Abstract
The α9α10 nicotinic cholinergic receptor (nAChR) is a ligand-gated pentameric cation-permeable ion channel that mediates synaptic transmission between descending efferent neurons and mechanosensory inner ear hair cells. When expressed in heterologous systems, α9 and α10 subunits can assemble into functional homomeric α9 and heteromeric α9α10 receptors. One of the differential properties between these nAChRs is the modulation of their ACh-evoked responses by extracellular calcium (Ca2+). While α9 nAChRs responses are blocked by Ca2+, ACh-evoked currents through α9α10 nAChRs are potentiated by Ca2+ in the micromolar range and blocked at millimolar concentrations. Using chimeric and mutant subunits, together with electrophysiological recordings under two-electrode voltage-clamp, we show that the TM2-TM3 loop of the rat α10 subunit contains key structural determinants responsible for the potentiation of the α9α10 nAChR by extracellular Ca2+. Moreover, molecular dynamics simulations reveal that the TM2-TM3 loop of α10 does not contribute to the Ca2+ potentiation phenotype through the formation of novel Ca2+ binding sites not present in the α9 receptor. These results suggest that the TM2-TM3 loop of α10 might act as a control element that facilitates the intramolecular rearrangements that follow ACh-evoked α9α10 nAChRs gating in response to local and transient changes of extracellular Ca2+ concentration. This finding might pave the way for the future rational design of drugs that target α9α10 nAChRs as otoprotectants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nicotinic acetylcholine receptors (nAChRs) belong to the Cys-loop family of the pentameric ligand-gated ion channels (pLGICs) superfamily, that also includes GABAA, GABAc, glycine, 5-hydroxytryptamine-3 (5-HT3) and some invertebrate anionic glutamate and histamine receptors [1,2,3,4,5]. Members of this family are composed of five subunits arranged around a central pore, each comprising an extracellular N-terminal region, four transmembrane domains (TM1–TM4), a variable intracellular region and a short C-terminus [3, 6]. To date, 19 different nAChR subunits have been described in vertebrates: α1–α10, β1–β4, δ, ε, and γ [1, 3], with α11 and β5 subunits only identified in some fish species [7].
Nicotinic receptors are allosteric proteins. A diversity of modulatory sites has been discovered, with either positive (PAM) or negative effects upon acetylcholine (ACh) signal transduction of the nAChR [8,9,10,11,12]. These modulatory sites are named ‘allosteric’, since they are topographically distinct from the orthosteric sites, and indirectly modulate channel gating transition of the nAChR in response to the orthosteric agonist. Thus, PAMs most likely decrease the energetic barrier between resting and activated conformations of the receptor. In this regard, extracellular calcium (Ca2+) has been reported to increase macroscopic currents and change the kinetic properties of several native and recombinant nAChRs and has been classified as an allosteric modulator of these receptors [13,14,15,16,17,18,19]. Moreover, previous studies revealed that Ca2+ binding leads to similar but smaller conformational changes than those due to agonist binding, that are consistent with a partially activated state [20, 21].
The α9 and α10 subunits were the latest nAChRs subunits to be identified [22,23,24]. Their main function as canonical ligand-gated ion channels is in the inner ear and the lateral line systems, where a pentameric α9α10 nAChR mediates synaptic transmission between descending efferent neurons and mechanosensory hair cells [25,26,27]. The α9α10 nAChR has a very high Ca2+ permeability and its activation is tightly modulated by extracellular Ca2+ [23, 28]. Moreover, contrary to other nAChRs, its net synaptic output is inhibitory, since Ca2+ entry through α9α10 receptors leads to activation of nearby Ca2+-activated SK potassium channels and hyperpolarization of hair cells [27]. A tight regulation of α9α10 nAChR activity is crucial for the maintenance of the inhibitory signature of the cholinergic efferent-hair cell synapse [29, 30].
Although included within the nAChR family of receptors, α9 and α10 subunits are distant and distinct members and have been referred to as “odd cousins within an old family” [5]. Due to their atypical features, the α9 and α10 subunits form a separate clade within the subfamily of nAChRs. Both have low amino acid sequence identity when compared to other nicotinic subunits [22, 23, 31,32,33,34,35] and very strict co-assembly rules, comprising only α9 and α10 subunits [22,23,24, 36]. Moreover, they exhibit a restricted expression pattern [22, 37,38,39] and a different evolutionary trajectory when compared to other nicotinic subunits [32, 33, 35]. When expressed in heterologous systems, α9 and α10 subunits can assemble into functional homomeric α9 and heteromeric α9α10 receptors [22, 23, 34, 35, 40]. In addition, a recent work has shown that functional human α10 homomeric receptors can be observed when expressed in Xenopus laevis oocytes, albeit only when incubated with the alkaloids strychnine, brucine, or methyllycaconitine [41]. Binding experiments utilizing α9/5HT3 and α10/5HT3 chimeric receptors have indicated that, although homomeric α9 as well as α10 protein subunits reach the membrane, expression is exponentially enhanced when both subunits are co-expressed [42]. Interestingly, homomeric α9 and heteromeric α9α10 receptors exhibit a similar peculiar pharmacological profile which they share with that of the hair cell nAChR [22,23,24, 43,44,45]. However, murine α9 and α9α10 nAChRs are differentially modulated by extracellular Ca2+ [18, 35, 46]. While rat α9 nAChRs responses are only blocked by this ion, ACh-evoked currents through α9α10 nAChRs are both potentiated and blocked by physiological concentrations of external Ca2+ (Fig. 1) [18, 46]. This suggests that, whereas α9 homomeric receptors do not require extracellular Ca2+ for channel gating by ACh, activation of α9α10 nAChRs is enhanced by this cation. Blockage of α9 and α9α10 nAChRs occurs in the millimolar range, is voltage-dependent, and proposed to occur as a result of Ca2+ permeation [18, 46]. On the other hand, potentiation of α9α10 receptors is voltage-independent [18], suggesting that, similar to that proposed for α7 receptors [17, 21, 47,48,49], Ca2+ most likely interacts at an extracellular binding site to allosterically modulate coupling between ligand-binding and gating [18].
Modulation of α9 and α9α10 nAChRs by extracellular Ca2+. a Top Representative responses evoked by 100 µM ACh in a α9 expressing oocyte at different Ca2+ concentrations (Vhold -70), reproduced from Elgoyhen et al. [23], Copyright (2001) National Academy of Sciences, U.S.A. Bottom Bar diagram illustrating the inhibition of responses to 100 µM ACh (Vhold -70) as a function of increasing extracellular Ca2+ (modified from Katz et al. [46]). Current amplitudes at different Ca2+concentrations were normalized to the amplitudes obtained at 0 mM Ca2+ (Imax). Values are the mean ± S.E.M. of data pooled from experiments of the type illustrated in a Top. Open circles represent individual oocytes (n = 3). b Top Representative responses evoked by 10 µM ACh in a α9α10 expressing oocyte at different Ca2+ concentrations (Vhold -90). Bottom Bar diagram illustrating the bell-shaped profile of responses to 10 µM ACh at different Ca2+ concentrations (Vhold -90). Current amplitudes recorded in each oocyte were normalized to the response obtained at 1.8 mM Ca2+ in the same oocyte. Bars represent the mean ± S.E.M and open circles the individual oocytes (n = 10). *p < 0.05, **p < 0.010, ***p < 0.001 and ****p < 0.0001, Kruskal-Wallis followed by Dunn’s multiple comparison-test, when comparing the Ca2+ concentration that evokes the highest response (0 and 0.5 mM for α9 and α9α10, respectively) versus the other Ca2+ concentrations
To date the biophysical and structural mechanisms behind Ca2+ potentiation of α9α10 nAChRs have not been elucidated. Thus, the differential modulation of α9 and α9α10 nAChRs by Ca2+ opens the unique opportunity for defining the molecular determinants that underlie the potentiation of the hair cell nAChR. Using chimeric and mutant subunits, together with electrophysiological recordings under two-electrode voltage-clamp, we report that the TM2-TM3 loop of the α10 subunit contains key structural determinants responsible for the potentiation of the α9α10 nAChR by extracellular Ca2+. Strikingly, molecular dynamics (MD) simulations show that the TM2-TM3 loop of α10 does not contribute to the Ca2+ potentiation phenotype through the formation of novel calcium binding sites not present in the α9 homomeric receptor. Taken together, these results most likely suggest that the TM2-TM3 loop of α10 might act as a control element that allosterically modulates coupling between ligand-binding and gating of α9α10 nAChRs in response to local and transient changes of extracellular Ca2+ concentration. This finding might pave the way for the future rational design of PAMs that target α9α10 nAChRs as otoprotectants [50,51,52,53].
Methods
Generation of mutant and chimeric subunits
All experiments were performed with rat wild-type, chimeric or mutant receptors. All mutant and chimeric subunits were confirmed by sequencing.
The α10α9Nt, α10α9Nt−TM1TM2loop and α10α9Nt−TM2TM3loop constructs were generated by overlap-extension PCR [54, 55]. For α10α9Nt, the DNA sequence of the α9 N-terminal domain was amplified from an α9 pGEMHE construct using primers 5’-GGGCGAATTAATTCGAGCTC-3’ and 5’-CACCTTCACTCTCCTTCTGAAGCGCCGCGCTGCAGCCTACGTGTG-3’, and the DNA sequence of the α10 subunit from TM1 to the C-terminal domain was amplified from an α10 pSGEM construct using primers 5’-CACACGTAGGCTGCAGCGCGGCGCTTCAGAAGGAGAGTGAAGGTG-3’ and 5’-GCTATGACCATGATTACGCC-3’. The chimeric subunit was amplified using the DNA fragments generated in the two PCRs described above and primers 5’-GGGCGAATTAATTCGAGCTC-3’ and 5’-GCTATGACCATGATTACGCC-3’. For α10α9Nt−TM1TM2loop, the DNA sequence of the α9 N-terminal domain plus the TM1 and the TM1-TM2 loop was amplified from an α9 pGEMHE construct using primers 5’-GGGCGAATTAATTCGAGCTC-3’ and 5’- CCCAGCTGCCTCTGGGGAGAAGGTGTCTCTGGGCGTCACCGTGCTCCTGG-3’, and the DNA sequence of the α10 subunit from TM2 to the C-terminal domain was amplified from an α10 pSGEM construct using primers 5’-TGACGCCCAGAGACACCTTCTCCCCAGAGGCAGCTGGGAGATAGAAATCTC-3’ and 5’-GCTATGACCATGATTACGCC-3’. The chimeric subunit was amplified using the DNA fragments generated in the two PCRs described above and primers 5’-GGGCGAATTAATTCGAGCTC-3’ and 5’-GCTATGACCATGATTACGCC-3’. For α10α9Nt −TM2TM3loop, the DNA sequence of the α9 N-terminal domain plus the TM1, TM2 and the TM2-TM3 loop was amplified from an α9 pGEMHE construct using primers 5’-GGGCGAATTAATTCGAGCTC3’ and 5’-GAGATCATGCCAGCCTCAGAAAATGTGCCACTCATCGGAAAGTACTATATG-3’, and the DNA sequence of the α10 subunit from the TM3 to the C-terminal domain was amplified from an α10 pSGEM construct using primers 5’-CATATAGTACTTTCCGATGAGTGGCACATTTTCTGAGGCTGGCATGATCTC-3’ and 5’-GCTATGACCATGATTACGCC-3’. The chimeric subunit was amplified using the DNA fragments generated in the two PCRs described above and primers 5’-GGGCGAATTAATTCGAGCTC-3’ and 5’-GCTATGACCATGATTACGCC-3’. Chimeric constructs were subcloned in the pSGEM vector.
The α10α9Nt−TM2 construct, containing the N-terminal, the TM1 and the TM2 domains of the rat α9 subunit and the remaining regions of rat α10 subunit, was generated by QuikChange® Multi Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA, USA), using the α10α9Nt−TM2TM3loop construct subcloned in pSGEM vector as template and primers 5´-CTAATGGTGGCAGAGAGCATGCCACCTGCAGAAAGTGTTCC-3´ and 5´-GGAACACTTTCTGCAGGTGGCATGCTCTCTGCCACCATTAG-3´.
The chimeric α10α9extra subunit, where the extracellular domains (Nt, preTM1 and TM2-TM3 loop) of rat α10 were replaced by the corresponding ones from rat α9, was generated by two rounds of QuikChange® Site-Directed Mutagenesis (Stratagene, La Jolla, CA, USA). In the first round QuikChange® Multi Site-Directed Mutagenesis (Stratagene, La Jolla, CA, USA) was performed using α10α9Nt construct subcloned in pSGEM vector as template and primers 5´-GGGCAGCAGGAGGTTGACGATGTAGAATGAAGAGCGGCGCTTCAG-3’ and 5´-GGCATGCTCTCGGCCACCATCAGCT-3´. In the second round QuikChange® XL II (Stratagene, La Jolla, CA, USA) was performed using the PCR product obtained in the first round as template and primers 5´-GATCCTGGCCGAGATCATGCCAGCTTCAGAGAATGTGC-3´ and 5´-GCACATTCTCTGAAGCTGGCATGATCTCGGCCAGGATC-3´.
