Abstract
Lipopolysaccharide (LPS) induces a strong pro-inflammatory reaction of macrophages upon activation of Toll-like receptor 4 (TLR4) with the assistance of CD14 protein. Considering a key role of plasma membrane rafts in CD14 and TLR4 activity and the significant impact exerted on that activity by endocytosis and intracellular trafficking of the both LPS acceptors, it seemed likely that the pro-inflammatory reaction could be modulated by flotillins. Flotillin-1 and -2 are scaffolding proteins associated with the plasma membrane and also with endo-membranes, affecting both the plasma membrane dynamics and intracellular protein trafficking. To verify the above hypothesis, a set of shRNA was used to down-regulate flotillin-2 in Raw264 cells, which were found to also become deficient in flotillin-1. The flotillin deficiency inhibited strongly the TRIF-dependent endosomal signaling of LPS-activated TLR4, and to a lower extent also the MyD88-dependent one, without affecting the cellular level of TLR4. The flotillin depletion also inhibited the pro-inflammatory activity of TLR2/TLR1 and TLR2/TLR6 but not TLR3. In agreement with those effects, the depletion of flotillins down-regulated the CD14 mRNA level and the cellular content of CD14 protein, and also inhibited constitutive CD14 endocytosis thereby facilitating its shedding. Ultimately, the cell-surface level of CD14 was markedly diminished. Concomitantly, CD14 recycling was enhanced via EEA1-positive early endosomes and golgin-97-positive trans-Golgi network, likely to compensate for the depletion of the cell-surface CD14. We propose that the paucity of surface CD14 is the reason for the down-regulated signaling of TLR4 and the other TLRs depending on CD14 for ligand binding.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipopolysaccharide (LPS) is the main constituent of the outer membrane of Gram-negative bacteria. Upon infection, LPS triggers pro-inflammatory signaling pathways activating Toll-like receptor 4 (TLR4) present in the plasma membrane of numerous immune cells, including macrophages, and some non-immune cells. If released to the cytosol, LPS binds to caspases 4/5/11 inducing programmed pro-inflammatory cell death called pyroptosis [1, 2]. While the local acute inflammation helps to combat the infection, an excessive response to the infection leads to sepsis with a potentially fatal outcome. On the other hand, a prolonged low-grade inflammation contributes to the development of type 2 diabetes, cardiovascular disease, and other diseases [3, 4]. Such chronic inflammation is potentiated by the activity of cytoplasmic NLRP3 inflammasome which can be activated downstream of TLR4 signaling [5].
The TLR4 activation is a multi-step process which starts from the recognition of LPS by lipopolysaccharide-binding protein (LBP) and next by the CD14 protein [6]. CD14 exists in two forms—a soluble protein (sCD14) found in serum, and a plasma membrane-bound glycosylphosphatidylinositol (GPI)-anchored protein (mCD14, CD14) present mainly in myeloid cells, including monocytes, macrophages, and dendritic cells. Both forms of CD14 bind LPS and are engaged in TLR4 activation. Notably, the involvement of the membrane-bound CD14 at this stage of TLR4 activation can be dispensable at higher LPS concentrations due to the likely contribution of albumin and sCD14 in delivering LPS to the receptor [7, 8]. Upon LPS binding, CD14 dissociates from the LPS/LBP complex and transfers LPS monomers to the MD2 protein of the TLR4/MD2 heterodimer. Thus activated, TLR4 recruits two adaptor proteins, TIRAP and MyD88, triggering a signaling pathway leading ultimately to the activation of the NFκB, AP-1, and CREB transcription factors. A hallmark pro-inflammatory cytokine expressed in this pathway is TNFα [9,10,11]. Also, glycolysis is induced in this MyD88-dependent way to provide cells with metabolic intermediates required for gene expression and cytokine production [12, 13]. Subsequently, the complex of TLR4/MD2 with LPS is internalized and in endosomes TLR4 recruits a second pair of adaptor proteins, TRAM and TRIF. This triggers the second signaling pathway of TLR4 which leads to the activation of the IRF3/7 transcription factors and the production of type I interferons, chemokine CCL5/RANTES, and proteins encoded by interferon-regulated genes, like IP-10. Also, so-called late-phase activation of NFκB takes place [9, 14, 15].
It has been established that TLR4/LPS endocytosis is governed by the membrane-bound CD14 and several regulators of the endocytosis have been revealed, including enzymes catalyzing the turnover of phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] [16,17,18]. We found that CD14 itself determines the synthesis of PI(4,5)P2 in LPS-stimulated cells [19]. Recently, a role of CD14 in the Ca2+ influx mediated by the plasma membrane TRPM7 channel necessary for TLR4 endocytosis in macrophages has been demonstrated [20]. Notably, also in unstimulated macrophages CD14 undergoes slow constitutive endocytosis and is degraded in lysosomes, and its de novo synthesis is required to replenish the cell surface pool of CD14. Upon LPS binding, the constitutive CD14 endocytosis is accelerated concomitant with TLR4/MD2/LPS uptake [21]. Additionally, our recent studies have revealed that a fraction of the endocytosed CD14 can recycle back to the plasma membrane, both in resting and in LPS-stimulated cells, via a pathway depending on sorting nexins SNX1, 2, and 6. This newly discovered pathway contributes to the maintenance of the cell surface pool of CD14 determining the extent of the activation of TLR4 by LPS [22]. This is a good illustration of how the ultimate reaction of macrophages to LPS is modulated by diverse proteins controlling the cellular trafficking of CD14. The CD14 trafficking through the endo-lysosomal route is divergent from that of TLR4, which increases the likelihood of a dysregulation of the inflammatory reactions to LPS by a malfunctioning of this compartment that is intrinsic to several human hereditary diseases [23].
The contribution of CD14 to the TLR4 activation and endocytosis in macrophages is likely related to its localization in distinct nanodomains of the plasma membrane enriched in sphingolipids and cholesterol called rafts. The ample data supporting this assumption include bio-imaging assays, the sensitivity of LPS-induced TLR4 signaling to changes of cell cholesterol level, and studies of the protein content of the so-called detergent-resistant membrane (DRM) fraction roughly corresponding to rafts [24]. Also, a selected group of proteins that associate with rafts, like the Lyn tyrosine kinase, have been proposed to regulate TLR4 activity [25]. We have recently found that stimulation of Raw264 macrophage-like cells with LPS up-regulates the level of S-palmitoylated flotillin-1 [26]. Flotillin-1 (reggie-2) and flotillin-2 (reggie-1) are widely expressed proteins of about 50 kDa often considered as raft markers. This association can be mediated by hydrophobic interactions of flotillins with membrane lipids, including sphingosine and cholesterol, and additionally facilitated by their S-palmitoylation [27]. Reciprocally, flotillins can contribute to the raft assembly by acting in cooperation with the scaffolding protein MPP1 [28]. The membrane-binding ability is attributed to the N-terminal fragment of the SPFH domain, so named after proteins containing a homologous sequence of ca. 200 amino acids. The following extended α-helical part of flotillins mediates their oligomerization and is also involved in numerous intermolecular interactions [27]. Due to their membrane binding on the one hand and the direct binding of numerous proteins on the other, homo- and hetero-oligomers of flotillins can serve as scaffolds enabling the assembly of multiprotein complexes involved in signal transduction, clathrin-/caveolin-independent endocytosis of plasma membrane proteins, and cytoskeleton remodeling [29,30,31,32,33,34]. Importantly, a growing set of recent data indicates that flotillins can also associate with endo-membranes, including the Golgi apparatus and the endo-lysosomal compartment, to take part in protein trafficking and thereby affect signaling of diverse plasma membrane receptors [35,36,37,38,39].
Surprisingly, the data on the contribution of flotillins to the signaling of TLRs are scarce. Flotillins are used as markers of rafts in immunohistochemical or biochemical analyses, and their co-localization with TLR4 suggested an association of activated TLR4 with these plasma membrane domains detected in liver samples and in Kupffer cells [40]. Flotillin-1 was found to modulate TLR3 signaling in HUVEC endothelial cells by affecting caveolin-1 level [41]. A down-regulation of stomatin-like protein-2, a member of the SPFH family, in Raw264 cells was detrimental to the raft integrity, as indicated, i.e., by a depletion of the DRM fraction of flotillins, CD14, and MyD88. As a result, activation of various TLRs, including TLR4, was inhibited [42].
In this work, we analyzed the involvement of flotillins in TLR4 pro-inflammatory activity and found that they are crucial for maximal TRIF-dependent endosomal signaling of TLR4 and also signaling of other selected TLRs due to their pleiotropic influence on the level, trafficking, and localization of CD14 protein.
Materials and methods
Cell culture and stimulation
Raw264.7 (further Raw264) cells and J774 cells (both from ATCC) were cultured in DMEM containing 10% FBS (Thermo Fisher Scientific) with 4.5 and 1 g/l glucose, respectively. Thioglycolate-elicited macrophages were isolated from 8- to 14-week-old male C57BL/6 mice, as described previously [22]. The procedure had been reviewed and approved by the Local Animal Ethics Committee (permission No. 394/2017). For stimulation, Raw264 cells were overlaid with fresh DMEM/10% FBS supplemented with the following ligands: 10 or 100 ng/ml smooth LPS from Escherichia coli O111:B4 (List Biological Laboratories), 10 or 100 ng/ml N-palmitoyl-S-[2,3-bis(palmitoyloxy)-propyl]-(R)-cysteinyl-(lysyl)3-lysine (Pam3CSK4), 10 or 100 ng/ml S-[2,3-bis(palmitoyloxy)-propyl]-(R)-cysteinyl-(lysyl)3-lysine (Pam2CSK4), 10 μg/ml polyinosinic-polycytidylic acid (poly(I:C), (the latter three from InvivoGen). Cells were stimulated for up to 6 h (5% CO2, 37 °C).
Preparation of Raw264 cells with stably silenced Flot2
Cells were infected separately with five different lentiviral transduction particles (at MOI = 5) containing five different shRNA species (Merck) specific for the mouse Flot2 gene (NM_008028, Table 1). Cells infected in two independent attempts with non-mammalian shRNA transduction particles (Merck) served as controls. The cells were cultured in the presence of 2 µg/ml puromycin (Merck) until the mortality of non-infected cells reached 100% leaving cells transfected with shRNA alive. The efficiency of the Flot2 silencing was verified with RT-qPCR using primers specific to the mouse Flot2 gene, with Tbp as reference (primers listed in Table 2).
ELISA
TNFα and CCL5/RANTES were quantified in cell culture supernatants using mouse ELISA kits (BioLegend, R&D Systems) according to manufacturers' instructions. Samples were run in triplicates and normalized against protein content in the cells determined by crystal violet staining. Total amount of sCD14 was determined in cell supernatants using a mouse ELISA kit (R&D System) according to the manufacturer’s instructions. The absorbance was read in a Sunrise plate reader (Tecan). Alternatively, supernatants from triplicate cultures were combined and analyzed using the Mouse Cytokine Array Kit, Panel A (R&D System). In this assay, the profile of secreted inflammatory markers was quantified by incubation with a cytokine array membrane followed by immunoblotting performed according to the manufacturer's instructions. Immunoreactive dots were subjected to a densitometric analysis using the ImageJ program and normalized against internal standards present on the membranes.
