stage by a red solid line for year 1 and a blue dashed line for year 2. Note the discharge is in sqrt-scale and y-axis for stages egg-instar 4 is 10 times larger than instar 5-pupae
Agapetus fuscipes Pupae started to appear from the end of March in the ST stream and the end of April in the BU, SE and OB stream (Fig. 4). In all streams, the majority of the individuals had pupated by September. Matching the start of the pupation period of the model to the data on A. fuscipes resulted in comparable timing between the model and the data for the larval instars 5–8. In all streams, the population density of larval instars 5–8 was lower in the first measurement year than in the second year, except for the OB stream where the population density was similar during both years.
In the BU stream, a high spate and several smaller spates occurred in the first year when primarily larvae of instar stages 1–3 were present (Fig. 4a). Here, the models including either a constant high impact by spates or a decreasing impact by spates were better able to represent the population development in larval instars 5–8 than the models including either a constant low impact by spates or an increasing impact by spates (Table 1). In the ST stream, spates of various intensities occurred during different life stages (Fig. 4b). The model including a decreasing impact by spates most accurately described the population development in larval instars 5–8 in this stream (Table 1). In the SE stream, several small spates occurred between instar stage 7 to instar stage 1 (Fig. 4c). The model including a constant high impact by spates most accurately described the population development in larval instars 5–8 (Table 1). In the OB stream, the population density of larval instars 5–8 was similar during both years (Fig. 4d). The high spate during instar stages 4–6 did not seem to be associated with a decrease in population density. The model including decreasing impact by spates most accurately described the population development in larval instars 5–8 in this stream (Table 1).
Discussion
For three of the four studied streams the stage-population model including a decreasing impact by spates with larval life stage most accurately described the A. fuscipes population development of larval instars 5–8, supporting our hypothesis. It must be stressed that the study was based on a ‘natural experiment’ where several high spates occurred during early stages, while there was only one data point for a high spate during later periods in the life cycle. In the fourth stream, the population density of larval instars 5–8 was most accurately described by the model including constant high impact by spates. In that stream only several small spates occurred between instar stage 7 to instar stage 1 in the first year. The lowered population density of larval instars 5–8 in that year may not have been directly related to the flow, but instead to the large amount of deposited sand, silt and detritus on the gravel beds during this period (extra observations in Supplementary material A, Figure S3). A. fuscipes larvae may endure respiratory difficulties when material is deposited upon them, in particular during later stages (Majecki et al. 1997). The effects of flow and sediment transport are, however, difficult to separate as both factors interact (Hynes 1970). Controlled experiments are needed to understand the mechanistic effects of flow and sediment transport on invertebrate species during a spate (e.g. Bond and Downes 2003; Gibbins et al. 2007), but to our knowledge such experiments have not been combined with testing for the effect on specific life stages.
Comparable to our study, Elliott (2006) assessed the effects of a severe spate on different life stages of four Elmid beetles species in a ‘natural experiment’. The effects of the spate were negligible for the larvae as they were buried in the gravel, which served as a refuge from the spate. In contrast, all adult densities were negatively affected by the spate, but the magnitude varied between species, presumably related to species specific habitat requirements (Elliott 2006). The same spate had limited effect on a Baetid mayfly, as the specimens present during the spate were in larval stages 2–3, and probably small enough to burrow between small stones in the substratum to avoid effects of the spate (Elliott 2013). In agreement with these studies, Sagnes et al. (2008) observed that the aquatic insect larvae can make use of different hydraulic habitats while growing, i.e. dependent on the species they may prefer higher or lower shear velocity conditions with increasing larval stage. Beside the timing of spates during the invertebrate life cycle, Lancaster (1992) concluded that the time of day at which a disturbance takes place should be taken into account when interpreting the effect of peak discharges on invertebrates, as the density of Baetis nymphs in her study was reduced significantly by the spate created after sunset, but not at dawn or mid-afternoon.