The α10α9TM2TM3loop chimeric subunit, in which only the TM2-TM3 loop of rat α10 was replaced by the corresponding region of rat α9, was generated by QuikChange® XL II kit (Stratagene, La Jolla, CA, USA), using an α10 pSGEM construct as template and primers 5´-GCTGATCCTGGCCGAGATCATGCCAGCTTCAGAGAATGTGCCACT-3´ and 5´-AGTGGCACATTCTCTGAAGCTGGCATGATCTCGGCCAGGATCAGC-3´.
Rat α9E72Q, α9E202Q, α10E71Q, α10E201Q and α10E287Q mutant subunits were generated by QuikChange® XL II kit (Stratagene, La Jolla, CA, USA) using either a α9 pGEMHE or α10 pSGEM construct as templates. Primers used were as follows: 5´- GGACATGGACGGGAGAAACCAGATTC-3´ and 5´-GAATCTGGTTTCCCTCGTCCATGTCC-3´ for α9E72Q; 5´- TGAAGATGTGGGATGGGAGGTCC-3´ and 5´- GGACCTCCCATCCCACATCTTCA-3´ for α9E202Q; 5´-ATTGTCTCAGATCATTGATATGGATCAGCGGAACCAGGTGC-3´ and 5´-GCACCTGGTTCCGCTGATCCATATCAATGATCTGAGACAAT-3´ for α10E71Q; 5´-GCCGACTTCGTGGAGAACGTTCAATGGCGGGTGCTGGGC-3´ and 5´-GCCCAGCACCCGCCATTGAACGTTCTCCACGAAGTCGGC-3´ for α10E201Q; 5´- GCT GATCCTGGCCCAGAGCATGCCACC-3´ and 5´- GGTGGCATGCTCTGGGCC for α10E287Q.
Expression of recombinant receptors in X. Laevis oocytes and electrophysiological recordings
For expression studies, rat α9, α10, α10α9X chimeras and mutant subunits were subcloned into modified pGEMHE or pSGEM vectors [22, 23, 33]. Capped cRNAs were in vitro transcribed from linearized plasmid DNA templates using RiboMAX™ Large Scale RNA Production System (Promega, Madison, WI, USA). The maintenance of X. laevis and the preparation and cRNA injection of stage V and VI oocytes have been described in detail elsewhere [46]. Typically, oocytes were injected with 50 nl of RNase-free water containing 0.01–1.0 ng of cRNA and maintained in Barth’s solution (in mM): NaCl 88, Ca(NO3)2 0.33, CaCl2 0.41, KCl 1, MgSO4 0.82, NaHCO3 2.4, HEPES 10, at 18° C. cRNA mixtures were injected at a 1:1 molar ratio, in order not to force the preferred assembly of any subunit into the pentamer. However, it should be noted that under our experimental conditions we cannot control for construct assembly preferences.
Electrophysiological recordings were performed 2–6 days after cRNA injection under two-electrode voltage-clamp with an Oocyte Clamp OC-725B or C amplifier (Warner Instruments Corporation, Hamden, CT, USA). Recordings were filtered at a corner frequency of 10 Hz using a 900 BT Tunable Active Filter (Frequency Devices Inc., Ottawa, IL, USA). Data acquisition was performed using a Patch Panel PP-50 LAB/1 interphase (Warner Instruments Corp., Hamden, CT, USA) at a rate of 10 points per second. Both voltage and current electrodes were filled with 3 M KCl and had resistances of ∼ 1 MΩ. Data were analyzed using Clampfit from the pClamp 6.1 software. During electrophysiological recordings, oocytes were continuously gravity fed (15 ml/min) with normal frog saline composed of: 115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, and 10 mM HEPES buffer, pH 7.2. ACh was applied along with the perfusion solution of the oocyte chamber. In the experiments in which the Ca2+ concentration was varied from 0 to 3 mM, the other components of the frog saline remained constant. To minimize the activation of the endogenous Ca2+-sensitive chloride current [23, 56], all experiments were carried out in oocytes incubated with the membrane-permeant Ca2+ chelator 1,2-bis (2-aminophenoxy) ethane-N, N,N0,N0-tetraacetic acid-acetoxymethyl ester (BAPTA-AM, 100 µM) for 3 h prior to electrophysiological recordings. All recordings were performed at -70 mV holding potential, unless otherwise stated.
Biophysical properties of nAChRs
Concentration–response curves were obtained by measuring responses to increasing concentrations of ACh. Current amplitudes were normalized to the maximal agonist response in each oocyte. The mean and S.E.M. values of the responses are represented. Agonist concentration–response curves were iteratively fitted, using Prism 6 software (GraphPad Software Inc.) with the equation: I/Imax= [A]nH/([A]nH+ EC50nH), where I is the peak inward current evoked by the agonist at concentration [A], Imax is the current evoked by the concentration of agonist eliciting a maximal response, EC50 is the concentration of agonist inducing half-maximal current response, and nH is the Hill coefficient.
The IC50 values for the Ca2+ blockage were calculated with the formula: 100*I/Imax = 100 / (1 + 10(log IC50− X)*nH), where I is the current obtained at the different concentrations of extracellular Ca2+, Imax is the current obtained at a nominally 0 mM Ca2+concentration, X is the logarithm of the Ca2+ concentration and nH is the Hill coefficient. IC50 values were obtained for each oocyte and then averaged. Data are presented as mean ± S.E.M.
The effects of extracellular Ca2+ on the ionic currents through nAChRs were studied by measuring the amplitudes of the responses to a near-EC50 concentration of ACh (10 µM for α9α10, α9α10α9Nt, and α9α10α9extra nAChRs; 50 µM for α9α10α9TM2TM3 loop ; 40 µM for α9α10α9Nt–TM1TM2loop ; 30 µM for α9α10α9Nt–TM2 nAChR; 5 µM for α9α10α9Nt–TM2TM3 loop; 20 µM for α9α10E287; 100 µM for homomeric α9, α9α10E71 and α9α10E201 nAChRs). Increasing Ca2+ concentrations ranging from nominally 0 to 3 mM were analyzed at a holding potential of -90 mV [18]. These experiments were carried out in oocytes injected with 7.5 ng of an oligonucleotide (5’- GCTTTAGTAATTCCCATCCTGCCATGTTTC-3’) antisense to connexin C38 mRNA to minimize the activation of the oocyte’s nonselective inward current through a hemigap junction channel that results from the reduction of external divalent cation concentration [57, 58]. In all experiments, oocytes were gravity fed for 2 min with the test solution (frog saline X mM Ca2+) before the application of ACh, and were transferred back to frog saline 1.8 mM Ca2+ for at least 3 min before changing to a different test solution. To evaluate the effects of current rundown along the experiment, current amplitudes at each Ca2+ concentration were normalized to the average of the ACh-evoked responses in 1.8 mM Ca2+obtained in the same oocyte before and after the ACh response elicited in each Ca2+concentration tested.
Current-voltage (I–V) relationships were obtained by applying 2 s voltage ramps from − 120 to + 50 mV from a holding potential of -70 mV, at the plateau response to ACh. Leakage correction was performed by digital subtraction of the I–V curve obtained by the same voltage-ramp protocol prior to the application of ACh. Generation of voltage protocols and data acquisition were performed using a Patch Panel PP-50 LAB/1 interface (Warner Instruments Corp.) at a rate of ten points per second and the pClamp 7.0 software (Axon Instruments Corp., Union City, CA).
Decay rate of ACh-evoked currents was evaluated via prolonged (1 min) agonist applications. The percentage of current remaining 20 s after the peak of the response was determined for each oocyte.
Molecular modeling
AlphaFold models were generated using AlphaFold-multimer [59] through the local version of colabfold_batch v1.3.0 [59, 60]. In addition to the default parameters, the relaxation of predicted structures using Amber force fields was applied. The input files for the structure prediction were FASTA files containing the amino acid sequences of all five subunits of nAChR pentamers from three receptor types, including α9 homopentamers, as well as wild-type α9α10 and chimeric α9α10α9TM2TM3loop heteropentamers. The models were generated allowing the use of structural templates in the prediction algorithm. We first built models of α9 and α9α10 receptors. The N-terminal region (α9: M1-A26, and α10: M1-A25) and the intracellular domain (α9: P332-E447, and α10: P331-D415) were predicted with very low confidence. As a result, these regions showed different conformational states in different subunits of the same receptor and model replicas. This conformational diversity was attributed to the uncertainty of structure prediction in these regions. Therefore, we deleted these regions in the input sequences and rebuilt the models using AlphaFold. The intracellular domain of each chain was replaced by a pentapeptide linker of sequence GSGSG to join the TM3 and TM4 domains. This strategy allowed the reduction of the number of atoms in the models to improve the efficiency of the simulations and to focus on the structural regions that are more relevant to the present work. The shaved structures thus obtained, encompass residues N27-R331-GSGSG-W448-D479 in α9 and E26-H330-GSGSG-W416-L447 in α10 and α10α9TM2TM3loop subunits. For the wild-type α9α10 and α9α10α9TM2TM3loop receptors, models were built in an alternated 10/9/10/9/10 configuration. Alphafold models, were validated using the provided confidence metrics such as the pLDDT (predicted Local Distance Difference Test) and PAE (predicted aligned errors) scores (Online Resource 1). Additionaly, the predicted models were compared to available experimental structures of related nAChRs, using RMSD (Online Resource 2). The comparison of the 3D structures of our AlphaFold models and those reported by Noviello et al. [61] revealed that our models more closely resemble the desensitized-like conformational state of α7.
A series of replicas of the AlphaFold predictions of each nAChRs were performed and the two best replicas of each receptor type, as measured by the QMEANbrane parameter [59, 60, 62] were selected for further analysis. In total six structural models were obtained, including the best two of α9, α9α10 and α9α10α9TM2TM3loop receptors.
Molecular dynamics simulations
Input files for molecular dynamics simulations were built with CHARMM-gui input generator interface [63]. The AlphaFold models of the different receptors were embedded in a 1-palmitoyl-2-oleoyl phosphatidylcholine (POPC) double layer membrane model using the membrane builder module [64,65,66].
Molecular dynamics (MD) simulations were performed using GROMACS v2023.2 software [67]. The CHARMM36 force field was employed for protein and lipid parameters [68]. The receptor complexes were solvated in a cubic TIP3 water box containing 83,000 water molecules. A newly developed multisite calcium model [69] configured using the CHARMM-GUI server was used for the simulations. Ca2+ was added to a final concentration of 50 mM. Sodium and chloride ions were added to neutralize the systems. Ions were added using the distance ion placing method implemented in CHARMM-GUI [63]. This method includes stochastic placement of ions, mimicking the natural environment where ions are not uniformly distributed but are rather governed by diffusion and electrostatic forces. The protonation states of ionizable residues (Asp, Glu, His, Lys, Arg) were assigned based on their default states at physiological pH, as defined by the CHARMM36 force field [68]. This approach ensures that the simulation conditions reflect biologically relevant protonation states. To maintain consistency across simulations, identical protonation states were applied to equivalent residues in similar monomers. This methodology avoids potential artifacts arising from differential protonation states and facilitates valid comparisons between structural variants.
The systems were first minimized with 10,000 steps of steepest descent and then equilibrated in the NVT and NPT ensembles over 250 and 1625 ps, respectively. The temperature was maintained at 310 K using a v-rescale thermostat with a coupling constant of 1.0 ps. In the NPT equilibration, pressure was controlled at 1 bar using a c-rescale barostat with a coupling constant of 5.0 ps and an isothermal compressibility of 4.5e− 5 bar − 1. During equilibration, the protein backbone, side chain, and lipid atoms were restrained with a harmonic force potential with force constant of 4000, 2000, and 1000 kJ mol− 1 nm− 2, respectively, to allow the solvent molecules and ions to relax around the protein and membrane structures. These restraints were gradually released during equilibration and completely removed before the production runs. The production runs were conducted over 100 ns with pressure coupling set at 1 atm using the Parrinello-Rahman barostat with a coupling constant of 5.0 ps and a constant temperature of 310 K using the Nose-Hoover thermostat with a coupling constant of 1.0 ps. The MD simulations used a time step of 2 fs and all bonds involving hydrogen atoms were maintained to their standard length using the LINCS algorithm. For all simulation stages a cutoff distance of 1.2 nm was applied for Van der Waals interactions, with the use of a Verlet scheme for neighbor searching. Long-range electrostatic interactions were treated with the Particle Mesh Ewald (PME) method, using a grid spacing of 0.12 nm. MD trajectories were analyzed using GROMACS analysis tools and VMD [67].
For each receptor, MD simulations were performed for the two best structural models predicted with AlphaFold. For each model, we ran two replicas, independently set up with stochastic variations in solvation, membrane generation, ion addition, and initial velocities. A random seed was used for each replica to ensure distinct initial velocities derived from a Maxwell-Boltzmann distribution at 310 K. As a result, a total of four replicas were run for each receptor, ensuring diverse sampling and robust results.
We employed GROMACS commands to cluster conformations sampled during the 100 ns MD simulations, and derive an average structure of the receptor over the trajectory. This structure served as a reference state for relaxation an equilibration assessment. We calculated the Root Mean Square Deviation (RMSD) of the receptor structure relative to this reference state throughout the simulation trajectory. This analysis enabled us to observe the relaxation of the receptor structure within the initial 20 ns of each run. To ensure the reliability of our results, we discarded this initial equilibration phase and retained the subsequent 80 ns trajectory of each replica for all further analyses.