CD14 endocytosis and shedding
A biotinylation-based assay was used to assess CD14 endocytosis essentially as described earlier [22]. Briefly, Raw264 cells (1.5 × 106 per 3.5-cm dish) were grown for 24 h, the culture medium was exchanged for a fresh one, and after 2 h the cells were washed and overlaid with ice-cold PBS containing 1 mM MgCl2 and 0.1 mM CaCl2, pH 8.2 (PBS+) supplemented with 0.5 mg/ml EZLink Sulfo-NHS-SS-Biotin (30 min on ice; Thermo Fisher Scientific). Subsequently, the excess of biotin was removed by washing the cells with PBS + containing 5% FBS and 25 mM lysine, and twice with ice-cold PBS+. At this stage of the procedure one of the samples of control and flotillin-depleted cells was lysed (30 min on ice) in 300 μl of a lysis buffer (25 mM HEPES, 0.5% SDS, 0.5% NP-40, 10% glycerol; pH 8.2) containing inhibitors (1 mM PMSF, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 1 mM Na3VO4, 50 μM PAO, 10 mM p-nitrophenyl phosphate) and 250 units of Benzonase (Merck). An aliquot of 20 μl was withdrawn from each lysate for 10% SDS-PAGE and immunoblotting analysis of the input CD14 (“input 0 min”), while the rest served to determine the level of surface-bound CD14, as described below. The other samples were incubated in a complete culture medium for 30, 60, or 90 min at 37 °C to induce endocytosis. To remove the biotin tag remaining on the cell surface, the cells were washed with PBS + and incubated twice (15 min each) with 50 mM MESNA (Merck) in 100 mM Tris, 100 mM NaCl, pH 8.6, followed by a wash with 5 mg/ml iodoacetamide in PBS+. Finally, the cells were washed with PBS + and lysed (30 min on ice) in 300 μl of the lysis buffer. An aliquot of 20 μl was withdrawn from each lysate to analyze the input CD14 as above (“input 30–90 min”). The remaining portion of these lysates, as well as of the lysates obtained after cell surface biotinylation, was diluted 5 times in the lysis buffer without detergents and incubated with streptavidin beads allowing isolation of internalized and cell surface proteins, respectively. For the incubation (overnight at 4 °C with agitation) 30 μl of the beads per sample was used (High Capacity Streptavidin Resin, Thermo Fisher Scientific). Next, the beads were washed three times with the lysis buffer containing 0.1% SDS and 0.1% NP-40, and bound proteins were eluted by incubation for 10 min at 95 °C in 70 μl 2 × SDS-sample buffer containing 4% β-mercaptoethanol, and the released proteins were subjected to 10% SDS-PAGE and immunoblotting. At 30–90 min of endocytosis, culture supernatants were collected, soluble biotinylated proteins were adsorbed on streptavidin beads and processed as above for immunoblotting detection of sCD14 shed from the cell surface.
CD14 recycling assay
Raw264 cells (0.7 × 105 per 15 × 15 mm coverslip) were grown for 24 h, next the culture medium was exchanged for a fresh one for 2 h. The assay for CD14 recycling was performed essentially as described earlier [22]. Briefly, cells were incubated with anti-mouse CD14 rat IgG2a (clone Sa14-2; BioLegend) for 30 min at 4 °C (all the antibodies used are listed in Table 3), and transferred to 37 °C for 30 min to induce CD14 uptake. Next, the cells were washed twice (2 min each) with ice-cold DMEM, pH 2.0, and twice with ice-cold PBS, pH 7.4, and incubated in the complete medium for 30 min at 37 °C to allow CD14/IgG recycling, and next for 30 min at 37 °C in the presence of chicken anti-rat IgG conjugated with Alexa Fluor 647 (Invitrogen) to stain CD14/IgG on the cell surface and allow its subsequent uptake. In control samples, anti-CD14 was replaced by the corresponding rat isotype IgG2a (BioLegend) or the antibody was omitted. The cells were then washed twice (2 min each) with ice-cold DMEM, pH 2.0, and twice with ice-cold PBS, pH 7.4, fixed with 4% formaldehyde (20 min, room temp.), permeabilized with 0.1% saponin in PBS and incubated with 4 μg/ml Hoechst 33342 (Merck) for 10 min at room temp. in PBS. To assess the initial surface level of CD14, the cells were incubated with anti-mouse CD14 rat IgG2a and after washing with PBS, with anti-rat IgG-Alexa Fluor 647 (30 min, 4 °C each). The cells were then fixed, incubated with 4 μg/ml Hoechst 33342 as above, and subjected to confocal microscopy.
The cells were examined under an inverted Axio Observer Z.1 microscope (Zeiss) with a CSU-X1 spinning-disc unit (Yokogawa) equipped with a 63 × oil immersion objective (NA 1.4) and an Evolve 512 EMCCD camera (Photometrics). Fluorescence was excited using 405- and 647-nm diode lasers and detected in the spectral range of 419–465 nm and 668–726 nm. Laser power and camera gain were adjusted to cover a 16-bit dynamic range. Optical sections were acquired using image format of 512 × 512 pixels and a voxel size of 0.212 μm in the x–y and 0.3 μm in the z-dimension. To determine the number of vesicles containing CD14 per cell, the obtained z-stacks were analyzed using Cell-Profiler 4.2. software (Broad Institute) [43]. To this end, images were converted to maximum z-projections and exported to a tiff format in ZEN 3.3 (Zeiss) software and processed in CellProfiler as follows. A median filter was applied, then nuclei were segmented basing on Hoechst 33342 fluorescence using the Otsu algorithm, and CD14-containing vesicles basing on Alexa Fluor 647 fluorescence using a manually adjusted threshold. The area covered by a particular cell was determined by expanding the nucleus mask by 40 pixels in each direction. The number of CD14-containing vesicles in a particular cell was thereby determined. To determine the amount of CD14 on the cell surface, z-stacks were converted to sum z-projections, and the average raw integral density per cell was calculated using ImageJ program [44] and a manually adjusted threshold.
CD14 localization with immunofluorescence microscopy
Raw264 cells were seeded onto coverslips (3 × 104 per 15 × 15 mm coverslip), grown for 24 h, washed in PBS and fixed in 3.7% formaldehyde in APHEM (60 mM Pipes, 25 mM HEPES, 10 mM EGTA, 4 mM MgCl2, pH 6.9). Subsequently, the cells were incubated with 15 mM NH4Cl in APHEM (10 min, room temp.), permeabilized with 0.005% digitonin in APHEM (10 min, room temp.) and blocked with 6% BSA in Tris-buffered saline (TBS; 30 min, room temp.). Some samples were additionally incubated with 2.5 µg/ml of a fusion protein containing the pleckstrin homology (PH) domain of human FAPP1 and the GST tag (FAPP1-PH-GST) [45] in 0.2% BSA/TBS for 60 min at room temp. and washed (five times × 5 min in 0.2% BSA/TBS). The cells were then incubated overnight with rat anti-CD14 IgG (clone Sa14-2; BioLegend) and goat anti-GST IgG (Rockland) or rabbit anti-Rab11 IgG or rabbit anti-golgin-97 IgG or rabbit anti-EEA1 IgG (all rabbit IgG from Cell Signaling Technology) in 0.2% BSA/TBS and, after washing as above, were exposed to appropriate secondary IgG: chicken anti-rat IgG-Alexa Fluor 647 (Invitrogen), donkey anti-goat IgG-FITC (Rockland) or donkey anti-rabbit IgG-FITC (Jackson ImmunoResearch) (Table 3) and 2 µg/ml Hoechst 33342 in 0.2% BSA/TBS for 60 min at room temp. After washing, samples were mounted in mowiol/DABCO and examined under an LSM800 inverted confocal microscope (Zeiss) using a 63 × oil objective (NA 1.4) with scan frame 553 × 553, speed 9, zoom 2.6, line averaging 2, pixel size 70 nm, and z interval 230 nm. Triple-stained images were obtained by sequential scanning for each channel to eliminate the crosstalk of the chromophores and to ensure a reliable quantification of colocalization. FITC fluorescence was excited at 488 nm, Alexa Fluor 647 at 640 nm, and Hoechst at 405 nm, and detected at 485–540, 637–700, and 400–487 nm, respectively. 16-bit z-stacks were used to build a 3D structure in Imaris 9.1.2. (Oxford Instruments) software. Regions of interest (ROI) related to peri-nuclear structures defined by staining with FAPP1-PH, golgin-97 or Rab11, and to EEA1-positive vesicles were selected using the surface detection algorithm of Imaris with a manually adjusted threshold. The sum of voxel intensities of CD14 staining and of the marker proteins [46] and also Pearson’s correlation coefficient (PCC) for quantifying the protein colocalization were calculated in the ROI. For a complete colocalization, PCC has the maximal value of 1. As found earlier [46], the values of the sum of voxel intensities did not follow a Gaussian distribution, therefore the median was used as a measure of average intensities and the nonparametric Mann–Whitney test to evaluate statistical significance. To measure the total intensity of CD14 staining in the cell, the z-stacks were converted to sum z-projections and raw integral intensity per cell was calculated using the ImageJ Analyze Particle feature and a manually adjusted threshold.
Flow cytometry
Raw264 cells were seeded at 1 × 106/well in 12-well culture plates, grown overnight in DMEM/10% FBS, left unstimulated or treated with 100 ng/ml LPS for up to 60 min, suspended in ice-cold PBS and incubated in 2% mouse serum (Jackson ImmunoReserach) containing 0.01% NaN3 (30 min, 4 °C). Cell-surface CD14 was labeled using rat anti-mouse CD14 IgG2a conjugated with FITC (clone Sa2-8; eBioscience) while TLR4 with rat anti-mouse TLR4 IgG2a conjugated with phycoerythrin (clone Sa15-21; BioLegend) (Table 3). The labeling was conducted for 30 min at 4 °C (in the darkness); after that the cells were washed twice with ice-cold PBS and fixed with 3% formaldehyde. In control samples, the anti-CD14 and anti-TLR4 IgG2a were omitted. After washing with PBS, cells were resuspended in PBS and their fluorescence was determined with a Becton Dickinson FACS Calibur flow cytometer. FITC and phycoerythrin fluorescence were detected using a 530/30 nm and a 585/42 filter with an FL-1 and FL-2 detector, respectively. Cell debris was gated out by establishing a region around the population of interest on a Forward Scatter (FSC) versus Side Scatter (SSC) dot plot. Data were analyzed using BD CellQuest Pro software (BD Biosciences) and the amounts of cell-surface CD14 and TLR4 were calculated based on the geometric mean of fluorescence intensity.
RNA isolation and RT-qPCR
RNA was isolated from cells using a Universal RNA purification kit (EURx) and reverse-transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) according to the manufacturers’ instructions. qPCR was performed in a StepOnePlus instrument using Fast SYBR Green Master Mix (Thermo Fisher Scientific). The primers used are shown in Table 2. The PCR conditions were: initial denaturation for 20 s at 95 °C followed by 40 cycles comprised of denaturation for 3 s at 95 °C and annealing/extension for 30 s at 60 °C. mRNA expression levels for investigated genes (relative to the mRNA level for the Tbp or Hprt gene, each variant run in triplicate) were calculated by the ∆∆CT method.