Similar to other aquatic ecological studies, the early life stages of A. fuscipes (larvae instar 1–4) were not sampled effectively in this study. The smallest larvae may have been present in a different (micro)habitat than the larger larvae, like the sand under the stones, as only the top layer of gravel was collected. Alternatively, they may have been mechanically damaged in the buckets during transport or passed through the sieve when sorting the samples, as the mesh of the sieve was larger than the head width of larval instars 1–4. To compensate for the ineffective sampling, we applied the stage-structured population model (i.e. StagePop), which proved to be a valuable tool to obtain an indication of the duration, timing and mortality patterns of the early life stages of A. fuscipes for which sampling was ineffective.
The natural life cycle of the population in the model was based on previously obtained parameter values, such as stage durations, of an un-impacted headwater stream in Central Germany. However, actual stage durations are dependent on water temperature and cohort (Castro 1975). This temperature dependency may have caused slight differences between the timing and duration of each life stage, potentially causing uncertainty in the sensitivity of the population model compared to the field situation. In future studies, the model could thus be improved by making each stage duration temperature dependent (Kettle and Nutter 2015). Additionally, in some streams (e.g. BU and SE stream) that were disturbed by peak discharges during the first year, the A. fuscipes population grew fast and recovered during the following stable year. Similar to previous studies, we observed a simultaneous presence of different life stages of A. fuscipes in each stream, which may spread the risk of high mortality to discharge peaks (Becker 2005). Nijboer (2004) proposed that after a reduction in the population density by hydrological disturbances, the remaining females may be able to lay more eggs than normal, as there is less competition for food. Such density-dependent processes would need to be studied further in experiments, and could be included in the model to gain a better understanding the influence of spates on the local extinction of populations. Despite these uncertainties in the stage-population model, we were able to show that population declines may have been associated with the timing of spates, coinciding with the presence of early life stages.
Our study supports previous findings that floods can result in severe declines in stream invertebrate densities (see studies in Lake 2000; Death 2008). Although recovery from floods by invertebrates is typically high, some previous studies observed changes in species composition following repeated, severe and/or unpredictable flooding (e.g. Giller et al. 1991; Scrimgeour et al. 1988; Robinson et al. 2003). It is generally accepted that the effects of spates depend greatly on the taxon, as taxa have different resistance (ability to tolerate disturbance) and resilience to flow (ability to recover after a disturbance) (Death 2008; De Brouwer et al. 2017). This study provided initial indication that the resistance to peak discharge of invertebrates not only depends on the taxon, but also varies between life stages. This may have implications for management and restoration of freshwater ecosystems, as the current single-life-stage-based assessments with a strong focus on late instar or aquatic adult life stages may not elucidate which stressors or disturbances actually constrain invertebrate population densities during their entire life cycle, which may lead to unsuccessful management efforts. Restoration measures might aim at environmental factors relevant for late instars or aquatic adult life stages, which may not be the limiting life stage for that species (Bond and Lake 2003; Lancaster and Downes 2010). The assessment of the critical life stages of a specific species to specific disturbances may help to identify the actual cause for the presence and absence of species and thereby aid more effective management and restoration of degraded aquatic systems.