Statistical analysis
All plotting and statistical tests were conducted using Prism 6 software (GraphPad Software). Statistical significance was determined using either parametric paired t-test or one-way ANOVA followed by Dunnett´s multiple comparison test. The non-parametric Mann–Whitney test was used to perform comparisons between two groups and Kruskal–Wallis one-way analysis of variance followed by Dunn’s tests for comparisons between multiple groups. A p < 0.05 was considered significant.
Reagents
All drugs were obtained from Sigma-Aldrich (Buenos Aires, Argentina). ACh chloride was dissolved in distilled water as 100 mM stocks and stored in aliquots at -20 °C. BAPTA-AM was stored at -20 °C as aliquots of a 100 mM stock solution in dimethylsulfoxide, thawed and diluted into Barth’s solution shortly before incubation of the oocytes. ACh solutions in Ringer’s saline were freshly prepared immediately before application.
Results
The TM2-TM3 loop of the α10 subunit renders a Ca2+ potentiation phenotype in α9α10 nAChRs
Extracellular Ca2+ ions modulate the activity of several nAChRs [14,15,16,17, 70]. As previously reported [18, 46], Fig. 1 shows the differential responses of rat homomeric α9 and heteromeric α9α10 nAChRs expressed in Xenopus laevis oocytes to changes in extracellular Ca2+ concentrations. Thus, as previously reported [46] and depicted with modifications in Fig. 1a for the sake of comparison, responses of homomeric α9 nAChRs to 100 µM ACh have a maximal amplitude in the absence of extracellular Ca2+ (182 ± 20 nA, n = 4 [46]), indicating that channel gating by ACh does not require the presence of this cation. In addition, α9 nAChRs responses are reduced in the presence of increasing (0.05–1.8 mM) Ca2+, being smallest (5.63 ± 0.58%, n = 3) at a 1.8 mM physiological extracellular Ca2+ concentration (Fig. 1a and [46]). At -70 mV the concentration of Ca2+ that produces a 50% of response block (IC50) is 0.10 ± 0.01 mM [46]. Moreover, Ca2+ block of α9 receptors is voltage-dependent, since it is more pronounced at hyperpolarized than at depolarized potentials [46]. On the contrary, when α9 assembles with α10, rendering α9α10 heteromeric nAChRs, a bell-shaped profile of ACh-evoked responses at different Ca2+ concentrations is observed (Fig. 1b and [18]). Thus, responses to 10 µM ACh are small in the presence of nominally zero Ca2+ (16.79 ± 6.47% of responses at 1.8 mM, n = 7), are potentiated by sub-millimolar Ca2+ concentrations reaching a maximum at 0.5 mM (142.98 ± 10.12% of responses at 1.8 mM, n = 10) and then onwards blocked by further increases in extracellular Ca2+ within the millimolar range. Whereas potentiation of α9α10 nAChRs by Ca2+ is voltage independent, blockage is more pronounced at hyperpolarized than at depolarized potentials [18]. Taken together, these results suggest that it is the α10 subunit the one that provides structural determinants that subserve a Ca2+ potentiation phenotype of α9α10 nAChRs.
In order to dissect the domains of the α10 subunit that are critical for the Ca2+ potentiation phenotype of rat α9α10 nAChRs, we engineered multiple α10α9X subunit chimeras constructed by sequentially exchanging amino acidic regions of α10 by their corresponding ones from the α9 nAChR subunit as indicated in Fig. 2. All chimeras were heterologously expressed in X. laevis oocytes, and their function tested by two-electrode voltage-clamp recordings. None of the chimeric subunits elicited any response to ACh when expressed alone or when co-expressed with the α10 subunit, but were functional when co-expressed with rat α9 (Table 1). The amplitude of responses observed for the chimeric receptors at 1.8 mM Ca2+ (Table 2) were ∼ 10–30 times higher than those reported for homomeric rat α9 receptors (range of responses from 5 to 20 nA, with some exceptional responses of 50 and 70 nA, [23, 46]), indicating that, when co-expressed, rat α9 and chimeric subunits form heteromeric assemblies. To perform a comparative analysis of Ca2+ modulation on the generated chimeras, responses to ACh for each chimeric receptor were recorded in normal Ringer’s solution at a range of Ca2+ concentrations (nominally zero to 3 mM Ca2+). A near-EC50 ACh concentration (Table 1) was chosen for each chimeric receptor, in order to provide an ample dynamic range for Ca2+ modulation, allowing for both potentiation and block [49]. Since amplitude responses to ACh vary across different experiments and oocyte batches (even under the same conditions), current amplitudes at different Ca2+ concentrations were normalized to the response at 1.8 mM Ca2+ for each individual oocyte.
a Schematic overview of the design of chimeric subunits. Amino acid regions of the α10 (blue) subunit were sequentially exchanged by the following corresponding regions of the α9 (red) subunit: α10α9Nt, N-terminal domain; α10α9Nt–TM1TM2loop, N-terminal domain plus TM1 and TM1-TM2 intracellular loop; α10α9Nt–TM2, same as α10α9Nt–TM1TM2loop plus TM2; α10α9Nt–TM2TM3loop, same as α10α9Nt–TM2 plus TM2-TM3loop; α10α9extra, N-terminal domain plus pre TM1 and TM2-TM3 extracellular loop; α10α9TM2TM3loop, TM2-TM3 extracellular loop. In all following experiments chimeric subunits were co-injected with α9. b Alignment of the amino acid sequences of the rat α9 and α10 nAChR subunits. The residues located at the junction where the α9 and α10 nAChR subunits join in each chimeric subunit are highlighted: α10α9Nt (yellow), α10α9Nt-TM1TM2loop (green), α10α9Nt-TM2 (orange), and α10α9Nt- TM2TM3loop (red). Tip up triangles denote α10 residues that have been mutated to replace the preTM1 of rat α10 by the corresponding region of rat α9 to generate the α10α9extra chimeric subunit. Tip down triangles denote α10 residues that have been mutated to replace the TM2-TM3loop of rat α10 by the corresponding region of rat α9 to generate the α10α9extra and the α10α9TM2TM3 loop chimeric subunits
Figure 3 shows the results obtained in α9α10α9x receptors in which the α10 chimeric subunits were engineered by the exchange of either the extracellular N-terminal domain (α10α9Nt, left panel) or the extracellular N terminus, the TM1 and TM1-TM2loop (α10α9Nt–TM1TM2loop, right panel) of α10 by those of the α9 nAChR subunit. Similar to that described for α9α10 nAChRs (Fig. 1 and [18]), Ca2+ exerted a dual effect and responses to a near-EC50 ACh concentration (10 µM and 40 µM, respectively) were potentiated by Ca2+ up to 0.2 mM and blocked by higher concentrations of this ion. Thus, ACh-evoked currents at 0.2 mM Ca2+ were significantly higher than those observed at nominally zero Ca2+: α9α10α9Nt, 115.2 ± 27.3% and 225.8 ± 18.9%, n = 6, (p = 0.0373); α9α10α9Nt–TM1TM2 loop, 95.2 ± 18.8% and 358.3 ± 32.4%, n = 4–6, (p = 0.0022), (Kruskal-Wallis followed by Dunn’s multiple comparison-test) in 0 and 0.2 mM Ca2+, respectively. On the other hand, responses to 3 mM were significantly smaller than those observed at 0.2 mM Ca2+: α9α10α9Nt, 225.8 ± 18.9% and 64.9 ± 5.9%, n = 6, (p = 0.0010); α9α10α9Nt–TM1TM2loop, 358.3 ± 32.4% and 73.5 ± 9.3%, n = 4–6, (p = 0.0011), (Kruskal-Wallis followed by Dunn’s multiple comparison-test) in 0.2 and 3 mM Ca2+, respectively. As described in Fig. 1 and [18] for wild-type α9α10 nAChRs, potentiation of chimeric receptors by Ca2+ was voltage-independent (Fig. 3c). Thus, the 0.2 / 0 Ca2+ ratios of response amplitudes were similar (α9α10α9Nt, 2.1 ± 0.4 and 2.5 ± 0.3, n = 5 (p = 0.076, paired t test); α9α10α9Nt–TM1TM2 loop, 5.4 ± 2.4 and 5.9 ± 2.6, n = 5 (p = 0.223, paired t test), at -90 and + 40 mV, respectively.
Responses to ACh of α9α10α9Nt and α9α10α9Nt–TM1TM2loop receptors are potentiated by sub-mM Ca2+ concentrations. a Illustration indicating the cRNAs injected in each experiment: α9 plus either α10α9Nt (left) or α9α10α9Nt–TM1TM2loop (right), respectively. b Top Representative responses evoked by either 10 µM (left) or 40 µM (right) ACh at different extracellular Ca2+ concentrations (Vhold -90). Bottom Bars represent the mean ± S.E.M and open circles the individual oocytes. Current amplitudes recorded in each oocyte were normalized to the response obtained at 1.8 mM Ca2+ in the same oocyte. (n = 4–6 per group). *p < 0.05 and **p < 0.01, Kruskal-Wallis test followed by Dunn’s multiple comparison-test, when comparing the Ca2+ concentration that evokes the highest response (0.2 mM Ca2+) versus the other Ca2+ concentrations. c Representative I-V curves (n = 5 for α9α10α9Nt and n = 5 for α9α10α9Nt–TM1TM2loop) obtained by the application of a voltage ramp protocol (-120 to + 50, 2 s), 10 s after the application of either 10 µM (left) or 40 µM ACh (right) at different Ca2+ concentrations. Potentiation by 0.1 and 0.2 mM extracellular Ca2+ is observed in both chimeric receptors, both at depolarized and hyperpolarized potentials
We subsequently extended the α10 for α9 subunit amino acid replaced region by adding the TM2 of α9 to α10α9Nt–TM1TM2 loop, rendering the α10α9Nt–TM2 chimeric subunit and co-expressed it with α9 (Fig. 4a, left panel). Similar to that observed in α9α10 (Figs. 1 and [18]) and in α9α10α9Nt and α9α10α9Nt–TM1TM2loop (Fig. 3) chimeric receptors, Ca2+ exerted a dual effect on α9α10α9Nt–TM2 and responses to 30 µM ACh were potentiated by Ca2+ up to 0.2 mM and blocked by higher concentrations of this ion. Thus, ACh-evoked currents at 0.2 mM Ca2+ (322.0 ± 40.0%) were significantly higher than those observed at nominally zero Ca2+ (132.4 ± 27.5%, n = 5–8, p = 0.039), (Fig. 4b, left panel). On the contrary, responses to 3 mM (69.5 ± 7.6%) were significantly smaller than those observed at 0.2 mM Ca2+ (322.0 ± 40.0%, n = 5–8, p = 0.0003, Kruskal-Wallis followed by Dunn’s multiple comparison-test). Moreover, potentiation was once again voltage-independent (Fig. 4c, left panel). Thus, the 0.2 / 0 Ca2+ ratio of response amplitudes was similar at -90 mV (4.1 ± 0.2) and + 40 mV (2.8 ± 0.4), (p = 0.1424, n = 5, paired t test). Taken together, experiments in Figs. 3 and 4 (left panel) indicate that the replacement of the N terminal region until the entire TM2 of α10 by the corresponding amino acid regions of α9, do not recover a Ca2+ modulation phenotype similar to that observed in α9 homomeric receptors. Thus, Ca2+ potentiation was still observed at sub-millimolar concentrations and was voltage-independent. However, it should be noted that different to α9α10 wild-type receptors, where responses at zero Ca2+ were only 16.79 ± 6.47% of responses at 1.8 mM (n = 7), chimeric receptors exhibited larger current amplitudes under similar conditions: 115.2 ± 27.3% (n = 6) p = 0.014, 95.2 ± 18.8% (n = 6), p = 0.0047 and 132.4 ± 27.5% (n = 6) p = 0.0012, for α10α9Nt, α10α9Nt–TM1TM2loop and α10α9Nt–TM2, Mann-Whitney test. Moreover, maximal responses were obtained at a lower 0.2 mM Ca2+ concentration, compared to 0.5 mM in the case of α9α10 wild-type receptors.