Cell fractionation
Cells (1.5 × 106 per sample) were grown overnight and subjected to fractionation essentially as described earlier [25]. Briefly, cells were lysed in 500 μl of 0.1% Triton X-100, centrifuged (10 000 × g, 5 min, 4 °C), supernatants collected as Triton X-100-soluble fraction while pellets were solubilized in 500 μl of 1.8% octyl β-D-glucoside and centrifuged as above. Supernatants were collected as the DRM fraction, while the remaining pellets were solubilized in 500 μl of 4% SDS. Equal volumes of the fractions were subjected to 10% SDS-PAGE.
Immunoblotting
To obtain whole cell lysates, cells (1.2 × 105/well in 48-well plate) were rinsed with ice-cold PBS and lysed in 2 × SDS-sample buffer. Protein concentration in the lysates was determined with BradfordUltra (Expedeon). After transfer onto nitrocellulose, the membrane was blocked and incubated with antibodies indicated in Table 3. Immunoreactive bands were visualized with chemiluminescence using SuperSignal WestPico substrate (Thermo Fisher Scientific) and analyzed densitometrically using the ImageJ program. Samples from cell fractioning were blotted with appropriate primary antibodies followed by secondary antibodies conjugated with near-infrared fluorescent dyes IRDye 800CW or 680RD shown in Table 3 (LI-COR), and analyzed using an Odyssey M scanner (LI-COR) and Empiria Studio 2.2 software.
Data analysis
The significance of differences was calculated using Student’s t-test or 1- or 2-way ANOVA and Scheffe's or Tukey’s post hoc test; for non-normal variables Mann–Whitney test or Kruskal–Wallis test and Dunn’s post hoc were used. The data analysis was performed using the JASP software environment. The statistical tests used in individual experiments are specified in figure legends. The p-values < 0.05 were taken as statistically significant and are shown in figures. Some data are presented as boxplots, where the box encompasses the first and third quartiles with a line through the median. The top and bottom whiskers denote the maximum and minimum data points, respectively, excluding data 1.5 times the interquartile range (IQR) which are represented by dots.
Results
Silencing of Flot2 with shRNA leads to depletion of flotillin-2 and flotillin-1 in Raw264 cells
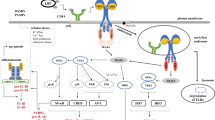
To assess the role of flotillins in LPS-induced signaling we aimed at obtaining macrophage cells with a stably silenced expression of respective gene(s). An RT-qPCR analysis indicated that the amount of flotillin-2 mRNA in Raw264 macrophage-like cells exceeded that of flotillin-1 about 4.2-fold. Also in J774 macrophage-like cells and in macrophages isolated from mouse peritoneum flotillin-2 mRNA prevailed over that of flotillin-1, 1.8- and 3.5-fold, respectively (Fig. 1a, b). Raw264 and J774 cells were relatively rich in CD14 mRNA in comparison to primary macrophages, while the TLR4 mRNA level was the highest in the latter cells (Fig. 1c, d). Since the expression of Flot2 dominated over Flot1 in all the cells tested, we silenced the expression of Flot2 in Raw264 cells and used them for further studies.
mRNA levels of flotillin-1, flotillin-2, CD14, and TLR4 in Raw264 and J774 cells, and mouse peritoneal macrophages (MΦ). Flot1 (A), Flot2 (B), and Tlr4 (D) transcripts were quantified by RT-qPCR relative to Tbp while that of Cd14 (C) relative to Hprt due to the comparable abundance of those transcripts. Data shown are mean ± SD from three experiments
For this purpose, Raw264 cells were transfected separately with five different lentiviral particles bearing five different shRNA sequences and twice with control shRNA bearing a non-mammalian shRNA. An RT-qPCR analysis revealed that four of the shRNAs used effectively silenced Flot2, expression with the shRNA variant No. 2 being the exception (Supplementary Fig. S1A). On average, the four effective shRNA species gave a reduction of the relative Flot2 mRNA level by 76% compared to the control cells transfected with the non-mammalian shRNAs, with most profound reduction, reaching 81%, observed for shRNA No. 5 (Fig. 2a, Supplementary Fig. S1A). Immunoblotting revealed that small amounts of flotillin-2 protein (on average about 12%, with only 4% in variant No. 5) were still present in the four Raw264 transformants (Fig. 2b, c, Supplementary Fig. S1B). Further experiments were performed using transformants No. 1, 3 and 5, all obtained with shRNA species targeting the 3′-untranslated region of flotillin-2 mRNA (Table 1). We found that these transformed cells were also depleted of flotillin-1 protein, and the extent of that depletion correlated with the reduction of flotillin-2 protein (Fig. 2b–d), in agreement with earlier studies [47, 48]. Also, the flotillin-1 mRNA level was reduced significantly, by about 7, 15 and 34% in the transformant lines 1, 3, and 5, respectively (Fig. 2e). Because we aimed to examine the effect of flotillin depletion on the response of the cells to LPS, we additionally determined the levels of CD14 and TLR4 mRNAs in those cells; they were decreased as well, on average by about 21% and 15%, respectively (Fig. 2f, g).
The effect of Flot2 knock-down on the expression of selected genes and flotillin-1 and -2 levels in Raw264 cells. Cells were transfected with Flot2-specific shRNA variants No. 1, 3, 5 (Flot2 shRNA) or control shRNA (NC1, NC2 shRNA). RT-qPCR analysis of Flot2 (A), Flot1 (E), Cd14 (F) and Tlr4 (G) transcripts quantified as in Fig. 1. B–D The abundance of flotillin-1 and -2 determined by immunoblotting (B) followed by densitometric analysis (C, D). The flotillins were quantified in indicated cells relative to actin. Positions of molecular weight markers are shown on the right in kDa. Jak1 and actin were visualized to verify equal loading of protein between wells. Data shown are mean ± SD from at least three experiments. Significantly different values as indicated by 1-way ANOVA with Scheffe's post hoc test are marked. In (A, C, D) the p values for individual Flot2 shRNA variants were < 0.001 relative to both NC variants
Depletion of flotillins reduces the CD14 level and inhibits LPS-induced signaling in Raw264 cells
To determine whether the flotillin depletion affected LPS-induced signaling, cells were stimulated with 10 or 100 ng/ml LPS for 1 h and the level of selected proteins of interest was examined by immunoblotting. The depletion of flotillins seen in unstimulated cells did not change upon their stimulation (Fig. 3a–c). The level of CD14 protein was reduced substantially, by about 35%, in resting cells. Upon stimulation with LPS, the amount of CD14 protein rose by about 14 or 25% (10 or 100 ng/ml LPS) in control cells and moderately also in the transformants. Ultimately, however, the level of CD14 in LPS-stimulated cells remained lower in the flotillin-depleted cells than in controls, by 28% and 42%, respectively (Fig. 3a, d).
The effect of Flot2 knock-down on the level of proteins involved in LPS-induced signaling. Cells were transfected with Flot2-specific shRNA variants No. 1 or 5 (shRNA Flot2) or control shRNA (NC1, NC2 shRNA). Cells were left unstimulated (NS) or were stimulated with 10 ng/ml or 100 ng/ml LPS (1 h, 37 °C). A Immunoblotting analysis of the abundance of indicated proteins in the cells. Positions of molecular weight markers are shown on the right in kDa. Jak1 and actin were visualized to verify equal loading of protein between wells. B–G Densitometric analysis of flotillin-1 (B), flotillin-2 (C), CD14 (D), TLR4 (E), phosphorylated IκB (pIκB) (F), phosphorylated IRF3 (pIRF3) (G) content relative to Jak1. Data shown are mean ± SD from at least three experiments. Significantly different values as indicated by 1-way ANOVA with Scheffe's post hoc test are marked
In striking contrast to CD14, the level of TLR4 in flotillin-depleted resting cells did not differ significantly from the control level, and a 1-h stimulation with LPS did not affect it in any of those cells either (Fig. 3a, e). Despite that, the deficiency of flotillins reduced the TLR4-dependent activation of two major transcription factors, NFκB and IRF3, triggered by LPS via, respectively, both the MyD88- and TRIF-dependent routes and the TRIF-dependent one exclusively [9, 14]. Notably, the activation of NFκB, indicated by phosphorylation of its regulatory subunit IκB, was reduced significantly only in flotillin-depleted cells stimulated with 10 ng/ml LPS, on average by 64% versus control cells, while at 100 ng/ml LPS the difference between flotillin-depleted and control cells (14% reduction) was non-significant. The IκB phosphorylation in controls was only slightly higher at 100 than at 10 ng/ml LPS (Fig. 3a, f). In contrast, the intensity of the TRIF-dependent phosphorylation of IRF3 in LPS-stimulated control cells correlated significantly with the LPS concentration used, as did the extant of its reduction in the flotillin-depleted cells, which amounted to 28% at 10 ng/ml and as much as 43% at 100 ng/ml LPS (Fig. 3a, g).
Taken together, the data show a negative influence of flotillin depletion on the abundance of CD14 but not of TLR4 in cells, and a resulting clear-cut reduction of the TRIF-dependent signaling of TLR4.
Depletion of flotillins limits LPS-induced production of cytokines
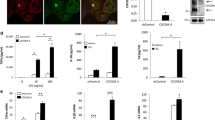
To determine the ultimate impact of the flotillin depletion on the pro-inflammatory responses of Raw264 cells, we analyzed the mRNA levels of TNFα, produced mainly in the MyD88-dependent manner, and of interferon β, CCL5/RANTES, and CCL2/MCP-1-cytokines produced strictly in the TRIF-dependent manner [11, 49]. We found that the abundance of TNFα mRNA was significantly reduced, by 59%, in flotillin-depleted cells stimulated with 10 ng/ml LPS only. On the other hand, the mRNA level of the three TRIF-dependent cytokines was reduced significantly in cells stimulated with both 10 and 100 ng/ml LPS, with the reduction of interferon β and CCL5/RANTES mRNAs reaching on average as much as 80% (Fig. 4a–d). The flotillin deficiency also reduced the mRNA level of interleukin 1β, and this reduction was more pronounced at 10 than at 100 ng/ml LPS (Fig. 4e). Expression of the Il1b gene is controlled by NFκB. The IL-1β precursor is produced, which after activation of the NLRP3 inflammasome matures into an active cytokine [50].