References
Becker G (1990) Comparison of the dietary composition of epilithic trichopteran species in a first-order stream. Archiv für Hydrobiologie 120:13–40
Becker G (2005) Life cycle of Agapetus fuscipes (Trichoptera, Glossosomatidae) in a first-order upland stream in central Germany. Limnologica 35(1–2):52–60. https://doi.org/10.1016/j.limno.2005.01.003
Boiten W (2000) Hydrometry. IHE Delft Lecture note series. Balkema, Rotterdam, p 246
Bond NR, Downes BJ (2003) The independent and interactive effects of fine sediment and flow on benthic invertebrate communities characteristic of small upland streams. Freshw Biol 48(3):455–465. https://doi.org/10.1046/j.1365-2427.2003.01016.x
Bond NR, Lake PS (2003) Local habitat restoration in streams: constraints on the effectiveness of restoration for stream biota. Ecol Manag Restor 4(3):193–198. https://doi.org/10.1046/j.1442-8903.2003.00156.x
Boulton AJ (2003) Parallels and contrasts in the effects of drought on stream macroinvertebrate assemblages. Freshw Biol 48(7):1173–1185. https://doi.org/10.1046/j.1365-2427.2003.01084.x
Castro LB (1975) Ökologie und Produktionsbiologie von A. fuscipes CUR. im Breitenbach 1971. Archiv für Hydrobiologie 45:305–375
Cummins KW, Wilzbach MA (1988) Do pathogens regulate stream invertebrate populations? Internationale Vereinigung für theoretische und angewandte Limnologie 23(2):1232–1243. https://doi.org/10.1080/03680770.1987.11899797
De Brouwer JHF, Besse-Lototskaya AA, Ter Braak CJF, Kraak MHS, Verdonschot PFM (2017) Flow velocity tolerance of lowland stream caddisfly larvae (Trichoptera). Aquat Sci 79(3):419–425. https://doi.org/10.1007/s00027-016-0507-y
Death RG (2008) The effect of floods on aquatic invertebrate communities. In: Lancaster J, Briers RA (eds) Aquatic insects: challenges to populations. CABI, Wallingford, pp 103–121
Elliott JM (2006) Critical periods in the life cycle and the effects of a severe spate vary markedly between four species of elmid beetles in a small stream. Freshw Biol 51(8):1527–1542. https://doi.org/10.1111/j.1365-2427.2006.01587.x
Elliott JM (2013) Contrasting dynamics from egg to adult in the life cycle of summer and overwintering generations of Baetis rhodani in a small stream. Freshw Biol 58(5):866–879. https://doi.org/10.1111/fwb.12093
Gibbins C, Vericat D, Batalla RJ (2007) When is stream invertebrate drift catastrophic? The role of hydraulics and sediment transport in initiating drift during flood events. Freshw Biol 52(12):2369–2384. https://doi.org/10.1111/j.1365-2427.2007.01858.x
Giller PS, Sangpradub N, Twomey H (1991) Catastrophic flooding and macroinvertebrate community structure. Internationale Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 24(3):1724–1729. https://doi.org/10.1080/03680770.1989.11899058
Hamner B, Frasco M, LeDell E (2018) Metrics r package. https://CRAN.R-project.org/package=Metrics. Accessed 14 Nov 2019
Hanna HM (1961) Selection of materials for case-building by larvae of caddis flies (Trichoptera). Proc R Entomol Soc Lond Ser A Gen Entomol 36(1–3):37–47. https://doi.org/10.1111/j.1365-3032.1961.tb00257.x
Harrison SSC, Harris IT, Croeze A, Wiggers R (2000) The influence of bankside vegetation on the distribution of aquatic insects. Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 27(3):1480–1484. https://doi.org/10.1080/03680770.1998.11901483
Hynes HBN (1970) The ecology of stream insects. Annu Rev Entomol 15(1):25–42. https://doi.org/10.1146/annurev.en.15.010170.000325
Jones NV, Litterick MR, Pearson RG (1977) Stream flow and behavior of caddis fly larvae. In: Proceedings of the second international symposium on Trichoptera, pp 259–266
Kettle H, Nutter D (2015) stagePop: modelling stage-structured populations in r. Methods Ecol Evol 6(12):1484–1490. https://doi.org/10.1111/2041-210X.12445