Potentiation by Ca2+ is lost in α9α10α9Nt–TM2TM3loop receptors. a Illustration indicating the cRNAs injected in each experiment: α9 plus either α10α9Nt–TM2 (left) or α10α9Nt–TM2TM3loop (right), respectively. b Top Representative responses evoked by either 30 µM (left) or 5 µM (right) ACh at different extracellular Ca2+ concentrations (Vhold -90). Bottom Bars represent the mean ± S.E.M and open circles the individual oocytes. Current amplitudes recorded in each oocyte were normalized to the response obtained at 1.8 mM Ca2+ in the same oocyte (n = 5–8 per group). Note the lack of potentiation when TM2-TM3 loop of α9 is incorporated in the α10α9Nt–TM2TM3loop chimera, where only a block of ACh-evoked responses is observed (right panel). *p < 0.05, **p < 0.010 and ***p < 0.001, Kruskal-Wallis test followed by Dunn’s multiple comparison-test, when comparing the Ca2+ concentration that evokes the highest response (0.2 and 0 mM Ca2+ for α9α10α9Nt–TM2 and α9α10α9Nt–TM2TM3loop, respectively) versus the other Ca2+ concentrations. c Representative I-V curves for each receptor (α9α10α9Nt–TM2, n = 5; α9α10α9Nt–TM2TM3loop, n = 7), obtained by the application of a voltage ramp protocol (-120 to + 50, 2 s), 10 s after the application of 30 µM (left) or 5 µM ACh (right) at different Ca2+ concentrations. Potentiation by 0.1 and 0.2 mM extracellular Ca2+ is observed in α9α10α9Nt–TM2 receptors both at depolarized and hyperpolarized potentials (left panel). Potentiation is lost in α9α10α9Nt–TM2TM3loop and a voltage-dependent block of ACh responses is observed in the presence of 0.2 mM and 3.0 mM extracellular Ca2+ (right panel)
As shown in Fig. 4a (right panel) the α10α9Nt–TM2TM3loop chimeric subunit was constructed by introducing the TM2-TM3 loop of α9 in replacement of the corresponding region of α10α9Nt–TM2 and co-expressed with α9 in Xenopus oocytes. Different from that observed for α9α10 wild-type receptors and the above chimeras, responses were not potentiated by Ca2+, indicating that ACh responses became independent of the presence of extracellular Ca2+. Similar to homomeric α9 receptors, responses to 5 µM ACh of α9α10α9Nt–TM2TM3loop were only blocked by increasing Ca2+ concentrations (Fig. 4b, right panel). Thus, responses at zero Ca2+ (611.3 ± 91.2%) were significantly higher than those at 3 mM Ca2+ (108.1 ± 10.7%), n = 5–8 (p = 0.0020, Kruskal-Wallis followed by Dunn’s multiple comparison-test). As for α9 receptors [46], block of ACh evoked responses by Ca2+ was voltage-dependent and more pronounced at hyperpolarized than at depolarized potentials, exhibiting a 3 / 0 Ca2+ ratio of response of 0.20 ± 0.03 and 1.2 ± 0.3 (p = 0.0323, n = 7, paired t test), at -90 and + 40 mV, respectively. Thus, whereas the allosteric potentiation by Ca2+ is lost in α9α10α9Nt–TM2TM3loop nAChRs, its blocking effect is still maintained. It should be noted that the Ca2+ inhibition curve was slightly shifted to the right in the α9α10α9Nt–TM2TM3loop chimeric receptor, presenting a higher IC50 (0.5 ± 0.1 mM, n = 6), that differed significantly from that of homomeric α9 receptors (0.10 ± 0.01 mM, n = 3, from [46], Mann-Whitney test, p = 0.0238). Taken together, these results suggest that amino acids located within the α10 TM2-TM3 loop are required for potentiation of α9α10 receptors by external Ca2+. However, the results so far do not preclude the possibility that the loss of Ca2+ potentiation, is due to the interchange of the α10 TM2-TM3 loop by the corresponding one from α9, within the overall amino acid context of a α9 N terminal-TM2 domain, which is also present in the α10α9Nt–TM2TM3loop chimeric subunit. Thus, it has been described that the consequences of a given amino acid substitution greatly depends upon the overall sequence (and hence structure) of the protein, a phenomenon that can be referred to as epistasis [71].
In order to dissect the role of the α10 TM2-TM3 loop in Ca2+ potentiation, chimeric α10α9x subunits which included reduced α9 domains were engineered. The fact that potentiation by Ca2+ in all chimeric receptors was always voltage-independent, indicates that Ca2+ modulation is not subject to the electric field of the membrane. Therefore, as suggested previously for other nAChRs [8], including α9α10 [18], it most likely depends on an extracellular binding site that allosterically modulates coupling between ligand binding and gating, rather than on transmembrane regions. Consequently, we constructed the chimeric α10α9extra subunit, where only the extracellular domains of α10 (the Nt, preTM1 and TM2-TM3 loop) involved in coupling agonist binding to channel gating [72] were replaced by the corresponding ones from α9 (Fig. 5a, left panel). Similar to that described for murine α9 nAChRs (Fig. 1 and [46]) and the α9α10α9Nt–TM2TM3loop chimeric receptor (Fig. 4b, right panel), responses to 10 µM ACh of the α9α10α9extra were not potentiated by Ca2+, and only blocked by increasing Ca2+ concentrations (Fig. 5b, left panel). Thus, responses were significantly higher at zero Ca2+ (217.3 ± 6.3%) than at 3 mM Ca2+ (72.3 ± 5.3%, n = 7, p = 0.020, Kruskal-Wallis followed by Dunn’s multiple comparison-test). It should be noted that the Ca2+ inhibition curve was slightly shifted to the right in α9α10α9extra with a Ca2+ IC50 (0.7 ± 0.2 mM, n = 6) that significantly differed (p = 0.0238, Mann-Whitney test) from that of homomeric α9 receptors (0.10 ± 0.01 mM, n = 3, from [46]). Moreover, as for α9 receptors [46] and α9α10α9Nt–TM2TM3loop (Fig. 5c, left panel), block of ACh evoked responses by Ca2+ was voltage-dependent and more pronounced at hyperpolarized than at depolarized potentials, exhibiting a 3 / 0 Ca2+ ratio of response of 0.20 ± 0.05 and 0.60 ± 0.08 (p = 0.0016, n = 4, paired t -test), at -90 and + 40 mV, respectively. Taken together, these results suggest that amino acids located within the extracellular domains of α10 (the Nt, preTM1 and TM2-TM3 loop) are required to render a potentiation phenotype of α9α10 receptors by external Ca2+.
Lack of Ca2+ potentiation in α9α10α9extra and α9α10α9TM2TM3loop receptors. a Illustration indicating the cRNAs injected in each experiment: α9 plus either α10α9extra (left) or α10α9TM2TM3loop (right), respectively. b Top Representative responses evoked by either 10 µM (left) or 50 µM (right) ACh at different extracellular Ca2+ concentrations (Vhold -90). Bottom Bars represent the mean ± S.E.M and open circles the individual oocytes. Current amplitudes recorded in each oocyte were normalized to the response obtained at 1.8 mM Ca2+ in the same oocyte (n = 6–7 per group). Note that the sole replacement of the TM2-TM3 loop of the α10 subunit by that of α9 is sufficient to abolish potentiation by extracellular Ca2+ (right panel). *p < 0.05 and ***p < 0.001 Kruskal-Wallis test followed by Dunn’s multiple comparison-test, when comparing the Ca2+ concentration that evokes the highest response (0 mM Ca2+) versus the other Ca2+ concentrations. c Representative I-V curves (α9α10α9extra, n = 4; α9α10α9TM2TM3loop; n = 6) for each receptor, obtained by the application of a voltage ramp protocol (-120 to + 50, 2 s), 10 s after the application of 10 µM (left) or 50 µM (right) ACh at different Ca2+ concentrations. Potentiation by extracellular Ca2+ is lost in α9α10α9extra (left) and α9α10α9TM2TM3loop (right) receptors at all holding potentials
In order to provide further insights into the role of the TM2-TM3 loop in Ca2+ potentiation of α9α10 nAChRs, we generated the α10α9TM2TM3loop chimeric subunit, in which only the TM2-TM3 loop of α10 was replaced by the corresponding region of α9 (Fig. 5a, right panel). Figure 5b, right panel, shows that similar to α9α10α9Nt–TM2TM3loop nAChRs, α9α10α9TM2TM3loop receptors were not potentiated by extracellular Ca2+, but instead blocked by this ion. Thus, responses were significantly higher at zero Ca2+ (401.0 ± 103.4%) than at 3 mM Ca2+ (71.3 ± 5.2%, n = 6, p = 0.0008, Kruskal-Wallis test followed by Dunn’s multiple comparison-test). It should be noted that the Ca2+ inhibition curve was slightly shifted to the right in α9α10α9TM2TM3loop with a Ca2+ IC50 (0.8 ± 0.3 mM, n = 5), that differed significantly from that of homomeric α9 receptors (0.10 ± 0.01 mM, n = 3, p = 0.0357, Mann-Whitney test). Moreover, similar to α9 [46] and α9α10α9Nt–TM2TM3loop (Fig. 5c, left panel) receptors, block of ACh evoked responses by Ca2+ was voltage-dependent and more pronounced at hyperpolarized than at depolarized potentials, exhibiting a 3 / 0 Ca2+ ratio of response of 0.14 ± 0.02 and 0.74 ± 0.14 (p = 0.0067, n = 6, paired t -test), at -90 and + 40 mV, respectively. Since potentiation by Ca2+ is more pronounced at low agonist concentrations ( [18] and Fig. 2), modulation by this ion was further assessed in α9α10α9TM2TM3loop at a sub-EC50 ACh concentration (25 µM) (Fig. 6). Once again, responses were not potentiated by Ca2+, and only block was observed (Fig. 6a), indicating that channel gating by ACh is independent of the presence of extracellular Ca2+, even at a sub-EC50 ACh concentration. Thus, responses at zero Ca2+ (668.7 ± 340.2%) were significantly higher than those at 3 mM Ca2+ (46.3 ± 20.9%, n = 5–6, p = 0.0412, Kruskal-Wallis followed by Dunn’s multiple comparison-test). It should be noted that responses of α9α10α9TM2TM3loop are the result of the co-assembly of the chimera with α9 and not that of α9 homomeric receptors, since the EC50 of homomeric α9 (11.4 ± 0.8 µM, [45]) differs from that of α9α10α9TM2TM3loop (78 ± 18 µM, p = 0.0106, Mann-Whitney test) receptors. Moreover, maximal responses to ACh at 1.8 mM α9α10α9TM2TM3loop (211 ± 55 nA, n = 7, p = 0.0061, Table 2) were significantly higher than those reported for α9 homomers (range of responses from 5 to 20 nA, with some exceptional responses of 50 and 70 nA [23, 46]). In addition, whereas α9 homomeric receptors have a slow decay rate of ACh evoked currents upon a prolonged application of a high ACh concentration (94.9 ± 1.6% of remaining current after 20 s of the peak response to ACh [23], α9α10α9TM2TM3loop exhibited a significant decay rate (61.9 ± 4.6% of remaining current after 20 s of the peak response to ACh, p < 0.0001, One-Way ANOVA followed by Dunnett´s multiple comparison test), similar to that observed for α9α10 receptors (57.1 ± 4.3% of remaining current after 20 s of the peak response to ACh, from [23], p ≤ 0.0001, one-way ANOVA followed by Dunnett´s multiple comparison test). The reverse chimera, α9α10TM2TM3loop, in which the TM2-TM3 loop of α9 was replaced by the corresponding region of α10 did not render functional receptors even at a high 1 mM ACh concentration, either when injected alone or co-injected with α9 subunits.
a Loss of Ca2+ potentiation of the α9α10α9TM2TM3loop receptor at a low (EC25) ACh concentration. Top Representative responses evoked by 25 µM ACh of α9α10α9TM2TM3loop, obtained at three different extracellular Ca2+ concentrations. Bottom Normalized ACh response amplitudes of α9α10α9TM2TM3loop as a function of extracellular Ca2+. Bar diagram illustrating the inhibition of responses to 25 µM ACh (Vhold -90) as a function of increasing extracellular Ca2+ (n = 5–6 per group, *p < 0.05 Kruskal-Wallis test followed by Dunn’s multiple comparison-test, when comparing the Ca2+ concentration that evokes the highest response versus the other Ca2+ concentrations). b The α9α10α9TM2TM3loop receptor exhibits different decay time of ACh evoked currents compared to α9. Top Responses to ACh in the continued presence of agonist in α9, α9α10 and α9α10α9TM2TM3loop receptors. Bottom Percentage of remaining current after 20 s of the peak response in α9, α9α10 and α9α10α9TM2TM3loop receptors (n = 7–12 per group, ****p < 0.0001, One-Way ANOVA. Data from the α9 and α9α10 receptors have been taken from Elgoyhen et al. [23]
Taken together, the results so far strongly support the hypothesis that the TM2-TM3 loop of α10 contains structural determinants that are critical to render the Ca2+ potentiation phenotype of α9α10 nAChRs.
Molecular dynamics simulations
The data derived from chimeric receptors revealed that the TM2-TM3 loop of α10 is a key player in the Ca2+ potentiation phenotype of the α9α10 nAChR. This finding prompts the hypothesis that Ca2+ potentiation of α9α10 nAChRs arises as a consequence of the differential binding of this cation to the environment of the TM2-TM3 loops of α9 and α10 subunits, perturbing the energy landscape of the closed to open state transition.
To test this hypothesis, we performed MD simulations. First, we evaluated Ca2+ interaction sites along the structure of α9 and α9α10 nAChRs. Given the lack of any experimental structure of these nAChRs, we used AlphaFold to build their theoretical structural models, CHARMM-gui to embed receptors in a POPC membrane and GROMACS for MD simulations, as described in Methods. With this setup, a series of simulations of the different receptors were performed, in the presence and absence of Ca2+, using a newly developed multisite calcium model [69].