Inhibition of LPS-induced cytokine production in flotillin-depleted cells. Cells were transfected with Flot2-specific shRNA or control (NC) shRNA. Cells were left unstimulated or were stimulated with 10 ng/ml or 100 ng/ml LPS (4 h at 37° C in A–E, H, I or 6 h at 37 °C in F, G or 1 h at 37 °C in J, K. A–E Transcripts of Tnf (A), Ifnb (B), Ccl5 encoding RANTES (C), Ccl2 encoding MCP-1 (D) and Il1b (E) were quantified by RT-qPCR relative to Hprt transcript. F, G The concentration of TNFα (F) and CCL5/RANTES (G) was determined in culture supernatants with ELISA. No cytokine production was found without cell stimulation. H, I Cytokine array detection (H) in cells stimulated with 10 ng/ml LPS followed by densitometric analysis of immunoblots (I). Dots left unmarked in the upper blot in H served as internal standards used to normalize signals between membranes. J, K The surface level of TLR4 determined by flow cytometry in cells unstimulated and stimulated with 100 ng/ml LPS (J) and expressed as percentage of the value in unstimulated control cells (K). In A–E and H–K cells were transfected with Flot2-specific shRNA variant No. 5 and control shRNA NC1 while in F, G the cell transformants used are indicated. Data shown are mean ± SD from four (A–E, K) or three (F, G, I) experiments. Significantly different values as indicated by 1-way ANOVA (A–G) with Tukey’s post hoc test or Student's t-test (I) or 2-way ANOVA (K) are marked
The observed strong dependence of cytokine expression in the TRIF-dependent pathway on the abundance of flotillins was further confirmed by an analysis of the amount of secreted TNFα and RANTES. In control cells, the production of TNFα was about twofold higher at 10 than at 100 ng/ml LPS (Fig. 4f), and flotillin depletion affected it only in cells stimulated with 10 ng/ml LPS; it was lower in transformants No. 1, 3, and 5 by 31–46% of the control level. In contrast, the CCL5/RANTES production was 1.5-fold higher in control cells stimulated with 100 ng/ml LPS than at 10 ng/ml LPS. In the flotillin-depleted cells it was lower by 36–58% upon stimulation with 10 ng/ml LPS and by at least 21% (37% in transformants No. 5) at 100 ng/ml LPS (Fig. 4g). Thus, the present results obtained using ELISA were consistent with the results of the immunoblotting analysis of IκB and IRF3 phosphorylation shown in Fig. 3 and mirrored the differences in the respective mRNA levels seen in Fig. 4a, c.
To get a more complete picture of how the LPS-induced cytokine production is affected by flotillin deficiency, we analyzed the production of an array of inflammatory markers by cells stimulated with 10 ng/ml LPS (Fig. 4h, i). Altogether, 13 inflammatory markers were secreted by Raw264 cells in these conditions. Among them, the secretion of IP-10, IL-1ra, CCL5/RANTES, GM-CSF, IL-6, and CD54/ICAM-1 was significantly lower for the flotillin-depleted cells (Fig. 4h, i). Notably, the production of IP-10, IL-1ra, and CCL5/RANTES depends strictly on the involvement of TRIF and IRF3 activation, and that of IL-6 and CD54/ICAM-1 has also been reported to depend strongly on this TLR4-triggered signaling pathway [14, 15, 51,52,53,54]. The production of those inflammatory markers was reduced by 40–66% and by as much as 82% in the case of IL-6 (Fig. 4h, i). The latter can result from a synergy of a lower Il6 expression and an enhanced instability of IL-6 mRNA, both being TRIF-dependent in macrophages [55]. Most of the factors affected by flotillin deficiency have a pro-inflammatory activity except for IL-1ra, an anti-inflammatory cytokine that can negatively regulate the pathogenesis of inflammation [54]. Notably, the secretion of CCL2/MCP-1 was not diminished in the flotillin-depleted cells, despite the significant reduction of the CCL2/MCP-1 mRNA level (Fig. 4h, i, compare with Fig. 4d). It has been found that the MyD88-dependent pathway has a positive effect on CCL2/MCP-1 mRNA stability [56], which may mitigate the negative impact of the inhibition of the TRIF-dependent signaling on the ultimate CCL2/MCP-1 protein level. Notably, the cytokine array assay revealed no effect of flotillin deficiency on the level of cytokines produced in the MyD88-dependent pathway of TLR4, including the MIP-family chemokines and TNFα (Fig. 4h, i), the latter in contrast to the ELISA results presented above (see Fig. 4f). This discrepancy could result from differences in the sensitivity and the mode of cytokine detection between these two assays. The cytokine array seems less suitable for assessing the level of relatively abundant cytokines, such as the MyD88-dependent ones, due to the high, saturated optical density of respective spots.
The above data indicate that the knock-down of flotillins affected mainly the TRIF-dependent signaling of TLR4; an effect on the MyD88-dependent pathway also seems possible, especially in cells stimulated with a low LPS concentration (10 ng/ml). Thus, the involvement of flotillins in LPS-induced signaling is manifested in conditions that require the engagement of CD14 for TLR4 activation and endocytosis [16, 18, 57]. To address the latter issue, we examined the surface level of TLR4 at the onset and following an LPS-induced stimulation. The TLR4 level was not significantly affected by flotillin depletion in resting cells. Stimulation with 100 ng/ml LPS led to a gradual disappearance of TLR4 from the surface of control cells to about 60% of the initial level after 60 min. Notably, in the flotillin-depleted cells, the amount of cell-surface TLR4 did not change upon the LPS stimulation indicating that its endocytosis was abrogated (Fig. 4j, k). To sum up, the obtained data indicate that flotillins are required for a maximal LPS-induced production of cytokines and act by affecting CD14-dependent TLR4 activation.
Flotillin depletion affects transcript levels of cytokines induced in a CD14-dependent manner by TLRs other than TLR4
We asked whether flotillin depletion affects only the TLR4-dependent cytokine generation or has an effect also on the signaling of other plasma membrane receptor complexes whose activation involves CD14, such as TLR2/TLR1 and TLR2/TLR6 [57]. Upon TLR2/TLR1 stimulation with 10 ng/ml Pam3CSK4, the level of TNFα mRNA was lower by 42% in flotillin-depleted cells compared to controls but showed no difference between the two types of cells at 100 ng/ml Pam3CSK4. The induction of IL-6 mRNA was nearly abolished by the flotillin depletion at the both Pam3CSK4 concentrations (Fig. 5a, b). In cells stimulated with Pam2CSK4, a TLR2/TLR6 ligand, the level of TNFα mRNA was not affected by flotillin depletion whereas the inducation of IL-6 mRNA was again diminished by about 65% at both 10 and 100 ng/ml Pam2CSK4 (Fig. 5c, d). These results are in agreement with our earlier data showing that TLR2/TLR1-induced TNFα secretion in Raw264 cells was inhibited by a CD14-neutralizing antibody to a higher extent than the TLR2/TLR6-induced one [57]. In contrast to that induced by TLR2 complexes, the expression of TNFα- and RANTES-encoding genes upon stimulation of endosomal TLR3 with poly(I:C) was not affected by flotillin depletion (Fig. 5e, f), likely reflecting the lack of a CD14 involvement in TLR3 activation [57].
Inhibition of cytokine expression induced by TLR2/TLR1 and TLR2/TLR6 in flotillin-depleted cells. Cells were transfected with Flot2-specific shRNA variant No. 5 or control (NC) shRNA variant No. 1. Cells were left unstimulated or were stimulated with 10 ng/ml or 100 ng/ml Pam3CSK4 (A, B), Pam2CSK4 (C, D) or 10 μg/ml poly(I:C) (E, F) for 4 h at 37 °C. Transcripts of Tnf (A, C, E), Il6 (B, D) and Ccl5 encoding RANTES (F) were quantified by RT-qPCR relative to Hprt transcript. In all unstimulated cell types tested, transcript levels of cytokines were negligible. Data shown are mean ± SD from three or four experiments. Significantly different values as indicated by 1-way ANOVA with Tukey’s post hoc test are marked
Taking into account all the above results indicating an association of flotillins with CD14 in TLR activation, we asked whether flotillins affect the cell surface level of CD14. It has been established recently that the latter determines the extent of the TLR4- and inflammasome-induced signaling [22, 58].
Flotillin depletion reduces the surface level of CD14
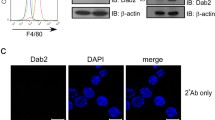
Using flow cytometry to determine the abundance of cell-surface CD14 we found that flotillin depletion in Raw264 cells decreased the surface pool of CD14 by about 26% in comparison with control cells (Fig. 6a, b). By fractionating the cells into Triton X-100-soluble and -insoluble (DRM) fractions, we found that flotillin depletion strongly reduced the amount of the mature form of CD14 (CD14 bands with slower gel migration due to glycosylation [22, 59, 60]) present in the DRM fraction despite a relatively modest content of flotillin-2 itself in this fraction in control cells (Fig. 6c). In contrast, another raft-associated protein, Lyn kinase, was present in comparable amounts in the DRM fraction in control and flotillin-depleted cells (Fig. 6c), indicating the specificity of the effect of flotillin depletion on raft-associated CD14. As expected, transferrin receptor (TfR) was found exclusively in the Triton X-100-soluble fraction regardless of the flotillin status of the cells (Fig. 6c). This fraction also contained the immature forms of CD14, and substantial amounts of flotillin-2 and actin, while small amounts of those proteins were found in the SDS-soluble (cytoskeletal) fraction (Fig. 6c).
The effect of Flot2 knock-down on cell-surface CD14 abundance, endocytosis and shedding. Cells were transfected with Flot2-specific shRNA variant No. 5 (Flot2 shRNA) or control shRNA NC1 (NC shRNA). A, B The cell-surface level of CD14 determined by flow cytometry (A) and expressed as percentage of the value in control cells (B). C Fractionation of Triton X-100 lysates of the cells. TX-100 sol.—fraction soluble in 0.1% Triton X-100, TX-100 insol.—fraction insoluble in Triton X-100, SDS—fraction soluble in 4% SDS. Numbers below blots show the relative content of the respective protein in each fraction determined by densitometry (OD). Results of one representative experiment of two run in duplicate are shown. D Scheme of the biotinylation-based assay of CD14 endocytosis and shedding. E Constitutive endocytosis of CD14 and TfR determined by immunoblotting (left panel) followed by densitometric analysis relative to actin content in the input samples (right panel). Three different amounts of the initial cell surface fraction of proteins were subjected to SDS-PAGE and after averaging, they were used as a reference point to calculate the percentage of CD14 and TfR internalized after 30–90 min and also the amount of sCD14 shed from the cell surface (F). In E, to balance the signal from CD14 between control and flotillin-depleted cells, different portions of cellular fractions (of the original 70 μl) were subjected to SDS-PAGE, as indicated above blots. Positions of molecular weight markers are shown on the right in kDa. The total amount of CD14 and TfR relative to actin in input cell lysates is shown in Supplementary Fig. S2. F, G The content of sCD14 in culture supernatants determined by the biotinylation assay (F) and by ELISA prior to and after stimulation of cells with 10 ng/ml LPS for 1 or 4 h at 37 °C (G). Data shown in B, E, F, G are mean ± SD from three or four experiments. Significantly different values as indicated by Student's t-test (B, E, F) and 1-way ANOVA with Tukey’s post hoc test (G) are marked
Since the reduction of the cell surface (mature) CD14 could be caused by its enhanced constitutive endocytosis, we determined CD14 uptake using an assay relying on the biotinylation of cell surface proteins. For this, we labeled the cell surface proteins of Raw264 cells with a membrane-impermeable biotin derivative at 4 °C preventing endocytosis and compared the amount of the labeled CD14 on the cell surface at the onset of the experiment with that found in the cell interior 30–90 min later, after warming the cells to 37 °C; the stronger the constitutive CD14 endocytosis, the larger its fraction would be expected in the latter pool (Fig. 6d). This assay confirmed a reduction of the amount of CD14 present on the surface of flotillin-depleted cells (Fig. 6e) and also a strong reduction of the total amount of the protein in input samples (Supplementary Fig. S2A, B). According to this assay, the cell surface level of CD14 was diminished in flotillin-depleted cells by as much as 81%, which exceeded the extent of the CD14 depletion detected on the surface of intact cells by flow cytometry (Fig. 6e, compare with Fig. 6a, b). We believe that this quantitative difference reflects the differences between the two assays run on intact versus lysed cells. In contrast to CD14, TfR was significantly up-regulated by nearly 50% on the surface of flotillin-depleted cells and an enrichment of the total cellular pool of TfR was also detected (Fig. 6e, Supplementary Fig. S2A, B). After 30 min of cell warming, about 12% of the labeled CD14 was internalized in control cells and this pool increased slightly after 60 min, while a subsequent 30 min led to a reduction of the amount of internal CD14 likely reflecting its degradation (Fig. 6e). In cells depleted of flotillins, the amount of internalized CD14 was lower, reaching 5–6% of its initial surface pool, with the most pronounced reduction relative to the control cells (about 60%) seen at 30 min of endocytosis (Fig. 6e). We also detected a tendency for an inhibition of TfR endocytosis in these cells (Fig. 6e).