Kohler SL, Hoiland WK (2001) Population regulation in an aquatic insect: the role of disease. Ecology 82(8):2294–2305. https://doi.org/10.1890/0012-9658(2001)082[2294:PRIAAI]2.0.CO;2
Lake PS (2000) Disturbance, patchiness, and diversity in streams. J N Am Benthol Soc 19(4):573–592. https://doi.org/10.2307/1468118
Lancaster J (1992) Diel variations in the effect of spates on mayflies (Ephemeroptera: Baetis). Can J Zool 70(9):1696–1700. https://doi.org/10.1139/z92-236
Lancaster J, Downes BJ (2010) Linking the hydraulic world of individual organisms to ecological processes: putting ecology into ecohydraulics. River Res Appl 26(4):385–403. https://doi.org/10.1002/rra.1274
Lytle DA, Poff NL (2004) Adaptation to natural flow regimes. Trends Ecol Evol 19(2):94–100. https://doi.org/10.1016/j.tree.2003.10.002
Majecki J, Schot J, Verdonschot PFM, Higler LWG (1997) Influence of sand cover on mortality and behavior of A. fuscipes larvae (Trichoptera: Glossosomatidae). In: Proceedings of the eighth international symposium on Trichoptera, pp 283–288
Marchant R, Hehir G (1999) Growth, production and mortality of two species of Agapetus (Trichoptera: Glossosomatidae) in the Acheron River, south-east Australia. Freshw Biol 42(4):655–671. https://doi.org/10.1046/j.1365-2427.1999.00505.x
McCahon CP, Pascoe D (1988) Use of Gammarus pulex (L.) in safety evaluation tests: culture and selection of a sensitive life stage. Ecotoxicol Environ Saf 15(3):245–252. https://doi.org/10.1016/0147-6513(88)90078-4
Miller AD, Roxburgh SH, Shea K (2012) Timing of disturbance alters competitive outcomes and mechanisms of coexistence in an annual plant model. Theor Ecol 5(3):419–432. https://doi.org/10.1007/s12080-011-0133-1
Nielsen A (1942) Über die Entwicklung und Biologie der Trichopteren mit besonderer Berücksichtigung der Quelltrichopteren Himmerlands. Archiv für Hydrobiologie 17:255–631
Nijboer RC (2004) The ecological requirements of Agapetus fuscipes Curtis (Glossosomatidae), a characteristic species in unimpacted streams. Limnologica 34(3):213–223. https://doi.org/10.1016/S0075-9511(04)80046-X
Nijboer RC, Van den Hoorn MW, Van den Hoek TH, Wiggers R, Verdonschot PFM (2003) Keylinks: ecologische processen in sloten en beken; II de relatie tussen afvoerdynamiek, temperatuur en de populatiegroei van Agapetus fuscipes (no. 1069). Alterra
Nisbet R, Gurney W (1983) The systematic formulation of population models for insects with dynamically varying instar duration. Theor Popul Biol 23(1):114–135. https://doi.org/10.1016/0040-5809(83)90008-4
Pandori LL, Sorte CJ (2018) The weakest link: sensitivity to climate extremes across life stages of marine invertebrates. Oikos 128(5):621–629. https://doi.org/10.1111/oik.05886
Pineda MC, McQuaid CD, Turon X, López-Legentil S, Ordóñez V, Rius M (2012) Tough adults, frail babies: an analysis of stress sensitivity across early life-history stages of widely introduced marine invertebrates. PLoS ONE 7(10):e46672. https://doi.org/10.1371/journal.pone.0046672
Poff NL (1992) Why disturbances can be predictable: a perspective on the definition of disturbance in streams. J N Am Benthol Soc 11(1):86–92. https://doi.org/10.2307/1467885
Poff NL, Allan JD, Bain MB, Karr JR, Prestegaard KL, Richter BD, Stromberg JC (1997) The natural flow regime. Bioscience 47(11):769–784. https://doi.org/10.2307/1313099
Resh VH, Brown AV, Covich AP, Gurtz ME, Li HW, Minshall GW, Wissmar RC (1988) The role of disturbance in stream ecology. J N Am Benthol Soc 7(4):433–455. https://doi.org/10.2307/1467300
Riis T, Suren AM, Clausen B, Sand-Jensen K (2008) Vegetation and flow regime in lowland streams. Freshw Biol 53(8):1531–1543. https://doi.org/10.1111/j.1365-2427.2008.01987.x