The MD simulation for the α9 pentamer revealed two Ca2+ binding sites in the environment of the TM2-TM3 loops (Fig. 7a and d). Each site presented the cation lodged at an interface between subunits and coordinated by anionic side chains. In site 1, Ca2+ was coordinated by E288, from the TM2-TM3 loop, at both the (+) and (-) faces. Although there are five potential Ca2+ binding sites 1, only two Ca2+ ions were simultaneously observed alternating between these sites. Ca2+ binding site 2 occurred in the exterior side of the interchain interface, where Ca2+ was coordinated by E294, from the TM2-TM3 loop, at the (+) face, and E204 and E199, from loop 9, and R236 of the pre TM1 domain, at the (–) face. In addition, a third Ca2+ binding site coordinated by residues D69, D71, E72, located in the β1–β2 loop, and E202, from the β8–β9 loop, from the same subunit, was revealed (Fig. 7i). This latter finding is in line with previous studies in α7 nAChRs [48, 61] that have proposed the existence of a binding site for Ca2+ that involves residues E44 (E72 rat α9 numbering) and E172 (E202 rat α9 numbering). Figure 8 shows a global view of the location of the three Ca2+ sites in relation to the ECD and the TM.
Calcium occupancy in the environment of the TM2-TM3 loops observed in MD simulations of the α9 (panels a, d and i), α9α10 (panels b, e, g, j and l), and α9α10α9TM2TM3loop (panels c, f, h, k and m) receptors. Ca2+ binding at site 1 (panels a-c), site 2 (panels d-h) and site 3 (panels i-m) are shown. α9 and α10 chains are colored in yellow and blue, respectively. Side chains are colored by residue type (red: acid, blue: basic). Ca2+ average occupancy along simulations is represented as a green wireframe. Ca2+ ions captured in the snapshots are represented as a cyan sphere with VDW radius
The MD simulations for α9α10 and α9α10α9TM2TM3loop receptors revealed Ca2+ binding coordinated by contiguous α9 E288 and α10 E287 side chains in all subunit interfaces, in direct homology to the Ca2+ binding site 1 observed in α9 nAChRs (Fig. 7b and c). On the other hand, Ca2+ binding at site 2 in α9α10 and α9α10α9TM2TM3loop receptors was only observed at the α10(+)/α9(-) interfaces (Fig. 7e and f, respectively), coordinated by α10 E293 and α9 E199, α9 E204 and α9 R236 residues. No Ca2+ binding was observed in the region homologous to site 2 in the α9(+)/α10(-) (Fig. 7g and h, respectively) and α10(+)/α10(-) interfaces, where the negative charge of residue α9 E204 is replaced by a positive charge of residue R203 when α10 is in the (-) face. A third Ca2+ binding site, homologous to the Ca2+ binding site 3, was also observed in α9α10 and α9α10α9TM2TM3loop receptors, coordinated by residues α9D69, α9D71, α9 E72 and α9 E202, and α10D68, α10D70, α10 E71 and α10E201 (Fig. 7j-m).
To gain further insight into the interaction of Ca2+ with binding sites 1, 2 and 3, we calculated the Ca2+ occupancy in these sites for the α9, α9α10 and α9α10α9TM2TM3loop receptors. Calcium occupancy was calculated as the fraction of time this ion is observed within a radius of 6 Å of specific residues that underlie the binding sites, during the simulations. To measure Ca2+ occupancy for site 1, α9E288 and α10E287 were evaluated. For site 2, α9 E294 or α10 E293, and α9 E204, E199 and R236 were analyzed. For site 3, α9D69, α9D71, α9 E72, α9 E202, α10D68, α10D70, α10 E71 and α10E201 were analyzed. Figure 9a and b show that the averaged Ca2+ occupancy of sites 1 and 2 were similar for the α9, α9α10 and α9α10α9TM2TM3loop nAChRs (Fig. 9a: α9, 0.65 ± 0.12; α9α10, 0.55 ± 0.11; α9α10α9TM2TM3loop, 0.60 ± 0.13, n = 4; Fig. 9b: α9, 0.78 ± 0.01; α9α10, 0.86 ± 0.07; α9α10α9TM2TM3 loop, 0.78 ± 0.05, n = 4; Kruskal Wallis followed by Dunn’s multiple comparison-test, α9 vs. α9α10 = α9 vs. α9α10α9TM2TM3loop = α9α10 vs. α9α10α9TM2TM3loop, p > 0.9999). In the case of site 3, the average Ca2+ occupancy of α9 receptors was higher than those of α9α10 and α9α10α9TM2TM3loop nAChRs, but similar for the α9α10 and α9α10α9TM2TM3loop receptors (Fig. 9c: α9, 0.89 ± 0.01; α9α10, 0.45 ± 0.07; α9α10α9TM2TM3loop, 0.46 ± 0.06, n = 3–4; Kruskal Wallis followed by Dunn’s multiple comparison-test, α9 vs. α9α10, p = 0.0298; α9 vs. α9α10α9TM2TM3loop, p = 0.0660; α9α10 vs. α9α10α9TM2TM3loop, p> 0.9999).
Calcium occupancy in the environment of the TM2-TM3 loops of the α9, α9α10 and α9α10α9TM2TM3loop receptors at sites 1 (panel a), 2 (panel b) and 3 (panel c). Site 1 was evaluated by measuring the Ca2+ occupancy in the environment of residues α9E288 and α10E287. Site 2 was evaluated by measuring the Ca2+ occupancy in the environment of residues α9 E294 or α10 E293, and α9 E204, E199 and R236. Site 3 was evaluated by measuring the Ca2+ occupancy in the environment of residues α9D69, α9D71, α9E72, α9E202, α10D68, α10D70, α10E71 and α10E201. The values correspond to the average of four independent 100 ns simulations carried out for each receptor. Error bars represent S.E.M of the four replicas
In summary, the MD simulations revealed that Ca2+ is coordinated by the same key anionic residues and with similar Ca2+ occupancy in the environment of the TM2-TM3 loops of α9, α9α10 and α9α10α9TM2TM3loop receptors. This result suggests that the TM2-TM3 loop of α10 does not contribute to the Ca2+ potentiation phenotype of the α9α10 nAChR through the formation of novel Ca2+ binding sites that are not present in the α9 homomeric receptor.
To test the predictions derived from the MD simulations we generated E72Q and E202Q (site 3) mutants in rat α9, and E71Q, E201Q (site 3) and E287Q (site 1) in rat α10 subunits, and determined their contribution to Ca2+ potentiation of α9α10 nAChRs. Each mutant subunit was co-expressed with its wild-type counterpart in Xenopus oocytes. Notably, α9E72Qα10 and α9E202Qα10 mutant receptors failed to respond to 1 mM ACh (n = 6). On the contrary, α9α10E71Q, α9α10E201Q and α9α10E287Q complexes formed functional channels (Table 1). As reported in Table 1, both α10E71Q and α10E201Q substitutions produced a shift of the ACh concentration-response curve to the right and an increase in the ACh EC50 (104 ± 20 µM and 135 ± 23 µM, respectively). The significant increase in the EC50 of mutant receptors compared to wild-type α9 (p = 0.0013 and 0.0007, student t test, Table 3) and α9α10 (p = 0.0286 and 0.0159, Mann-Whitney test, Table 1) receptors indicate that both α9α10E71Q and α9α10E201Q receptors are assembly competent and that responses do not derive from homomeric α9 wild-type receptors. In the case of α10E201Q receptors, the significant increase in the Imax compared to wild-type α9 (p = 0.0159, Mann-Whitney test, Table 2) receptors indicate that α9α10E287Q receptors are assembly competent and that responses do not derive from homomeric α9 wild-type receptors. Figure 10 shows the modulation profile of α9α10E71Q, α9α10E201Q and α9α10E287Q receptors obtained at a near EC50 concentration of ACh (100 µM for α9α10E71Q and α9α10E201Q, and 20 µM for α9α10E287Q) and increasing concentrations of extracellular Ca2+. Different to that observed for α9α10 wild-type receptors, responses to ACh of α9α10E71Q, α9α10E201Q and α9α10E287Q receptors were not potentiated by Ca2+, indicating that channel gating by ACh became independent of the presence of extracellular Ca2+. Similar to homomeric α9 receptors, responses to ACh of α9α10E71Q, α9α10E201Q and α9α10E287Q receptors were only blocked by increasing Ca2+ concentrations (Fig. 10d-f). Thus, α9α10E71Q responses were highest at zero Ca2+ (211.0 ± 35.6%) and lowest at 3 mM Ca2+ (64.8 ± 2.7%, n = 4, p = 0.0069, Kruskal-Wallis followed by Dunn’s multiple comparison-test). It should be noted that the inhibition curve was shifted to the right with a Ca2+ IC50 (0.8 ± 0.1 mM, n = 6) that differed significantly (Mann-Whitney test, p = 0.0238) from that of homomeric α9 receptors (0.10 ± 0.01 mM, n = 3, from [46]). In addition, like α9 nAChRs [46], block of ACh evoked responses by Ca2+ was voltage-dependent and more pronounced at hyperpolarized than at depolarized potentials, exhibiting a 3 / 0 Ca2+ ratio of response of 0.25 ± 0.05 and 1.05 ± 0.29 (Fig. 10g, p = 0.0302, n = 5, paired t -test), at -90 and + 40 mV, respectively. Similarly, responses to ACh of α9α10E201Q were highest at zero Ca2+ (201.6 ± 39.7%) and lowest at 3 mM Ca2+ (74.3 ± 11.2%, n = 7, p = 0.0071, Kruskal-Wallis followed by Dunn’s multiple comparison-test), with a Ca2+ IC50 (0.9 ± 0.3, n = 6) that differed significantly (Mann-Whitney test, p = 0.0238) from that of homomeric α9 receptors (0.10 ± 0.01 mM, n = 3, from [46]). Moreover, as for α9 receptors [46], block of ACh evoked responses by Ca2+ was voltage-dependent and more pronounced at hyperpolarized than at depolarized potentials, exhibiting a 3 / 0 Ca2+ ratio of response of 0.18 ± 0.05 and 0.53 ± 0.12 (Fig. 10h, p = 0.0358, n = 6, paired t-test), at -90 and + 40 mV, respectively. Similarly, α9α10E287Q responses were highest at zero Ca2+ (320.7 ± 97.4%) and lowest at 3 mM Ca2+ (67.6 ± 5.6%, n = 5, p = 0.0010, Kruskal-Wallis followed by Dunn’s multiple comparison-test) with a Ca2+ IC50 (0.3 ± 0.1, n = 4) that did not differ significantly (Mann-Whitney test, p = 0.400) from that of homomeric α9 receptors (0.10 ± 0.01 mM, n = 3, from [46]). Like α9 nAChRs [46], block of ACh evoked responses by Ca2+ was voltage-dependent and more pronounced at hyperpolarized than at depolarized potentials, exhibiting a 3 / 0 Ca2+ ratio of response of 0.26 ± 0.02 and 0.48 ± 0.04 (Fig. 10i, p = 0.0031, n = 10, paired t -test), at -90 and + 40 mV, respectively. Taken together, these results suggest that α10E71Q, α10E201Q and α10E287 are required for potentiation of α9α10 receptors by external Ca2+ and are compatible with those obtained in MD simulations, indicating that α10E71Q, α10E201Q and α10E287 form part of Ca2+ binding sites.
α10E71, E201 and E287 are key residues involved in Ca2+ potentiation a-c Diagram of an α10 (blue) subunit illustrating the position of the E71Q (a), E201Q (b) and E287Q (c) mutations, which were co-injected with α9 (red). d-f Top Representative responses evoked by 100 µM ACh in α9α10E71Q (d) and α9α10E201Q (e), and by 20 µM ACh in α9α10E287Q (f) receptors, respectively, obtained at three different extracellular Ca2+ concentrations. Bottom Bars represent the mean ± S.E.M and open circles the individual oocytes (n = 4–7). Normalized ACh response amplitudes of α9α10E71Q (d), α9α10E201Q (e), and α9α10E287Q (f) as a function of extracellular Ca2+. The potentiation by extracellular calcium was lost in all mutant receptors. **p < 0.01, Kruskal-Wallis test followed by Dunn’s multiple comparison-test, when comparing the Ca2+ concentration that evokes the highest response (0 mM Ca2+) vs. the other Ca2+ concentrations. (g-i) Representative I-V curves (α9α10E71Q, n = 5; α9α10E201Q, n = 6; α9α10E287Q, n = 10) obtained by the application of a voltage ramp protocol (-120 to + 50, 2 s), 10 s after the application of 100 µM ACh in α9α10E71Q and α9α10E201Q receptors, and 20 µM ACh in α9α10E287Q receptors, at different Ca2+ concentrations. The lack of potentiation by extracellular Ca2+ was maintained at different voltages in all mutant receptors
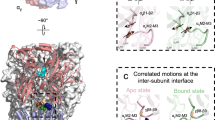
Previous work applied MD simulations to a model of the full-length α7 nAChR and evidenced conformational changes in the TM2-TM3 loops, in the presence of Ca2+ [21]. Since conformational changes in the TM2-TM3 loops might have implications for their role in channel gating, we examined the potential impact of Ca2+ on the structure and motions of the TM2-TM3 loops of α9, α9α10 and α9α10α9TM2TM3 loop receptors. For that purpose we measured the root-mean-square-fluctuation (RMSF) profile of the TM2-TM3 loops of α9 and α10 subunits from α9, α9α10 and α9α10α9TM2TM3loop receptors, in the presence and absence of Ca2+ (Fig. 11). No changes in the flexibility of the TM2-TM3 loops of α9 (Fig. 11a), α9α10 (Fig. 11b and c) and α9α10α9TM2TM3loop (Fig. 11d and e) receptors, were observed in the presence of Ca2+. These results suggest that changes in structural flexibility of the α9 and α10 TM2-TM3 loops most likely do not account for the differential calcium potentiation phenotype between homomeric α9 and α9α10 heteromeric nAChRs.