As the reduced endocytosis was concomitant with the reduced cell-surface level of CD14, we analyzed CD14 shedding by following the amount of sCD14 in culture supernatants during 30–90 min of constitutive endocytosis in relation to CD14 biotinylated on the cell surface at the onset of the experiment (Fig. 6d). The shedding of CD14 was enhanced 1.5-fold in flotillin-depleted cells versus controls after 60 min and as much as twofold after 90 min (Fig. 6f). Since sCD14 can be generated by shedding from the cell surface and by the exocytosis of sCD14 produced intracellularly [61,62,63], we used an ELISA assay to determine the total amount of sCD14 in the culture supernatants of control and flotillin-depleted cells. The flotillin depletion did not reduce the amount of total sCD14 released by resting cells while in LPS-stimulated cells it was slightly higher than in control cells (Fig. 6g). Given the reduction of Cd14 expression and of the total cellular CD14 abundance, the release of sCD14 could in fact have been more efficient in the flotillin-depleted cells to reach and even surpass the control level, but it was the shedding of sCD14 that was unequivocally up-regulated. Taken together, these results indicate that the reduced cell-surface level of CD14 in flotillin-depleted cells is a net result of two changes: a reduced constitutive endocytosis and an enhanced shedding.
Flotillin depletion enhances CD14 recycling
The data presented above prompted us to analyze the influence of flotillin depletion on the recycling of CD14. For this purpose, we applied a microscopic assay developed by us recently [22] which is based on the labeling of cell-surface CD14 with specific antibodies. Following successive incubations of the cells with the anti-CD14 antibody and a secondary fluorescent antibody, separated by cell warming and removal of the CD14-bound antibodies remaining on the cell surface, only the recycling pool of CD14 is eventually visible in vesicles inside the cells (Fig. 7a). In the flotillin-depleted cells the number of vesicles containing recycling CD14 was significantly higher (about twofold) than in control cells (Fig. 7b and Supplementary Fig. S3A, compare lanes 3 and 1).
Enhancement of CD14 recycling in flotillin-depleted cells. Cells were transfected with Flot2-specific shRNA variant No. 5 (Flot2 shRNA) or control shRNA NC1 (NC shRNA). A Scheme of the fluorescent antibody-based assay of CD14 recycling with a micrograph showing CD14-containing vesicles (red) and nuclei (blue). B Quantitation of CD14 recycling. The cells were examined under a fluorescence microscope and vesicles containing fluorescently labeled CD14 representing the recycling CD14 were counted in n number of cells. When indicated, the cells were pretreated with 20 μg/mL CHX prior to the assay and CHX was also present during the assay. In control samples, rat isotype IgG2a was used instead of the CD14-specific antibody or the antibody was omitted. Box plots represent the median (light green lines) and 25th/75th quartiles of the number of vesicles containing fluorescently labeled CD14 per cell in one experiment of two. Significantly different values as indicated by Kruskal–Wallis test followed by Dunn's multiple comparisons post hoc test are marked
Parenthetically, this assay further confirmed the reduction of the cell surface level of CD14 in flotillin-depleted cells (Supplementary Fig. S3B). To establish to what extent the recycling of CD14 in both cell types was affected by newly synthesized CD14, the cells were pretreated with 20 μg/ml cycloheximide (CHX) for 30 min prior to the assay. CHX decreased the rate of CD14 recycling in control cells (the median number of CD14-containing vesicles decreased by 1/3; Fig. 7b and Supplementary Fig. 3A, compare lanes 1 and 2) and unexpectedly abolished it fully in flotillin-depleted cells (Fig. 7b and Supplementary Fig. 3A, compare lanes 3 and 4). This indicated that in the flotillin-depleted cells only the newly synthesized CD14 protein underwent a single round of endocytosis and recycling following its initial transport to the plasma membrane. Moreover, taking into account the low surface level of CD14 in these cells and a decreased rate of its endocytosis, the CD14 recycling per se seems to be strongly enhanced in the absence of flotillins.
Flotillin depletion leads to accumulation of CD14 in EEA1- and golgin-97-positive compartments
We broadened the investigation of the CD14 recycling in flotillin-depleted cells by analyzing its distribution in cellular compartments involved in protein sorting/recycling, i.e., early endosomes marked by EEA1, Rab11-positive endocytic recycling compartments (ERC), and the trans-Golgi network (TGN) identified by the presence of golgin-97. For comparison, we also determined the distribution of CD14 in the Golgi apparatus which accommodates newly synthesized CD14 en route to the plasma membrane. As a marker for this compartment we used the PH domain of the FAPP1 protein which binds to the PI(4)P/Arf1 complex in the Golgi [45, 64, 65]. Optical sections of triple-labeled cells (including nucleus labeling) were used to reconstruct 3D images of studied structures which were identified on the basis of the presence of the marker proteins in combination with their cellular localization. Namely, the ERC, TGN, and Golgi were defined as perinuclear structures enriched in respective marker proteins, whereas early endosomes were defined as dispersed EEA1-positive vesicles. The amount of CD14 in each compartment was determined by calculating the sum of its voxel intensities in each cell via 3D image analysis [46] and related to the total fluorescence signal for CD14 in the sum z-projections of the same cell. The latter ratio reflected the fraction of CD14 localized to a given compartment. We also assessed the co-localization of CD14 with the respective protein markers of each compartment by calculating PCC, and additionally determined the sum of voxel intensities representing the marker protein to reveal whether flotillin depletion affected the compartment itself (Table 4).
We found that flotillin deficiency facilitated the accumulation of CD14 in EEA1-positive endosomes, which was reflected both by an increase of the median sum of CD14 voxel intensities and by an increased fraction (by 35% and 107% in two experiments) of CD14 found in this compartment (Fig. 8a–c, Table 4). The colocalization of CD14 and EEA1 was low (Table 4), as CD14 was visible inside vesicles decorated on their surface with EEA1 (Fig. 8A4, B4). It is worth noting that the median sum of EEA1 voxel intensities also tended to be increased in the flotillin-depleted cells (Table 4), suggesting that the early endosomal compartment was larger in those cells and accommodated more CD14 than in control cells.
Enrichment of CD14 in EEA1-positive early endosomes in flotillin-depleted cells. A Raw264 cells transfected with control shRNA, variant NC1. B Cells transfected with shRNA specific against Flot2, variant No. 5. Localization of CD14 (A1, B1), EEA1 (A2, B2) and merged images of CD14 and EEA1 localization (A3, B3). z-Stack images of three optical sections taken in the middle of a cell are shown. Colocalized CD14 and EEA1 appear as white spots. A4, B4 Enlarged images of EEA1-positive endosomes indicated by arrows in A3 and B3 with CD14 visible in their interior. A5, B5 Reconstructed 3D images of an EEA1-positive compartment delineated in green with CD14 visualized in pink. Contours of the nucleus detected by Hoechst 33342 staining are shown in grey. ROI as these were used for quantitative analysis of CD14 localization in the compartment—see Table 4. C Ratio of the fluorescence intensity of CD14 in EEA1-positive endosomes calculated as the sum of CD14 voxel intensities to the total CD14 fluorescence detected in z-projections of the respective cell. n, number of cells analyzed. Results of two independent experiments are shown. Significantly different values as indicated by Student's t-test are marked
The enrichment of CD14 in early endosomes in the flotillin-depleted cells did not augment its localization in Rab11-positive ERC. On the contrary, all the ERC parameters analyzed were decreased or remained unaffected in those cells (Supplementary Fig. S4A–C, Table 4), suggesting that this compartment is not involved in the enhanced recycling of CD14. In marked contrast, the fraction of total CD14 found in the golgin-97-marked TGN was higher by up to 60% in the flotillin-depleted cells than in the control ones. In agreement, a moderate tendency for an increase of the median sum of CD14 voxel intensities in this compartment was observed in the flotillin-depleted cells (Fig. 9a–c, Table 4). Also the colocalization of CD14 with golgin-97 tended to be higher in those cells, albeit without a reproducible change in the median sum of golgin-97 voxel intensities itself (Fig. 9a–c, Table 4). These data indicate that the deficiency of flotillins facilitated CD14 recycling via the TGN rather than via the Rab11-positive compartment.
Enrichment of CD14 in golgin-97-positive TGN in flotillin-depleted cells. A Raw264 cells transfected with control shRNA, variant NC1. B Cells transfected with shRNA specific against Flot2, variant No. 5. Localization of CD14 (A1, B1), golgin-97 (A2, B2) and merged images of CD14 and golgin-97 localization (A3, B3). z-Stack images of three optical sections taken in the middle of a cell are shown. Colocalized CD14 and golgin-97 appear as white spots. A4, B4 Enlarged images of golgin-97-positive TGN indicated by arrowheads in A3, B3. A5, B5 Reconstructed 3D images of golgin-97-positive TGN delineated in green with CD14 visualized in pink. Contours of the nucleus detected by Hoechst 33342 staining are shown in grey. ROI as these were used for quantitative analysis of CD14 localization in the compartment—see Table 4. C Ratio of the fluorescence intensity of CD14 in golgin-97-positive TGN calculated as the sum of CD14 voxel intensities to the total CD14 fluorescence detected in z-projections of the respective cell. n, number of cells analyzed. Results of two independent experiments are shown. Significantly different values as indicated by Student's t-test are marked
Finally, a fraction of CD14 was detected in the FAPP1-PH-decorated Golgi apparatus. Neither this fraction nor the median sum of CD14 voxel intensities in this compartment, nor the median sum of FAPP1-PH voxel intensities were consistently affected by the deficiency of flotillins (Supplementary Fig. S5A–C, Table 4) making it difficult to assess the impact of the flotillin depletion on the anterograde transport of CD14 via the Golgi apparatus. Interestingly, CD14 colocalized with FAPP1-PH to the highest degree among all the marker proteins investigated (PCC around 0.3), similarly in the flotillin-depleted and control cells (Table 4).
Discussion
In this study we aimed to determine the role of flotillins in the CD14- and TLR4-mediated pro-inflammatory response of macrophages to LPS. For this purpose, we used shRNA transfection to obtain a set of Raw264 cells stably depleted of flotillin-2, finding that the cells were depleted of flotillin-1 as well. The phenomenon of a loss of the both flotillins after a directed down-regulation of one of them has already been reported and seems associated with the hetero-dimerization of flotillins increasing each other’s stability [32, 47, 66, 67]. Moreover, we also detected a down-regulation of flotillin-1 mRNA in Raw264 cells after Flot2 silencing, which could contribute to the flotillin-1 depletion. Therefore, the reduction of LPS-induced inflammatory response of Raw264 cells described in this study needs to be ascribed to the depletion of the both flotillins even though some specific functions are attributed to flotillin-1 owing to its distinct S-palmitoylation pattern [66, 68].