Robinson CT, Uehlinger U, Monaghan MT (2003) Effects of a multi-year experimental flood regime on macroinvertebrates downstream of a reservoir. Aquat Sci 65(3):210–222. https://doi.org/10.1007/s00027-003-0663-8
Sagnes P, Merigoux S, Péru N (2008) Hydraulic habitat use with respect to body size of aquatic insect larvae: case of six species from a French Mediterranean type stream. Limnologica 38(1):23–33. https://doi.org/10.1016/j.limno.2007.09.002
Sangpradub N, Giller PS, O'Connor JPO (1999) Life history patterns of stream-dwelling caddis. Archiv für Hydrobiologie 146(4):471–493. https://doi.org/10.1127/archiv-hydrobiol/146/1999/471
Schnute J, Couture‐Beil A, Haigh R, Kronlund A (2013) PBSmodelling r package. https://CRAN.R-project.org/package=PBSmodelling. Accessed 14 Nov 2019
Scrimgeour GJ, Davidson RJ, Davidson JM (1988) Recovery of benthic macroinvertebrate and epilithic communities following a large flood, in an unstable, braided, New Zealand river. N Z J Mar Freshw Res 22(3):337–344. https://doi.org/10.1080/00288330.1988.9516306
Southwood TRE (1978) Ecological methods with particular reference to the study of insect populations. Chapman & Hall, London
Stuijfzand SC, Poort L, Greve GD, van der Geest HG, Kraak MHS (2000) Variables determining the impact of diazinon on aquatic insects: taxon, developmental stage, and exposure time. Environ Toxicol Chem 19(3):582–587. https://doi.org/10.1002/etc.5620190309
Verdonschot PFM, Van den Hoorn M (2010) Using discharge dynamics characteristics to predict the effects of climate change on macroinvertebrates in lowland streams. J N Am Benthol Soc 29(4):1491–1509. https://doi.org/10.1899/09-154.1
Wesner J (2019) Using stage-structured food webs to assess the effects of contaminants and predators on aquatic–terrestrial linkages. Freshw Sci 38(4):928–935. https://doi.org/10.1086/706103
Wieser A, Reuss F, Niamir A, Müller R, O’Hara RB, Pfenninger M (2019) Modelling seasonal dynamics, population stability, and pest control in Aedes japonicus japonicus (Diptera: Culicidae). Parasites Vectors 12(1):142. https://doi.org/10.1186/s13071-019-3366-2
Yarra AN, Magoulick DD (2019) Modelling effects of invasive species and drought on crayfish extinction risk and population dynamics. Aquat Conserv Mar Freshw Ecosyst 29(1):1–11. https://doi.org/10.1002/aqc.2982
Acknowledgements
We would like to thank Rebi Nijboer for coordinating the field work and preparing the data; Dorine Dekkers, Marie-Claire Boerwinkel, Ruud van Kats, Matthijs Bassie, Jennie van Iwaarden, Jasper Wijkamp and Dimitri Huntink for assistance with the fieldwork and measuring the head capsule widths of the caddisflies; Theo Jacobs, Rini Schuiling and Co Onderstal for digitalizing the substrate coverage; Isabel Smallegange for valuable discussions on population ecology models; Helen Kettle for writing an example model script in stagePop; Sven van der Lee for assisting with R; Staatsbosbeheer, Stichting Geldersch Landschap and several private landowners for access to their properties. Two anonymous reviewers provided valuable suggestions to improve the manuscript.
Author information
Authors and Affiliations
Contributions
PV contributed to the study conception and design. Material preparation and data collection were performed by PV, RV and others (see Acknowledgements). GL performed the data analysis. The first draft of the manuscript was written by GL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Lee, G.H., Kraak, M.H.S., Verdonschot, R.C.M. et al. Persist or perish: critical life stages determine the sensitivity of invertebrates to disturbances. Aquat Sci 82, 24 (2020). https://doi.org/10.1007/s00027-020-0698-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-020-0698-0