Structural flexibility of the TM2-TM3 loops of α9 (panel a), α9α10 (panel b-c) and α9α10α9TM2TM3loop (panels d-e) receptors in the absence (cyan) and presence (magenta) of Ca2+. The flexibility is evaluated through the RMSF of the alpha carbons of the protein from the average structure observed during the simulations. The RMSF is discriminated for α9 (panels a, b and d) and α10 subunits (panels c and e). Solid lines represent the average over all subunits in four replicas for each receptor type. The S.E.M. is shown as colored fill
The electrophysiology results show that α10E71 and α10E201 are required for potentiation of α9α10 receptors by external Ca2+. MD simulations of the α9α10 pentamer revealed Ca2+ binding sites in the environment of α10E71, α10E201, α9E72 and α9E202. Previous work has shown that in α7 receptors these residues establish electrostatic interactions with the invariant residue R209 [61, 73,74,75], a key component of the pathway linking agonist binding to channel gating [76, 77]. Similarly, our MD data show that α9E72 and α9E202 establish electrostatic interactions with the conserved residue α9R235, and α10E71 and α10E201 with α10R234 (Fig. 12). This result might suggest that these glutamates contribute to coupling of Ca2+ binding to potentiation in addition of being part of a Ca2+ binding site.
Discussion
In the present study, we have identified structural determinants responsible for the potentiation by Ca2+ of the α9α10 nicotinic receptor. We have found that the TM2-TM3 loop of α10 markedly influences the modulation of ACh-evoked responses of α9α10 nAChRs by Ca2+. Thus, the replacement of the TM2-TM3 loop of the α10 by that of the α9 subunit eliminates the Ca2+ potentiating phenotype observed in α9α10 nAChRs, rendering a α9-like receptor in which ACh-evoked responses are only blocked by this cation. Molecular dynamics simulations reveal that the TM2-TM3 loop of α10 does not contribute to the Ca2+ potentiation phenotype through the formation of novel Ca2+ binding sites not present in the α9 homomeric receptor. These results suggest that the TM2-TM3 loop of α10 acts as a control element that determines the activity of α9α10 nAChRs in response to local and transient changes of extracellular Ca2+, most likely facilitating transduction of Ca2+-induced molecular rearrangements to channel gating.
Our results show that potentiation by Ca2+ in all chimeric receptors was always voltage-independent, indicating that Ca2+ modulation is not subject to the electric field of the membrane. In this regard, the observation that in the case of α9α10α9Nt–TM2, in which the five TM2 regions of the pentameric chimeric receptor are provided by the α9 subunit, Ca2+ potentiation is still observed, indicates that it is not the pore-forming TM2 region of the α10 subunit the one responsible for the Ca2+ potentiating phenotype of the α9α10 nAChR. This is in accordance with previous work [15, 16, 18, 78], where potentiation is independent of Ca2+ influx and membrane potential and thus is not subjected to the electric field of the plasma membrane. Therefore, as suggested previously for other nAChRs [8, 18], Ca2+ potentiation most likely depends on an extracellular binding site to allosterically modulate coupling between ligand binding and gating, rather than in transmembrane regions. In this regard, a leftward shift in the ACh concentration response curve has been described in α9α10 nAChRs in the presence of Ca2+, indicating that potentiation by Ca2+ involves changes in the apparent affinity for ACh [18, 78]. This could be the result of an increase in ACh binding affinity to its orthosteric binding site, a facilitation of the gating of the channel following agonist binding and/or changes in the kinetic properties of these receptors. The former mechanism has not been described for other nAChRs. The observation that both TM2 L9´T and V13´T α9α10 mutant receptors, that exhibit spontaneous openings and therefore a reduced energetic barrier for channel gating, do not require calcium for activation by ACh [79], speaks towards an increased channel gating of the α9α10 nAChR in the presence of calcium.
We show that ACh evoked responses of α9α10α9Nt–TM2TM3loop, α9α10α9extra and α9α10α9TM2TM3loop receptors become independent of the presence of extracellular Ca2+ and are not potentiated by this ion. Common to all these chimeric receptors is the lack of a α10 TM2-TM3 loop which was replaced by that of the α9 subunit. This result strongly suggests that the TM2-TM3 loop of α10 is responsible for the simultaneous need of Ca2+ and ACh for α9α10 receptor activation and thus its potentiation by external calcium. This is in agreement with that described in previous work for other nAChRs. Thus, the gating reaction couples local structural changes at the binding sites with changes in the ion channel leading to gate opening [80,81,82,83,84]. The extracellular-transmembrane interface is key in this process since it mediates a bi-directional allosteric interaction between the binding site and the pore domain. The functional coupling process is mediated by a network of loops from both domains including: β1β2 loop, Cys-loop, β8β9 loop and the end of β10, all from the extracellular region, and the pre-TM1 region, TM2–TM3 loop, and the C-terminal end of TM4 from the transmembrane region [72, 85,86,87,88]. Most importantly, the TM2–TM3 loop and flanking regions play a key role in channel gating in all Cys-loop receptors [89,90,91,92,93], regulating the receptor conformation and activation fine-tuning [91, 92, 94, 95]. In addition, the TM2-TM3 loop has an obligatory role in the positive allosteric modulation by PAMs of α7 nAChRs [96].
By leveraging the three-dimensional structures for the α9, α9α10 and α9α10α9TM2TM3loop nAChRs and subjecting them to MD simulations in the presence of Ca2+, we identified two novel sites of Ca2+ association involving residues within the TM2-TM3 loops. Even though the simulations reveal that both Ca2+ binding sites are present in all three receptors studied, in α9α10 nAChRs Ca2+ binding at site 2 only occurred at the α10(+)/α9(-) interfaces, coordinated by α10E293 and α9E199, E204 and R236 residues. No Ca2+ binding was observed in the α9(+)/α10(-) and α10(+)/α10(-) interfaces, where the negative charge of residue α9E204 is replaced by a positive charge of residue R203 when α10 is in the (-) face. Thus, the presence of α10R203 might result in a positively charged environment that would perturb the access of Ca2+ to the binding site. This indicates that the contribution of α9 and α10 subunits to the complementary face of the Ca2+ binding site 2 is nonequivalent. Likewise, in α9α10 nAChRs both α9 and α10 subunits contribute to the principal face of the ligand binding site but their contribution to the complementary face is nonequivalent, with α10 not contributing to the complementary face of the binding pocket or inefficiently providing the (-) face [42]. This phenotype results from the adaptive evolutionary amino acid changes acquired by mammalian α10, which rendered a divergent branch within the clade of vertebrate α10 subunits [33]. These findings might contribute to understand why rat homomeric α10 receptors are non-functional when expressed in Xenopus laevis oocytes [23, 24].
An overall difference in total calcium bound to the TM2-TM3 loop is not the underlying mechanism that accounts for the differential Ca2+ potentiating phenotype of α9 homomeric versus α9α10 heteromeric receptors. This is based on the observation that the total occupancy of the two Ca2+ association sites identified through MD simulations involving residues within the TM2-TM3 loops did not significantly differ between homomeric α9 and heteromeric α9α10 and α9α10α9 TM2TM3loop receptors. Thus, even if the binding of calcium to the two sites within the TM2-TM3 loop of the α9α10 nAChR were necessary for the potentiation by Ca2+ of ACh responses, a mechanism other than the binding per se is responsible for the differences in calcium modulation between α9 homomeric and α9α10 heteromeric receptors. Suresh et al. [21] applied MD simulations to a model of the full-length α7 nAChR and evidenced conformational changes in the TM2-TM3 loops, in the presence of Ca2+. In addition, they showed that in the presence of Ca2+ the TM2-TM3 loops are more flexible and their flexibility extends significantly down into the middle of the TM2 helix, causing pore widening. These observations suggest that Ca2+ serves to shift the receptor toward a conformation that more closely resembles an active state that can more easily transition to a fully active state upon agonist binding. On the contrary, our MD simulations show that the TM2-TM3 loops of α9, α9α10, and α9α10α9 TM2TM3loop nAChRs do not undergo conformational changes, leading to an increase in flexibility, in the presence of Ca2+. However, it should be noted that the time length of our simulations is likely insufficient to reveal the full range of conformational changes induced by Ca2+ binding.
Our combined in silico and experimental observations suggest that the TM2-TM3 loop of α10 is responsible for the simultaneous need of Ca2+ and ACh for α9α10 receptor activation and thus its potentiation by external calcium. A possible explanation based on the Monod–Wyman–Changeux model [97] is that the TM2-TM3 loop of α10 may contribute to the setpoint for activation, causing an increase of the energy barrier between the resting and active state of the α9α10 nAChR. In that scenario, after agonist binding a further reduction of the energy barrier, caused by exposure to Ca2+, is needed to open the channel. Thus, in the α9α10 wild-type background, the TM2-TM3 loop of α10 creates a greater energetic barrier between closed and open states. This could slow channel kinetics and lead to a requirement for binding of both ACh and Ca2+ to efficiently drive the conformational transitions, leading to channel opening. Further studies are needed to determine the effects of Ca2+ on the structure and dynamics of α9α10 nAChRs.
It should be noted that α9α10α9x chimeras did not fully recapitulate a homomeric α9 phenotype. Thus, ACh EC50 and Ca2+ IC50 values were shifted in some chimeric receptors. This could be the result of an epistatic phenomenon where the consequences of a given amino acid substitution greatly depends upon the overall sequence (and hence structure) of the protein [71]. In this regard, chimeric receptors were engineered in the overall context of a α10 subunit, compared to wild-type α9 homomeric receptors. Interestingly, α9α10α9Nt, α9α10α9Nt–TM1TM2loop and α9α10α9Nt–TM2 receptors exhibited robust ACh evoked responses in the absence of Ca2+, but these responses were still potentiated by Ca2+. These results might suggest that other domains of α10, like the Nt, contain structural determinants that play a role in the gating of the α9α10 receptor, but that these domains are not sufficient to explain the Ca2+ potentiation phenotype in α9α10 nAChRs.
An extracellular Ca2+ interaction site has been reported in an α7/5-HT3 chimera [17], where mutation of several negatively charged residues near the agonist binding site, including E44 and E172 analyzed in the present work, altered or prevented Ca2+ potentiation of ACh-elicited macroscopic currents. The participation of E172 in α7 modulation by Ca2+ has been further observed in an additional study [47]. Moreover, molecular modeling of the α7 extracellular domain indicates that E44 of the (+) subunit is close to E172 of the (-) subunit, and can form an intersubunit Ca2+-binding site [48]. In addition, cryo-EM structures of the α7 nAChR revealed five putative Ca2+ densities coordinated by four carboxylate-containing side chains including E44 and E172 [61]. Interestingly, these putative Ca2+ binding sites were present in all three major conformational states of the gating cycle—resting, activated, and desensitized—but were most prominent in the activated state [61]. Our present results when point-mutating E44 and E172 are similar to those of Galzi et al. [17], since mutation of these residues in the α10 subunit (α10E71Q and α10E201Q) abolished Ca2+ potentiation of the α9α10 nAChR. Moreover, our MD simulations further indicate that both α10E71 and α10E201 are direct participants in a Ca2+ binding site in the α9α10 nAChR. However, an additional study using barium as a modulator has questioned the participation of E44 and E172 in the allosteric potentiation of α7 nAChRs by divalent cations [98]. Moreover, it has been suggested that the local environment of α7 E172 appears incompatible with a Ca2+ binding site and that α7 E172 may instead contribute to coupling of Ca2+ binding to potentiation [49]. In this regard, the participation of α7 E44 and E172 in channel gating in nAChRs has been extensively reported [61, 76, 77, 99, 100]. Moreover, α7 E44 and E172 are situated in loops β1–β2 and β8–β9, respectively, known to form part of the structural determinants of channel gating [72, 76, 101]. High-resolution structures of several neuronal heteromeric nAChRs show that E44 and E172 establish electrostatic interactions with the invariant residue R209 of the preTM1 region [61, 73,74,75], a key component of the principal coupling pathway linking agonist binding to channel gating [76, 77]. Similarly, our MD data show that α9E72 and α9E202 establish electrostatic interactions with the conserved residue α9R235, while α10E71 and α10E201, with α10R234. Thus, in addition to forming a calcium binding site, these residues could contribute to coupling of Ca2+ binding to potentiation in the α9α10 nAChR. If this were the case, the lack of Ca2+ potentiation observed in the α10E71Q and E201Q mutants might result from a receptor with either impaired coupling of Ca2+ binding to potentiation or one in which the allosteric movements resulting from the effect of Ca2+ are mimicked by the mutation. In this regard, it has been described that in the Torpedo nAChR some E45 mutants enable per se channel gating by increasing the channel opening rate constant, while others impair gating [100]. Further single channel recordings are necessary to define the mechanism underlying the α10E71Q and E201Q mutant phenotypes.