We found that the depletion of flotillins diminished predominantly the TRIF-dependent endosomal signaling of TLR4 while the MyD88-dependent one was affected mainly in cells stimulated with the lower LPS concentration (10 ng/ml). Furthemore, we revealed that flotillins modulate the TLR4-triggered signaling and also the pro-inflammatory activity of TLR2/TLR6 and TLR2/TLR1 by affecting the abundance of CD14. The flotillin depletion decreased the CD14 mRNA level and reduced the levels of total and cell surface CD14. Flotillins and mature CD14 were both present in the DRM fraction confirming the possibility of their transbilayer interaction dependent on cholesterol and phosphatidylserine [69, 70]. Our unpublished observations show that CD14 cross-linking in the plasma membrane induces S-palmitoylation of flotillin-1 and -2 further underscoring the interdependence of these proteins. On the other hand, the observed reduction of Tlr4 expression, minor compared to that of Cd14, did not lead to a depletion of TLR4 at the protein level, arguing against a TLR4 deficiency being the cause of the impaired response to LPS.
Flotillins have been indicated to affect gene expression through their influence on the cellular level of sphingosine-1-phosphate (S1P) which in turn inhibits histone deacetylases HDAC1 and HDAC2 [67]. Previous studies of our group indicated that silencing of the gene encoding sphingomyelin synthase 1 (an enzyme converting ceramide into sphingomyelin) in J774 macrophage-like cells also reduced the CD14 mRNA level [71]. Therefore, an influence of flotillin depletion on Cd14 (and possibly other genes) expression mediated by sphingolipids seems likely. The Cd14 promoter is regulated (positively and negatively) by the transcription factors Sp1–Sp3, whose activity, in turn, is regulated by ceramide, reinforcing the possibility of its dysregulation by disturbances of the flotillins—sphingolipids axis [72,73,74].
The reduced expression of Cd14 could be the reason for the paucity of CD14 protein in flotillin-depleted cells, however, a disturbance in CD14 trafficking and shedding also seemed likely. Indeed, we detected an inhibition of the constitutive endocytosis of CD14 in the flotillin-depleted Raw264 cells paired with its enhanced shedding from the cell surface and increased recycling. Taking into account the colocalization of flotillins and GPI-anchored proteins in rafts, these proteins were originally considered the most likely cargo of flotillin-dependent clathrin-independent uptake [29, 75] and our current data are in agreement with this line of data. However, another study casts doubt on the flotillin involvement in this process [76]. Furthemore, flotillin depletion did not inhibit the endocytosis of the T-cell receptor (TCR), a hallmark receptor relying on rafts for signal transduction [36]. Instead, a crucial role of flotillins in fluid phase marker internalization and also in clathrin-dependent uptake of some proteins has been observed [77,78,79], indicating that the cellular functions of flotillins go beyond their contribution to raft dynamics. In agreement, an analysis of the DRM composition in HEK293 cells has revealed that flotillins are found in DRM fractions of higher density resembling “heavy” protein-rich DRM involved in TCR signaling [80]. Also in our analyzes flotillins were present in the DRM, Triton X-100-soluble, and SDS-soluble (cytoskeletal) fractions of Raw264 cells. It seems likely that the contribution of flotillins to various pathways of endocytosis, including fluid-phase uptake can result from their primary involvement in modulating the organization of membrane lipids and also from their interactions with some intracellular proteins engaged in these pathways [27].
We found that the inhibition of constitutive CD14 endocytosis was paired with its intensified shedding from the cell surface of the flotillin-depleted cells. Interestingly, while the inhibition of CD14 endocytosis was most pronounced at the onset of the assay, the enhanced shedding was somewhat delayed, being the most intensive after 60–90 min and suggesting that the down-regulated endocytosis facilitated the removal of CD14 from the cell surface. It has been established that CD14 shedding takes place in resting macrophages and is enhanced after LPS stimulation [61, 62], and that matrix metalloproteinases (MMP)-9 and -12 catalyze this process [81]. As mentioned above, sCD14 can transfer LPS monomers to the TLR4/MD-2 complex, therefore the up-regulated shedding of CD14 in flotillin-depleted cells can explain the minor effect of the deficiency of membrane-bound CD14 on the MyD88-dependent signaling.
Despite the inhibition of CD14 internalization, the depletion of flotillins markedly enhances its recycling. A growing line of reports shows that flotillins participate in the recycling of various proteins, including TCR, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, TfR, E-cadherin, and integrin α-5/β-1. Their flotillin-mediated recycling was required for sustained TCR and AMPA signaling, and the assembly of adherens junctions and focal adhesions [35, 36, 82,83,84]. Recent proteomic studies have identified flotillins in EEA1-positive early endosomes and in lysosomes [38], and the flotillin-mediated recycling of the above-mentioned proteins involved EEA1/Rab5- and Rab11a-positive endosomes [35, 36, 82,83,84]. Also, a direct binding of flotillin-2 to Rab11a has been shown [82]. It has been proposed that a flotillin-dependent preselection of proteins to cholesterol- and phosphatidylserine-rich plasma membrane domains is maintained after endocytosis and determines the sorting of those proteins in the EEA1/Rab5-positive compartment for their recycling via Rab11-positive endosomes [85]. Our current microscopic analysis of the CD14 distribution in Raw264 cells indicated that flotillin depletion led to an enrichment of CD14 in EEA1-positive early endosomes and the TGN compartment involved in some protein recycling routes, while its abundance in the Rab11-positive ERC was down-regulated. On the other hand, our earlier studies indicated that CD14 recycling is also dependent on sorting nexins SNX1, 2, and 6 potentially interacting with the retromer allowing CD14 recycling via TGN [22]. Taken together, one can assume that while the recycling of plasma membrane proteins via EEA1/Rab5- and Rab11-positive endosomes requires a flotillin participation, flotillins seem dispensable for the CD14 recycling mediated by SNX1, 2, and 6. In fact, a deficit of flotillins augments this latter CD14 trafficking pathway, possibly at the expense of compromised flotillin-dependent protein trafficking. Moreover, we observed an enhanced decoration of early endosomes with EEA1 in flotillin-depleted cells. These data suggest that the observed changes in CD14 recycling possibly stem from changes in the organization of early endosomes and disturbances in protein sorting in this compartment, as has been revealed earlier for TCR recycling in flotillin-depleted Jurkat T cells [36].
Paradoxically, despite the enhanced recycling of CD14 in the flotillin-depleted Raw264 cells, its surface level was actually reduced. It seems likely that the enhanced recycling could be a compensatory mechanism striving to maintain the cell surface level of CD14, similarly as it has been suggested for the accelerated recycling of integrin α-5/β-1 in flotillin-depleted cells [83]. The augmented recycling of CD14 relied exclusively on the newly synthesized CD14, while in control cells only a fraction of the recycling CD14 derived from de novo synthesis. Based on these data one can infer that in flotillin-depleted cells after a single round of recycling CD14 is removed from the cell surface by shedding or is directed from EEA1-positive endosomes to degradation in lysosomes.
In conclusion, our data indicate that flotillin depletion leads to a depletion of the total and cell-surface CD14 by a down-regulation of Cd14 expression and reduction of CD14 constitutive endocytosis accompanied by its enhanced shedding from the cell surface. Concomitantly, the recycling of newly synthesized CD14 through EEA1-positive early endosomes and TGN is enhanced, possibly aiming to compensate for the deficiency of CD14 on the cell surface. The paucity of surface CD14 weakens the endosomal TRIF-dependent inflammatory signaling of TLR4 after stimulation of cells with LPS and also the pro-inflammatory signaling of other CD14-dependent TLRs. Figure 10 summarizes these findings. Our results strengthen the earlier suggestion that the cell-surface level of CD14 gauges the pro-inflammatory response of macrophages [22, 58].
Flotillins modulate the cell-surface level of CD14 thereby determining the pro-inflammatory signaling of CD14-dependent TLRs. Depletion of flotillins down-regulates the level of CD14 mRNA and inhibits constitutive CD14 endocytosis. The latter facilitates the shedding of CD14 from the cell surface accompanied by enhanced recycling of CD14 aiming to compensate for the deficiency of CD14. The paucity of cell-surface CD14 resulting from flotillin depletion inhibits the signaling of TLR4, TLR2/TLR1, and TLR2/TLR6
Data availability
All data generated or analyzed during this study are included in this article (and its Supplementary Information files) and are available from the corresponding authors on reasonable request.
Change history
30 May 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00018-024-05288-y
References
Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085–2088. https://doi.org/10.1126/science.282.5396.2085
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhunag Y, Cai T, Wang F, Shao F (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526:660–665. https://doi.org/10.1038/nature15514
Virzi GM, Mattiotti M, de Cal M, Ronco C, Zanella M, De Rosa S (2022) Endotoxin in sepsis: methods for LPS detection and the use of omics techniques. Diagnostics (Basel) 13:79. https://doi.org/10.3390/diagnostics13010079
Violi F, Cammisotto V, Bartimoccia S, Pignatelli P, Carnevale R, Nocella C (2023) Gut-derived low-grade endotoxemia, atherothrombosis and cardiovascular disease. Nat Rev Cardiol 20:24–37. https://doi.org/10.1038/s41569-022-00737-2
Sharma BR, Kanneganti TD (2021) NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol 22:550–559. https://doi.org/10.1038/s41590-021-00886-5
Ryu JK, Kim SJ, Rah SH, Ji K, Jung HE, Lee D, Lee HK, Lee J-O, Park BS, Yoon T-Y, Kim HM (2017) Reconstruction of LPS transfer cascade reveals structural determinants within LBP, CD14, and TLR4-MD2 for efficient LPS recognition and transfer. Immunity 46:38–50. https://doi.org/10.1016/j.immuni.2016.11.007
Pugin J, Schurer-Maly CC, Leturcq D, Moriarty A, Ulevitch RJ, Tobias PS (1993) Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA 90:2744–2748. https://doi.org/10.1073/pnas.90.7.2744
Esparza GA, Teghanemt A, Zhang D, Gioannini TL, Weiss JP (2012) Endotoxin albumin complexes transfer endotoxin monomers to MD-2 resulting in activation of TLR4. Innate Immun 18:478–491. https://doi.org/10.1177/1753425911422723
Kawai T, Adachi O, Ogawa T, Takeda K, Akira S (1999) Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11:115–122. https://doi.org/10.1016/s1074-7613(00)80086-2
Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, McMurray D, Smith DE, Sims JE, Bird TA, O’Neill LA (2001) Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413:78–83. https://doi.org/10.1038/35092578
Bjorkbacka H, Fitzgerald KA, Huet F, Li HX, Gregory JA, Lee MAL, Ordija CM, Dowley NE, Golenbock DG, Freemen MW (2004) The induction of macrophage gene expression by LPS predominantly utilizes Myd88-independent signaling cascades. Physiol Genomics 19:319–330. https://doi.org/10.1152/physiolgenomics.00128.2004
Everts B, Amiel E, Huang SC-C, Smith AM, Chang C-H, Lam WY, Redmann V, Freitas TC, Blagih J, van der Windt GJW, Artyomov MN, Jones RG, Pearce EL, Pearce EJ (2014) TLR driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation. Nat Immunol 15:323–332. https://doi.org/10.1038/ni.2833
Tan Y, Kagan JC (2019) Innate immune signaling organelles display natural and programmable signaling flexibility. Cell 177:384-398.e11. https://doi.org/10.1016/j.cell.2019.01.039
Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, Hoshino K, Akira S (2001) Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol 167:5887–5894. https://doi.org/10.4049/jimmunol.167.10.5887
Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S (2003) TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol 4:1144–1150. https://doi.org/10.1038/ni986
Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, Freudenberg M, Beutler B (2005) CD14 is required for MyD88-independent LPS signaling. Nat Immunol 6:565–570. https://doi.org/10.1038/ni1207
Husebye H, Halaas R, Stenmark H, Tunheim G, Sandanger R, Bogen B, Brech A, Latz E, Espevik T (2006) Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J 25:683–692. https://doi.org/10.1038/sj.emboj.7600991
Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC (2011) CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147:868–880. https://doi.org/10.1016/j.cell.2011.09.051
Płóciennikowska A, Hromada-Judycka A, Dembińska J, Roszczenko P, Ciesielska A, Kwiatkowska K (2016) Contribution of CD14 and TLR4 to changes of the PI(4,5)P2 level in LPS-stimulated cells. J Leukoc Biol 100:1363–1373. https://doi.org/10.1189/jlb.2VMA1215-577R
Schappe MS, Szteyn K, Stremska ME, Mendu SK, Downs TK, Seegren PV, Mahoney MA, Dixit S, Krupa JK, Stipes EJ, Rogers JS, Adamson SE, Leitinger N, Desai BN (2018) Chanzyme TRPM7 mediates the Ca2+ influx essential for lipopolysaccharide-induced Toll-like receptor 4 endocytosis and macrophage activation. Immunity 48:59-74.e5. https://doi.org/10.1016/j.immuni.2017.11.026
Tan Y, Zanoni I, Cullen TW, Goodman AL, Kagan JC (2015) Mechanisms of Toll-like receptor 4 endocytosis reveal a common immune-evasion strategy used by pathogenic and commensal bacteria. Immunity 43:909–922. https://doi.org/10.1016/j.immuni.2015.10.008
Ciesielska A, Krawczyk M, Sas-Nowosielska H, Hromada-Judycka A, Kwiatkowska K (2022) CD14 recycling modulates LPS-induced inflammatory responses of murine macrophages. Traffic 23:310–330. https://doi.org/10.1111/tra.12842
Ciesielska A, Matyjek M, Kwiatkowska K (2021) TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci 78:1233–1261. https://doi.org/10.1007/s00018-020-03656-y
Płóciennikowska A, Hromada-Judycka A, Borzęcka K, Kwiatkowska K (2015) Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci 72:557–581. https://doi.org/10.1007/s00018-014-1762-5
Borzęcka-Solarz K, Dembinska J, Hromada-Judycka A, Traczyk G, Ciesielska A, Ziemlińska E, Świątkowska A, Kwiatkowska K (2017) Association of Lyn kinase with membrane rafts determines its negative influence on LPS-induced signaling. Mol Biol Cell 28:1147–1159. https://doi.org/10.1091/mbc.E16-09-0632
Sobocińska J, Roszczenko-Jasińska P, Zaręba-Koziol M, Hromada-Judycka A, Matveichuk OV, Traczyk G, Łukasiuk K, Kwiatkowska K (2018) Lipopolysaccharide upregulates palmitoylated enzymes of the phosphatidylinositol cycle: An insight from proteomic studies. Mol Cell Proteomics 17:233–254. https://doi.org/10.1074/mcp.RA117.000050
Kwiatkowska K, Matveichuk OV, Fronk J, Ciesielska A (2020) Flotillins: at the intersection of protein S-palmitoylation and lipid-mediated signaling. Int J Mol Sci 21:2283. https://doi.org/10.3390/ijms21072283
Biernatowska A, Augoff K, Podkalicka J, Tabaczar S, Gajdzik-Nowak W, Czogalla A, Sikorski AF (2017) MPP1 directly interacts with flotillins in erythrocyte membrane—possible mechanism of raft domain formation. Biochim Biophys Acta Biomembr 1859:2203–2212. https://doi.org/10.1016/j.bbamem.2017.08.021
Glebov OO, Bright NA, Nichols BJ (2006) Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol 8:46–54. https://doi.org/10.1038/ncb1342
Neumann-Giesen C, Fernow I, Amaddii M, Tikkanen R (2007) Role of EGF-induced tyrosine phosphorylation of reggie-1/flotillin-2 in cell spreading and signaling to the actin cytoskeleton. J Cell Sci 120:395–406
Frick M, Bright NA, Riento K, Bray A, Merrified C, Nichols BJ (2007) Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr Biol 17:1151–1156. https://doi.org/10.1242/jcs.03336
Ludwig A, Otto GP, Riento K, Hams E, Fallon PG, Nichols BJ (2010) Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment. J Cell Biol 191:771–781. https://doi.org/10.1083/jcb.201005140
Koh M, Yong HY, Kim ES, Son H, Jeon YR, Hwang JS, Kim M, Cha Y, Choi WS, Noh D-Y et al (2016) A novel role for flotillin-1 in H-Ras-regulated breast cancer aggressiveness. Int J Cancer 138:1232–1245. https://doi.org/10.1002/ijc.29869
Banning A, Babuke T, Kurrle N, Meister M, Ruonala MO, Tikkanen R (2018) Flotillins regulate focal adhesions by interacting with α-actinin and by influencing the activation of focal adhesion kinase. Cells 7:28. https://doi.org/10.3390/cells7040028
Bodrikov V, Pauschert A, Kochlamazashvili G, Stuermer CAO (2017) Reggie-1 and reggie-2 (flotillins) participate in Rab11a-dependent cargo trafficking, spine synapse formation and LTP-related AMPA receptor (GluA1) surface exposure in mouse hippocampal neurons. Exp Neurol 289:31–45. https://doi.org/10.1016/j.expneurol.2016.12.007
Redpath GMI, Ecker M, Kapoor-Kaushik N, Vartoukian H, Carnell M, Kempe D, Biro M, Ariotti N, Rossy J (2019) Flotillins promote T cell receptor sorting through a fast Rab5–Rab11 endocytic recycling axis. Nat Commun 10:4392. https://doi.org/10.1038/s41467-019-12352-w
Genest M, Comunale F, Planchon D, Govindin P, Noly D, Vacher S, Bièche I, Robert B, Malhotra H, Schoenit A, Tashireva LA, Casas J, Gauthier-Rouvière C, Bodin S (2022) Upregulated flotillins and sphingosine kinase 2 derail AXL vesicular traffic to promote epithelial-mesenchymal transition. J Cell Sci 135:jcs259178. https://doi.org/10.1242/jcs.259178
Singh J, Elhabashy H, Muthukottiappan P, Stepath M, Eisenacher M, Kohlbacher O, Giesielman V, Winter D (2022) Cross-linking of the endolysosomal system reveals potential flotillin structures and cargo. Nat Commun 13:6212. https://doi.org/10.1038/s41467-022-33951-0
Wisniewski DJ, Liyasova MS, Korrapati S, Zhang X, Ratnayake S, Chen Q, Gilbert SF, Catalano A, Voeller D, Meerzaman D, Guha U, Porat-Shliom N, Annunziata CM, Lipkowitz S (2023) Flotillin-2 regulates epidermal growth factor receptor activation, degradation by Cbl-mediated ubiquitination, and cancer growth. J Biol Chem 299:102766. https://doi.org/10.1016/j.jbc.2022.102766
Dattaroy D, Seth RK, Das S, Alhasson F, Chadrashekaran V, Michelotti G, Fan D, Nagarkatti M, Nagarkatti P, Diehl AM, Chatterje S (2016) Sparstolonin B attenuates early liver inflammation in experimental NASH by modulating TLR4 trafficking in lipid rafts via NADPH oxidase activation. Am J Physiol Gastrointest Liver Physiol 310:G510–G525. https://doi.org/10.1152/ajpgi.00259.2015
Fork C, Hitzel J, Nichols BJ, Tikkanen R, Brandes RP (2014) Flotillin-1 facilitates Toll-like receptor 3 signaling in human endothelial cells. Basic Res Cardiol 109:439. https://doi.org/10.1007/s00395-014-0439-4
Chowdhury SM, Zhu X, Aloor JJ, Azzam KM, Gabor KA, Ge W, Addo KA, Tomer KB, Parks JS, Fessler MB (2015) Proteomic analysis of ABCA1-null macrophages reveals a role for stomatin-like protein-2 in raft composition and Toll-like receptor signaling. Mol Cell Proteomics 14:1859–1870. https://doi.org/10.1074/mcp.M114.045179
McQuin C, Goodman A, Chernyshev V, Kamentsky L, Cimini BA, Karhohs KW, Doan M, Ding L, Rafelski SM, Thirstrup D, Wiegraebe W, Singh S, Becker T, Caicedo JC, Carpenter AE (2018) Cell Profiler 3.0: next-generation image processing for biology. PLoS Biol 16:e2005970. https://doi.org/10.1371/journal.pbio.2005970
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Hammond GR, Schiavo G, Irvine RF (2009) Immunocytochemical techniques reveal multiple, distinct cellular pools of PtdIns4P and PtdIns(4,5)P2. Biochem J 422:23–35. https://doi.org/10.1042/BJ20090428
Husebye H, Aune MH, Stenvik J, Samstad E, Skjeldal F, Halaas O, Nilsen NJ, Stenmark H, Latz E, Lien E, Mollnes TE, Bakke O, Espevik T (2010) The Rab11a GTPase controls Toll-like receptor 4-induced activation of interferon regulatory factor-3 on phagosomes. Immunity 33:583–596. https://doi.org/10.1016/j.immuni.2010.09.010
Solis GP, Hoegg M, Munderloh C, Schrock Y, Malaga-Trillo E, Rivera-Milla E, Stuermer CA (2007) Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem J 403:313–322. https://doi.org/10.1042/BJ20061686