In summary, this study demonstrates that the α10 TM2–TM3 loop is key for the positive allosteric modulation effect of Ca2+ and allows the α9α10 nAChR to function as a coincidence detector for both ACh and Ca2+, enabling fine tuning for receptor activation. Thus, the α10 TM2–TM3 loop acts as a control element that determines the activity of α9α10 receptors in response to local and transient changes of extracellular Ca2+ concentration. The identification of the α10 TM2–TM3 loop as a modulatory site of the α9α10 nAChR, if validated for the human counterpart, might offer opportunities for developing pharmacotherapeutics targeting this region for indications such as protection from acoustic trauma, where functional enhancement of the α9α10 receptor is thought to be beneficial [50,51,52,53].
Data availability
The vast majority of the data generated or analyzed during this study are included in this published article. Any remaining data for the current study is available from the corresponding authors on reasonable request.
Abbreviations
- 5-HT3A :
-
5-Hydroxy tryptamine type 3 subunit A
- ACh:
-
Acetylcholine
- ANOVA:
-
Analysis of variance
- Ca2+ :
-
Calcium
- cRNA:
-
Copy ribonucleic acid
- D:
-
Aspartate
- E:
-
Glutamate
- EC50 :
-
Concentration of agonist that induces half-maximal current response
- ECD:
-
Extracellular domain
- GABA:
-
γ-Aminobutyric acid
- IC50 :
-
Concentration of antagonist that reduces 50% of the maximal response of the agonist
- Imax :
-
Maximal current amplitude
- I–V:
-
Current-voltage relationship
- MD:
-
Molecular dynamics
- nAChR:
-
Nicotinic acetylcholine receptor
- nH:
-
Hill coefficient
- Nt:
-
N-terminal domain
- PAM:
-
Positive allosteric modulator
- PCR:
-
Polymerase chain reaction
- Q:
-
Glutamine
- RMSF:
-
Root-mean-square-fluctuation
- S.E.M.:
-
Standard error of the mean
- s:
-
Second
- TM:
-
Transmembrane domain
- Vhold :
-
Holding potential
References
Corringer PJ, Poitevin F, Prevost MS et al (2012) Structure and pharmacology of pentameric receptor channels: from bacteria to brain. Structure 20:941–956. https://doi.org/10.1016/j.str.2012.05.003
Dent JA (2006) Evidence for a diverse cys-loop ligand-gated ion channel superfamily in early bilateria. J Mol Evol 62:523–535. https://doi.org/10.1007/s00239-005-0018-2
Karlin A (2002) Ion Channel Structureemerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci 3:102–114. https://doi.org/10.1038/nrn731
Jaiteh M, Taly A, Hénin J (2016) Evolution of pentameric ligand-gated ion channels: pro-loop receptors. PLoS ONE 11:1–24. https://doi.org/10.1371/journal.pone.0151934
Lipovsek M, Marcovich I, Elgoyhen AB (2021) The hair cell α9α10 nicotinic acetylcholine receptor: Odd Cousin in an Old Family. Front Cell Neurosci 15:1–17. https://doi.org/10.3389/fncel.2021.785265
Unwin N (2005) Refined structure of the nicotinic acetylcholine receptor at 4 Å resolution. J Mol Biol 346:967–989. https://doi.org/10.1016/j.jmb.2004.12.031
Pedersen JE, Bergqvist CA, Larhammar D (2019) Evolution of vertebrate nicotinic acetylcholine receptors. BMC Evol Biol 19:38. https://doi.org/10.1186/s12862-018-1341-8
Changeux JP (2018) The nicotinic acetylcholine receptor: a typical ‘allosteric machine’. Philos Trans R Soc B Biol Sci 373. https://doi.org/10.1098/rstb.2017.0174
Wang J, Lindstrom J (2018) Orthosteric and allosteric potentiation of heteromeric neuronal nicotinic acetylcholine receptors. Br J Pharmacol 175:1805–1821. https://doi.org/10.1111/bph.13745
Williams DK, Wang J, Papke RL (2011) Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem Pharmacol 82:915–930. https://doi.org/10.1016/j.bcp.2011.05.001
Chatzidaki A, Millar NS (2015) Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol 97:408–417. https://doi.org/10.1016/j.bcp.2015.07.028
Grupe M, Grunnet M, Bastlund JF, Jensen AA (2015) Targeting α4β2 nicotinic acetylcholine receptors in central nervous system disorders: perspectives on positive allosteric modulation as a therapeutic approach. Basic Clin Pharmacol Toxicol 116:187–200. https://doi.org/10.1111/bcpt.12361
Amador M, Dani JA (1995) Mechanism for modulation of nicotinic acetylcholine receptors that can influence synaptic transmission. J Neurosci 15:4525–4532. https://doi.org/10.1523/jneurosci.15-06-04525.1995
Decker ER, Dani JA (1990) Calcium permeability of the nicotinic acetylcholine receptor: the single-channel calcium influx is significant. J Neurosci off J Soc Neurosci 10:3413–3420. https://doi.org/10.1523/JNEUROSCI.10-10-03413.1990
Mulle C, Léna C, Changeux JP (1992) Potentiation of nicotinic receptor response by external calcium in rat central neurons. Neuron 8:937–945. https://doi.org/10.1016/0896-6273(92)90208-U
Vernino S, Amador M, Luetje CW et al (1992) Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron 8:127–134. https://doi.org/10.1016/0896-6273(92)90114-S
Galzi JL, Bertrand S, Corringer PJ et al (1996) Identification of calcium binding sites that regulate potentiation of a neuronal nicotinic acetylcholine receptor. EMBO J 15:5824–5832. https://doi.org/10.1002/j.1460-2075.1996.tb00969.x
Weisstaub N, Vetter DE, Elgoyhen AB et al (2002) The alpha9alpha10 nicotinic acetylcholine receptor is permeable to and is modulated by divalent cations. Hear Res 167:122–135. https://doi.org/S0378595502003805 [pii]
Natarajan K, Mukhtasimova N, Corradi J et al (2020) Mechanism of calcium potentiation of the α7 nicotinic acetylcholine receptor. J Gen Physiol 152. https://doi.org/10.1085/JGP.202012606
Li S-X, Huang S, Bren N et al (2011) Ligand-binding domain of an α7-nicotinic receptor chimera and its complex with agonist. Nat Neurosci 14:1253–1259. https://doi.org/10.1038/nn.2908
Suresh A, Hung A (2019) Structural effects of divalent calcium cations on the α7 nicotinic acetylcholine receptor: a molecular dynamics simulation study. Proteins Struct Funct Bioinforma 87:992–1005. https://doi.org/10.1002/prot.25761
Elgoyhen AB, Johnson DS, Boulter J et al (1994) α9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 79:705–715. https://doi.org/10.1016/0092-8674(94)90555-X
Elgoyhen a B, Vetter DE, Katz E et al (2001) Alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci U S A 98:3501–3506. https://doi.org/10.1073/pnas.051622798
Sgard F, Charpantier E, Bertrand S et al (2002) A novel human nicotinic receptor subunit, α10, that confers functionality to the α9-subunit. Mol Pharmacol 61:150–159. https://doi.org/10.1124/mol.61.1.150
Plazas PV, Elgoyhen AB (2021) The cholinergic lateral line efferent synapse: structural, functional and molecular similarities with those of the Cochlea. Front Cell Neurosci 15:1–15. https://doi.org/10.3389/fncel.2021.765083
Elgoyhen AB (2023) The α9α10 acetylcholine receptor: a non-neuronal nicotinic receptor. Pharmacol Res 190:106735. https://doi.org/10.1016/j.phrs.2023.106735
Elgoyhen AB, Katz E (2012) The efferent medial olivocochlear-hair cell synapse. J Physiol Paris 106:47–56. https://doi.org/10.1016/j.jphysparis.2011.06.001
Weisstaub N, Vetter DE, Elgoyhen AB, Katz E (2002) The alpha9alpha10 nicotinic acetylcholine receptor is permeable to and is modulated by divalent cations. Hear Res 167:122–135
Moglie MJ, Fuchs PA, Elgoyhen AB, Goutman JD (2018) Compartmentalization of antagonistic Ca2 + signals in developing cochlear hair cells. Proc Natl Acad Sci U S A 115:E2095–E2104. https://doi.org/10.1073/pnas.1719077115
Moglie MJ, Wengier DL, Elgoyhen AB, Goutman JD (2021) Synaptic contributions to cochlear outer hair cell Ca2 + dynamics. https://doi.org/10.1523/JNEUROSCI.3008-20.2021. J Neurosci JN-RM-3008-20
Taranda J, Maison SF, Ballestero JA et al (2009) A point mutation in the hair cell nicotinic cholinergic receptor prolongs cochlear inhibition and enhances noise protection. PLoS Biol 7. https://doi.org/10.1371/journal.pbio.1000018
Franchini LF, Elgoyhen AB (2006) Adaptive evolution in mammalian proteins involved in cochlear outer hair cell electromotility. Mol Phylogenet Evol 41:622–635. https://doi.org/10.1016/j.ympev.2006.05.042
Lipovsek M, Im GJ, Franchini LF et al (2012) Phylogenetic differences in calcium permeability of the auditory hair cell cholinergic nicotinic receptor. Proc Natl Acad Sci 10–15. https://doi.org/10.1073/pnas.1115488109
Lipovsek M, Fierro A, Pérez EG et al (2014) Tracking the molecular evolution of calcium permeability in a nicotinic acetylcholine receptor. Mol Biol Evol 31:3250–3265. https://doi.org/10.1093/molbev/msu258
Marcovich I, Moglie MJ, Carpaneto Freixas AE et al (2020) Distinct evolutionary trajectories of neuronal and hair cell nicotinic acetylcholine receptors. Mol Biol Evol 37:1070–1089. https://doi.org/10.1093/molbev/msz290
Scheffer D, Sage C, Plazas PV et al (2007) The α1 subunit of nicotinic acetylcholine receptors in the inner ear: transcriptional regulation by ATOH1 and co-expression with the γ subunit in hair cells. J Neurochem 103:2651–2664. https://doi.org/10.1111/j.1471-4159.2007.04980.x
Morley BJ, Li H, Hiel H et al (1998) Identification of the subunits of the nicotinic cholinergic receptors in the rat cochlea using RT–PCR and in situ hybridization. Mol Brain Res 53:78–87. https://doi.org/10.1016/S0169-328X(97)00272-6
Morley BJ, Whiteaker P, Elgoyhen AB (2018) Commentary: nicotinic acetylcholine receptor α9 and α10 subunits are expressed in the brain of mice. Front Cell Neurosci 12:104. https://doi.org/10.3389/fncel.2018.00104
Atlas AB (2013) Accelerating Progress Toward Understanding the Brain. https://portal.brain-map.org/
Freixas AEC, Moglie MJ, Castagnola T et al (2021) Unraveling the molecular players at the cholinergic efferent synapse of the zebrafish lateral line. J Neurosci 41:47–60. https://doi.org/10.1523/JNEUROSCI.1772-20.2020
Hone AJ, McIntosh JM (2022) Alkaloid ligands enable function of homomeric human α10 nicotinic acetylcholine receptors. Front Pharmacol 13:1–16. https://doi.org/10.3389/fphar.2022.981760
Boffi JC, Marcovich I, Gill-Thind JK et al (2017) Differential Contribution of Subunit interfaces to α 9 α 10 nicotinic acetylcholine receptor function. Mol Pharmacol 91:250–262. https://doi.org/10.1124/mol.116.107482
Rothlin CV, Katz E, Verbitsky M, Belén Elgoyhen A (1999) The α9 nicotinic acetylcholine receptor shares pharmacological properties with type A γ-aminobutyric acid, glycine, and type 3 serotonin receptors. Mol Pharmacol 55:248–254. https://doi.org/10.1124/mol.55.2.248
Rothlin CV, Lioudyno MI, Silbering AF et al (2003) Direct interaction of serotonin type 3 receptor ligands with recombinant and native α9α10-containing nicotinic cholinergic receptors. Mol Pharmacol 63:1067–1074. https://doi.org/10.1124/mol.63.5.1067
Verbitsky M, Rothlin CV, Katz E, Belén Elgoyhen A (2000) Mixed nicotinic-muscarinic properties of the α9 nicotinic cholinergic receptor. Neuropharmacology 39:2515–2524. https://doi.org/10.1016/S0028-3908(00)00124-6
Katz E, Verbitsky M, Rothlin CV et al (2000) High calcium permeability and calcium block of the α9 nicotinic acetylcholine receptor. Hear Res 141:117–128. https://doi.org/10.1016/S0378-5955(99)00214-2
Eddins D, Sproul AD, Lyford LK et al (2002) Glutamate 172, essential for modulation of L247T alpha 7 ACh receptors by Ca2+, lines the extracellular vestibule. AJP Cell Physiol 283:C1454–C1460. https://doi.org/10.1152/ajpcell.00204.2002
Le Novère N, Grutter T, Changeux J-P (2002) Models of the extracellular domain of the nicotinic receptors and of agonist- and Ca2+-binding sites. Proc Natl Acad Sci U S A 99:3210–3215. https://doi.org/10.1073/pnas.042699699