Berger T, Ueda T, Arpaia E, Chio C II, Shirdel EA, Jurisica I, Hamada K, You-Ten A, Haight J, Wakeham A, Cheung CC, Mak TW (2013) Flotillin-2 deficiency leads to reduced lung metastases in a mouse breast cancer model. Oncogene 32:4989–4994. https://doi.org/10.1038/onc.2012.499
Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S (2003) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301:640–643. https://doi.org/10.1126/science
Guo H, Callaway JB, Ting JP (2015) Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21:677–687. https://doi.org/10.1038/nm.3893
Hirotani T, Yamamoto M, Kumagai Y, Uematsu S, Kawase I, Takeuchi O, Akira S (2005) Regulation of lipopolysaccharide-inducible genes by MyD88 and Toll/IL-1 domain containing adaptor inducing IFN-β. Biochem Biophys Res Commun 328:383–392. https://doi.org/10.1016/j.bbrc.2004.12.184
Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R (2008) TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol 2008(9):361–368. https://doi.org/10.1038/ni1569.4
Yanai H, Chiba S, Hangai S, Kometani K, Inoue A, Kimura Y, Abe T, Kiyonari H, Nishio J, Taguchi-Atarashi N, Mizushima Y, Negishi H, Grosschedl R, Taniguchi T (2018) Revisiting the role of IRF3 in inflammation and immunity by conditional and specifically targeted gene ablation in mice. Proc Natl Acad Sci USA 115:5253–5258. https://doi.org/10.1073/pnas.1803936115
Liu Y, Mo CF, Luo XY, Li H, Guo HJ, Sun H, Hu S, Li LM, Wang YT, Yang SX, Chang S, Zou Q (2020) Activation of Toll-Like receptor 3 induces interleukin-1 receptor antagonist expression by activating the interferon regulatory factor 3. J Innate Immun 12:304–320. https://doi.org/10.1159/000504321
Nyati KK, Kishimoto T (2022) Recent advances in the role of Arid5a in immune diseases and cancer. Front Immunol 12:827611. https://doi.org/10.3389/fimmu.2021.827611
Cekic C, Casella CR, Eaves CA, Matsuzawa A, Ichijo H, Mitchell TC (2009) Selective activation of the p38 MAPK pathway by synthetic monophosphoryl lipid A. J Biol Chem 284:31982–31991. https://doi.org/10.1074/jbc.M109.046383
Borzęcka K, Płóciennikowska A, Bjorkelund H, Sobota A, Kwiatkowska K (2013) CD14 mediates binding of high doses of LPS but is dispensable for TNF-α production. Mediators Inflamm 2013:824919. https://doi.org/10.1155/2013/824919
Zanoni I, Tan Y, Di Gioia M, Springstead JR, Kagan JC (2017) By capturing inflammatory lipids released from dying cells, the receptor CD14 induces inflammasome-dependent Phagocyte hyperactivation. Immunity 47:697-709.e3. https://doi.org/10.1016/j.immuni.2017.09.010
Stelter F, Pfister M, Bernheiden M, Jack RS, Bufler P, Engelmann H, Schutt C (1996) The myeloid differentiation antigen CD14 is N- and O-glycosylated. Eur J Biochem 236:457–464. https://doi.org/10.1111/j.1432-1033.1996.00457.x
Meng J, Parroche P, Golenbock DT, McKnight CJ (2008) The differential impact of disulfide bonds and N-linked glycosylation on the stability and function of CD14. J Biol Chem 283:3376–3384. https://doi.org/10.1074/jbc.M707640200
Bazil V, Strominger JL (1991) Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J Immunol 147:1567–1574. https://doi.org/10.4049/jimmunol.147.5.1567
Delgado M, Leceta J, Abad C, Martinez C, Ganea D, Gomariz RP (1999) Shedding of membrane-bound CD14 from lipopolysaccharide-stimulated macrophages by vasoactive intestinal peptide and pituitary adenylate cyclase activating polypeptide. J Neuroimmunol 99:61–71. https://doi.org/10.1016/s0165-5728(99)00105-8
Arai Y, Mizugishi K, Nonomura K, Naitoh K, Takaori-Kondo A, Yamashita K (2015) Phagocytosis by human monocytes is required for the secretion of presepsin. J Infect Chemother 21:564–569. https://doi.org/10.1016/j.jiac.2015.04.011
Godi A, Di Campli A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, De Matteis MA (2004) FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol 6:393–404. https://doi.org/10.1038/ncb1119
Weixel KM, Blumental-Perry A, Watkins SC, Aridor M, Weisz OA (2005) Distinct Golgi populations of phosphatidylinositol 4-phosphate regulated by phosphatidylinositol 4-kinases. J Biol Chem 280:10501–10508. https://doi.org/10.1074/jbc.M414304200
Jang D, Kwon H, Jeong K, Lee J, Pak Y (2015) Essential role of flotillin-1 palmitoylation in the intracellular localization and signaling function of IGF-1 receptor. J Cell Sci 128:2179–2190. https://doi.org/10.1242/jcs.169409
Riento K, Zhang Q, Clark J, Begum F, Stephens E, Wakelam MJ, Nichols BJ (2018) Flotillin proteins recruit sphingosine to membranes and maintain cellular sphingosine-1-phosphate levels. PLoS ONE 13:e0197401. https://doi.org/10.1371/journal.pone.0197401
Jang D, Kwon H, Choi M, Lee J, Pak Y (2019) Sumoylation of Flotillin-1 promotes EMT in metastatic prostate cancer by suppressing Snail degradation. Oncogene 38:3248–3260. https://doi.org/10.1038/s41388-018-0641-1
Maekawa M, Fairn GD (2015) Complementary probes reveal that phosphatidylserine is required for the proper transbilayer distribution of cholesterol. J Cell Sci 128:1422–1433. https://doi.org/10.1242/jcs.164715
Raghupathy R, Anilkumar AA, Polley A, Singh PP, Yadav M, Johnson C, Suryawanshi S, Saikam V, Sawant SD, Panda A, Guo Z, Vishwakarma RA, Rao M, Mayor S (2015) Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell 161:581–594. https://doi.org/10.1016/j.cell.2015.03.048
Prymas K, Światkowska A, Traczyk G, Ziemlińska E, Dziewulska A, Ciesielska A, Kwiatkowska K (2020) Sphingomyelin synthase activity affects TRIF-dependent signaling of Toll-like receptor 4 in cells stimulated with lipopolysaccharide. Biochim Biophys Acta Mol Cell Biol Lipids 1865:158549. https://doi.org/10.1016/j.bbalip.2019.158549
Zhang DE, Hetherington CJ, Tan S, Dziennis SE, Gonzales DA, Chen HM, Tenen DG (1994) Sp1 is a critical factor for the monocytic specific expression of human CD14. J Biol Chem 269:11425–11434
LeVan TD, Bloom JW, Bailey TJ, Karp CL, Halonen M, Martinez FD (2001) A common single nucleotide polymorphism in the CD14 promoter decreases the affinity of Sp protein binding and enhances transcriptional activity. J Immunol 167:5838–5844. https://doi.org/10.4049/jimmunol.167.10.5838
Wooten LG, Ogretmen B (2005) Sp1/Sp3-dependent regulation of human telomerase reverse transcriptase promoter activity by the bioactive sphingolipid ceramide. J Biol Chem 280:28867–28876. https://doi.org/10.1074/jbc.M413444200
Ait-Slimane T, Galmes R, Trugnan G, Maurice M (2009) Basolateral internalization of GPI-anchored proteins occurs via a clathrin-independent flotillin-dependent pathway in polarized hepatic cells. Mol Biol Cell 20:3792–3800. https://doi.org/10.1091/mbc.e09-04-0275
Langhorst MF, Reuter A, Jaeger FA, Wippich F, Luxenhofer G, Plattner H, Stuermer CA (2008) Trafficking of the microdomain scaffolding protein reggie-1/flotillin-2. Eur J Cell Biol 87:211–226. https://doi.org/10.1016/j.ejcb.2007.12.001
Schneider A, Rajendran L, Honsho M, Gralle M, Donnert G, Wouters F, Hell SW, Simons M (2008) Flotillin-dependent clustering of the amyloid precursor protein regulates its endocytosis and amyloidogenic processing in neurons. J Neurosci 28:2874–2882. https://doi.org/10.1523/JNEUROSCI.5345-07.2008
Otto GP, Nichols BJ (2011) The roles of flotillin microdomains–endocytosis and beyond. J Cell Sci 124:3933–3940. https://doi.org/10.1242/jcs.092015
Fekri F, Abousawan J, Bautista S, Orofiamma L, Dayam RM, Antonescu CN, Karshafian R (2019) Targeted enhancement of flotillin-dependent endocytosis augments cellular uptake and impact of cytotoxic drugs. Sci Rep 9:17768. https://doi.org/10.1038/s41598-019-54062-9
Otahal P, Angelisova P, Hrdinka M, Brdicka T, Novak P, Drbal K, Horejsi V (2010) A new type of membrane raft-like microdomains and their possible involvement in TCR signaling. J Immunol 184:3689–3696. https://doi.org/10.4049/jimmunol.0902075
Senft AP, Korfhagen TR, Whitsett JA, Shapiro SD, LeVine AM (2005) Surfactant protein-D regulates soluble CD14 through matrix metalloproteinase-12. J Immunol 174:4953–4959. https://doi.org/10.4049/jimmunol.174.8.4953
Solis GP, Hülsbusch N, Radon Y, Katanaev VL, Plattner H, Stuermer CA (2013) Reggies/flotillins interact with Rab11a and SNX4 at the tubulovesicular recycling compartment and function in transferrin receptor and E-cadherin trafficking. Mol Biol Cell 24:2689–2702. https://doi.org/10.1091/mbc.E12-12-0854
Hulsbusch N, Solis GP, Katanaev V, Stuermer CA (2015) Reggie-1/Flotillin-2 regulates integrin trafficking and focal adhesion turnover via Rab11a. Eur J Cell Biol 94:531–545. https://doi.org/10.1091/mbc.E12-12-0854
Rossatti P, Redpath GMI, Ziegler L, Samson GPB, Clamagirand CD, Legler DF, Rossy J (2022) Rapid increase in transferrin receptor recycling promotes adhesion during T cell activation. BMC Biol 20:189. https://doi.org/10.1186/s12915-022-01386-0
Redpath GMI, Betzler VM, Rossatti P, Rossy J (2020) Membrane heterogeneity controls cellular endocytic trafficking. Front Cell Dev Biol 8:757. https://doi.org/10.3389/fcell.2020.00757
Acknowledgements
We thank Dr. Jan Fronk (retired, formerly from the Faculty of Biology, University of Warsaw) for helpful comments. Confocal imaging was performed at the Laboratory of Imaging Tissue Structure and Function, core facility at the Nencki Institute of Experimental Biology and a part of the infrastructure of the Polish Euro-BioImaging Node. Flow Cytometry was performed at the Laboratory of Cytometry at the Nencki Institute of Experimental Biology.
Funding
This work was supported by the National Science Centre, Poland, Grant Nos. 2018/29/B/NZ3/00407 to K.K. and 2020/39/B/NZ3/02517 to A.C. This work received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie Grant No. 665735 and was supported by the project financed by the Ministry of Education and Science based on contract No. 2022/WK/05 (Polish Euro-BioImaging Node “Advanced Light Microscopy Node Poland”).
Author information
Authors and Affiliations
Contributions
OVM—Conceptualization, Investigation, Formal analysis, Writing—review and editing. AC—Conceptualization, Methodology, Investigation, Formal analysis, Project administration, Writing—original draft, review and editing. AH-J—Investigation, Formal analysis, Writing—review and editing. NN—Investigation, Writing—review and editing. IBA—Investigation, Writing—review and editing. GT—Investigation, Writing—review and editing. KK—Conceptualization, Supervision, Project administration, Writing—original draft, review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this paper.
Consent for publication
All the authors have read the manuscript and agreed to submit the paper to the Journal.
Ethics approval
The procedure of obtaining peritoneal macrophages from mice has been reviewed and approved by the Local Animal Ethics Committee (Permission No. 394/2017). No research involving humans was conducted and no relevant ethics approval or consent to participate and publish are required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to update the error in the typo in the author name Ichrak Ben Amor
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matveichuk, O.V., Ciesielska, A., Hromada-Judycka, A. et al. Flotillins affect LPS-induced TLR4 signaling by modulating the trafficking and abundance of CD14. Cell. Mol. Life Sci. 81, 191 (2024). https://doi.org/10.1007/s00018-024-05221-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-024-05221-3