Natarajan K, Mukhtasimova N (2020) Mechanism of calcium potentiation of the α 7 nicotinic acetylcholine receptor. 152
Elgoyhen AB (2022) The α9α10 nicotinic acetylcholine receptor: a compelling drug target for hearing loss? Expert Opin Ther Targets 26:291–302. https://doi.org/10.1080/14728222.2022.2047931
Boero LE, Castagna VC, Di Guilmi MN et al (2018) Enhancement of the medial olivocochlear system prevents hidden hearing loss. J Neurosci 38:7440–7451. https://doi.org/10.1523/JNEUROSCI.0363-18.2018
Elgoyhen AB, Katz E, Fuchs PA (2009) The nicotinic receptor of cochlear hair cells: a possible pharmacotherapeutic target? Biochem Pharmacol 78:712–719. https://doi.org/10.1016/j.bcp.2009.05.023
Boero LE, Castagna VC, Terreros G et al (2020) Preventing presbycusis in mice with enhanced medial olivocochlear feedback. 1–9. https://doi.org/10.1073/pnas.2000760117
Horton RM, Hunt HD, Ho SN et al (1989) Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension sequences ; frequency of errors ; exon ; intron ; mosaic fusion protein ; mouse histocompatibility genes). Sci (80-) 77:61–68. https://doi.org/10.1016/0378-1119(89)90359-4
Szewczyk E, Nayak T, Oakley CE et al (2006) Fusion PCR and gene targeting in aspergillus nidulans. Nat Protoc 1:3111–3120. https://doi.org/10.1038/nprot.2006.405
Gerzanich V, Anand R, Lindstrom J (1994) Homomers of alpha 8 and alpha 7 subunits of nicotinic receptors exhibit similar channel but contrasting binding site properties
Arellano RO, Woodward RM, Miledi R (1995) A monovalent cationic conductance that is blocked by extracellular divalent cations in Xenopus oocytes. J Physiol 484:593–604. https://doi.org/10.1113/jphysiol.1995.sp020689
Ebihara L (1996) Xenopus connexin38 forms hemi-gap-junctional channels in the nonjunctional plasma membrane of Xenopus oocytes. Biophys J 71:742–748. https://doi.org/10.1016/S0006-3495(96)79273-1
Evans R, O’Neill M, Pritzel A et al (2022) Protein complex prediction with AlphaFold-Multimer. https://doi.org/10.1101/2021.10.04.463034. bioRxiv 2021.10.04.463034
Mirdita M, Schütze K, Moriwaki Y et al (2022) ColabFold: making protein folding accessible to all. Nat Methods 19:679–682. https://doi.org/10.1038/s41592-022-01488-1
Noviello CM, Gharpure A, Mukhtasimova N et al (2021) Structure and gating mechanism of the α7 nicotinic acetylcholine receptor. Cell 184:2121–2134e13. https://doi.org/10.1016/j.cell.2021.02.049
Studer G, Biasini M, Schwede T (2014) Assessing the local structural quality of transmembrane protein models using statistical potentials (QMEANBrane). Bioinformatics 30:i505–i511. https://doi.org/10.1093/bioinformatics/btu457
Jo S, Kim T, Iyer VG, Im W (2008) CHARMM-GUI: a web-based graphical user interface for CHARMM. J Comput Chem 29:1859–1865. https://doi.org/10.1002/JCC.20945
Jo S, Kim T, Im W (2007) Automated builder and database of protein/membrane complexes for molecular dynamics simulations. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0000880. 2:
Wu EL, Cheng X, Jo S et al (2014) CHARMM-GUI membrane Builder toward realistic biological membrane simulations. J Comput Chem 35:1997–2004. https://doi.org/10.1002/JCC.23702
Park S, Choi YK, Kim S et al (2021) CHARMM-GUI membrane Builder for lipid nanoparticles with Ionizable Cationic lipids and PEGylated lipids. J Chem Inf Model 61:5192–5202. https://doi.org/10.1021/ACS.JCIM.1C00770
Abraham MJ, Murtola T, Schulz R et al (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25. https://doi.org/10.1016/J.SOFTX.2015.06.001
Brooks BR, Brooks CL, Mackerell AD et al (2009) CHARMM: the biomolecular simulation program. J Comput Chem 30:1545–1614. https://doi.org/10.1002/JCC.21287
Zhang A, Yu H, Liu C, Song C (2020) The Ca2 + permeation mechanism of the ryanodine receptor revealed by a multi-site ion model. Nat Commun 11. https://doi.org/10.1038/S41467-020-14573-W
Mulle C, Choquet D, Korn H, Changeux JP (1992) Calcium influx through nicotinic receptor in rat central neurons: its relevance to cellular regulation. Neuron 8:135–143. https://doi.org/10.1016/0896-6273(92)90115-t
Miton CM, Buda K, Tokuriki N (2021) Epistasis and intramolecular networks in protein evolution. Curr Opin Struct Biol 69:160–168. https://doi.org/10.1016/j.sbi.2021.04.007
Bouzat C, Gumilar F, Spitzmaul G et al (2004) Coupling of agonist binding to channel gating in an ACh-binding protein linked to an ion channel. Nature 430:896–900. https://doi.org/10.1038/nature02753
Walsh RMJ, Roh S-H, Gharpure A et al (2018) Structural principles of distinct assemblies of the human α4β2 nicotinic receptor. Nature 557:261–265. https://doi.org/10.1038/s41586-018-0081-7
Gharpure A, Teng J, Zhuang Y et al (2019) Agonist selectivity and Ion Permeation in the α3β4 Ganglionic Nicotinic receptor. Neuron 104:501–511e6. https://doi.org/10.1016/j.neuron.2019.07.030
Rahman MM, Teng J, Worrell BT et al (2020) Structure of the Native Muscle-type Nicotinic Receptor and Inhibition by Snake Venom Toxins. Neuron 1–11. https://doi.org/10.1016/j.neuron.2020.03.012
Lee WY, Sine SM (2005) Principal pathway coupling agonist binding to channel gating in nicotinic receptors. Nature 438:243–247. https://doi.org/10.1038/nature04156
Mukhtasimova N, Sine SM (2013) Nicotinic receptor transduction zone: invariant arginine couples to multiple electron-rich residues. Biophys J 104:355–367. https://doi.org/10.1016/j.bpj.2012.12.013
Gómez-Casati ME, Fuchs PA, Elgoyhen AB, Katz E (2005) Biophysical and pharmacological characterization of nicotinic cholinergic receptors in rat cochlear inner hair cells. J Physiol 566:103–118. https://doi.org/10.1113/jphysiol.2005.087155
Plazas PV, De Rosa MJ, Gomez-Casati ME et al (2005) Key roles of hydrophobic rings of TM2 in gating of the α9α10 nicotinic cholinergic receptor. Br J Pharmacol 145:963–974. https://doi.org/10.1038/sj.bjp.0706224
Miyazawa A, Fujiyoshi Y, Unwin N (2003) Structure and gating mechanism of the acetylcholine receptor pore. Nature 423:949–955. https://doi.org/10.1038/nature01748
Law RJ, Henchman RH, Andrew McCammon J (2005) A gating mechanism proposed from a simulation of a human 7 nicotinic acetylcholine receptor
Paas Y, Gibor G, Grailhe R et al (2005) Pore conformations and gating mechanism of a cys-loop receptor. Proc Natl Acad Sci U S A 102:15877–15882. https://doi.org/10.1073/pnas.0507599102
Cymes GD, Grosman C (2008) Pore-opening mechanism of the nicotinic acetylcholine receptor evinced by proton transfer. Nat Struct Mol Biol 15:389–396. https://doi.org/10.1038/nsmb.1407
Cymes GD, Ni Y, Grosman C (2005) Probing ion-channel pores one proton at a time. Nature 438:975–980. https://doi.org/10.1038/nature04293
Sine SM, Engel AG (2006) Recent advances in Cys-loop receptor structure and function. Nature 440:448–455. https://doi.org/10.1038/nature04708
Bartos M, Corradi J, Bouzat C (2009) Structural basis of activation of cys-loop receptors: the extracellular-transmembrane interface as a coupling region. Mol Neurobiol 40:236–252. https://doi.org/10.1007/s12035-009-8084-x
Taly A, Corringer PJ, Guedin D et al (2009) Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov 8:733–750. https://doi.org/10.1038/nrd2927
Thompson AJ, Lester HA, Lummis SCR (2010) The structural basis of function in cys-loop receptors. Q Rev Biophys 43:449–499. https://doi.org/10.1017/S0033583510000168
Grosman C, Salamone FN, Sine SM, Auerbach A (2000) The extracellular linker of muscle acetylcholine receptor channels is a gating control element. J Gen Physiol 116:327–340. https://doi.org/10.1085/jgp.116.3.327
Kash TL, Kim T, Trudell JR, Harrison NL (2004) Evaluation of a proposed mechanism of ligand-gated ion channel activation in the GABAA and glycine receptors. Neurosci Lett 371:230–234. https://doi.org/10.1016/j.neulet.2004.09.002
Grutter T, de Carvalho LP, Dufresne V et al (2005) Molecular tuning of fast gating in pentameric ligand-gated ion channels. Proc Natl Acad Sci U S A 102:18207–18212. https://doi.org/10.1073/pnas.0509024102
Castillo M, Mulet J, Bernal JA et al (2006) Improved gating of a chimeric α7-5HT3A receptor upon mutations at the M2-M3 extracellular loop. FEBS Lett 580:256–260. https://doi.org/10.1016/j.febslet.2005.12.010
Jha A, Cadugan DJ, Purohit P, Auerbach A (2007) Acetylcholine receptor gating at extracellular transmembrane domain interface: the cys-Loop and M2-M3 linker. J Gen Physiol 130:547–558. https://doi.org/10.1085/jgp.200709856
Rovira JC, Ballesta JJ, Vicente-Agulló F et al (1998) A residue in the middle of the M2-M3 loop of the β4 subunit specifically affects gating of neuronal nicotinic receptors. FEBS Lett 433:89–92. https://doi.org/10.1016/S0014-5793(98)00889-8
Campos-caro A, Sala S, José Ballesta J et al (1996) A single residue in the M2-M3 loop is a major determinant of coupling between binding and gating in neuronal nicotinic receptors
Grønlien JH, Ween H, Thorin-Hagene K et al (2010) Importance of M2-M3 loop in governing properties of genistein at the α7 nicotinic acetylcholine receptor inferred from α7/5-HT3A chimera. Eur J Pharmacol 647:37–47. https://doi.org/10.1016/j.ejphar.2010.08.027
Monod J, Wyman J, Changeux JP (1965) On the nature of allosteric transitions: a plausible model. J Mol Biol 12:88–118. https://doi.org/10.1016/s0022-2836(65)80285-6
Mclaughlin JT, Fu J, Sproul AD et al (2006) Role of the outer  -Sheet in Divalent Cation Modulation of ␣ 7 nicotinic receptors. 70:16–22. https://doi.org/10.1124/mol.106.023259.with
Sala F, Mulet J, Sala S et al (2005) Charged amino acids of the N-terminal domain are involved in coupling binding and gating in α7 nicotinic receptors. J Biol Chem 280:6642–6647. https://doi.org/10.1074/jbc.M411048200
Purohit P, Auerbach A (2007) Acetylcholine receptor gating at extracellular transmembrane domain interface: the pre-M1 linker. J Gen Physiol 130:559–568. https://doi.org/10.1085/jgp.200709857
Bouzat C (2012) New insights into the structural bases of activation of cys-loop receptors. J Physiol Paris 106:23–33. https://doi.org/10.1016/j.jphysparis.2011.09.012
Acknowledgements
This work used computational resources from Centro de Computación de Alto Desempeño (CCAD), Universidad Nacional de Córdoba (UNC), particularly Mendieta and Serafin Clusters, part of SNCAD-MinCyT, Argentina. The authors want to thank Franco Santin (INGEBI-CONICET) for his helpful insight with molecular biology protocols and Dario Fernandez Do Porto for his help with the AlphaFold models.
Funding
This work was supported by Agencia Nacional de Promoción Científica y Técnica, Argentina (Grant PICT 2021 − 00818 to A.B.E.)
Author information
Authors and Affiliations
Contributions
PVP and ABE conceived and coordinated the research; SLG, LA, PB, VD and PVP performed and analyzed molecular biology and electrophysiology experiments; POC designed, performed and analyzed molecular dynamics experiments; GS generated AlphaFold models, JCB, IM and AC provided reagents; PVP, POC and ABE wrote the paper. All authors edited and approved the final version.
Corresponding authors
Ethics declarations
Ethical approval
The animal study was reviewed and approved by Instituto de Investigaciones en Ingeniería Genética y Biología Molecular ‘‘Dr. Héctor N. Torres’’ (INGEBI) Institutional Animal Care and Use Committee.
Consent to participate and consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gallino, S.L., Agüero, L., Boffi, J.C. et al. Key role of the TM2-TM3 loop in calcium potentiation of the α9α10 nicotinic acetylcholine receptor. Cell. Mol. Life Sci. 81, 337 (2024). https://doi.org/10.1007/s00018-024-05381-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-024-05381-2