Abstract
Rhes (Ras homolog enriched in the striatum), a multifunctional protein that regulates striatal functions associated with motor behaviors and neurological diseases, can shuttle from cell to cell via the formation of tunneling-like nanotubes (TNTs). However, the mechanisms by which Rhes mediates diverse functions remain unclear. Rhes is a small GTPase family member which contains a unique C-terminal Small Ubiquitin-like Modifier (SUMO) E3-like domain that promotes SUMO post-translational modification of proteins (SUMOylation) by promoting “cross-SUMOylation” of the SUMO enzyme SUMO E1 (Aos1/Uba2) and SUMO E2 ligase (Ubc-9). Nevertheless, the identity of the SUMO substrates of Rhes remains largely unknown. Here, by combining high throughput interactome and SUMO proteomics, we report that Rhes regulates the SUMOylation of nuclear proteins that are involved in the regulation of gene expression. Rhes increased the SUMOylation of histone deacetylase 1 (HDAC1) and histone 2B, while decreasing SUMOylation of heterogeneous nuclear ribonucleoprotein M (HNRNPM), protein polybromo-1 (PBRM1) and E3 SUMO-protein ligase (PIASy). We also found that Rhes itself is SUMOylated at 6 different lysine residues (K32, K110, K114, K120, K124, and K245). Furthermore, Rhes regulated the expression of genes involved in cellular morphogenesis and differentiation in the striatum, in a SUMO-dependent manner. Our findings thus provide evidence for a previously undescribed role for Rhes in regulating the SUMOylation of nuclear targets and in orchestrating striatal gene expression via SUMOylation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mRNA of Rhes (Ras homolog enriched in the striatum) has significant expression in the striatum, a region that control motor skills, cognitive function, and emotion [1,2,3,4]. The expression of Rhes mRNA is stimulated by thyroid hormones. Additionally, Rhes has the ability to suppress the cAMP/PKA pathway and N-type Ca2+ channels (Cav 2.2) [5,6,7,8]. We have found several new roles for Rhes in the striatum. Rhes, in conjunction with RAS guanyl nucleotide-releasing protein guanine nucleotide exchange factor (RasGRP1-GEF), has the ability to suppress amphetamine-induced hyperactivity by establishing a protein–protein interaction network known as the "Rhesactome." [8]. Rhes and RasGRP1 facilitate the development of L-DOPA-induced dyskinesia (LID) through the involvement of mammalian target of rapamycin (mTOR) signaling in a pre-clinical model of Parkinson's disease (PD) [9, 10]. Rhes modulates autophagy through Beclin1 in a manner that is independent of mTOR [11]. We have also shown that Rhes has a crucial function in the striatal damage associated with Huntington's disease (HD). We found that Rhes interacts with mutant huntingtin, the genetic risk factor for HD, and enhances its solubility and striatal toxicity through modification by the post-translational small ubiquitin-like modifier (SUMO) [9, 12, 13].
In addition to PD and HD, Rhes is associated with tauopathy and mental disorders. Rhes has a crucial function in developing mutant tau-induced pathology through SUMO modification [14, 15]. Single nucleotide polymorphism (SNP) of the Rhes gene, RASD2, s were found associated with schizophreniapatients [16, 17], and a rare and conserved somatic mutation (R57H) was found in the RASD2 in twins diagnosed with autism [18]. However, the molecular process through which Rhes participate in diverse brain disorders remains unclear. Our recent discovery that Rhes promotes the formation of tubular or tunneling nanotube (TNT) moves from cell-to-cell via TNTs [19] strongly indicates an involvement of Rhes in cell–cell communication functions in the brain [20].
TNTs are delicate and inconspicuous membranous structures that connect two cells. TNTs have a diameter ranging from 50–200 nm and a length of 5–125 µm and have been observed in several types of cells [21]. Using mutagenesis, we have found that the membrane-binding site of Rhes (C263) is critical for the formation for TNTs. Furthermore, a distinct role for the GTPase domain (1-171aa) and the SUMO E3 ligase domain of Rhes (171-266aa) was identified in TNT formation [21]. The SUMO E3 ligase domain is necessary for TNT formation but is not on its own sufficient for the cell-to-cell transport. By contrast, the GTPase domain is defective in both the formation of TNT and in cell-to-cell transport [16].
Overall, the current evidence indicates that Rhes can execute more than one function at the molecular, cellular, and organismal levels. Multifunctional proteins like Rhes are not very rare in biology, as network topological information with protein annotations indicate that approximately 430 human proteins, including tumor suppressor protein p53 and cytochrome C, can be classed as extreme multifunctional proteins [22,23,24]. Despite the multiple roles and molecular complexity of Rhes, there is a lack of fundamental knowledge about the mechanisms by which Rhes operates. We previously reported that Rhes physiologically regulates SUMO modification in vivo and mechanistically promotes the transfer of SUMO from thioester to lysine residues (“cross-SUMOylation”) between E1 (Aos1) and E2 (Ubc9) SUMO enzymes [25]. However, the SUMO substrates of Rhes remain unidentified. In the present study, we have used biochemical, proteomics, and RNA- seq tools to investigate the SUMO substrates of Rhes and the putative role of Rhes in striatal gene expression.
Materials and methods
Reagents, plasmids and antibodies
Unless otherwise noted, reagents were obtained from Sigma. Full length Rhes rat and human cDNA constructs, Myc, GST or GFP tagged, were cloned in pCMV vectors (Clontech) [12, 19, 26]. Mass spectrometry detection compatible His6-mSUMO1, His6-mSUMO2 and His6-mSUMO3 were cloned as described [27, 28]. p181 pK7-HDAC1 (GFP) (Addgene; #11054) were from Ramesh Shivdasani. H2B-mCherry (Addgene; #20972) were from Robert Benezra. mH2A1.2-CT-GFP (Addgene, #45169) were from Brian Chadwick, Hunt Willard. pT7-V5-SBP-C1-HshnRNPM (Addgene; #64924) were from Elisa Izaurralde. GFP-PBRM1 (Addgene; #65387) were from Kyle Miller. Flag-hPIASy (Addgene; #15208) were from Ke Shuai. GFP-NPM WT (Addgene; 15578) were from Xin Wang. DGK beta (Addgene; #35405) were from Robert Lefkowitz and Stephen Prescott. pCMV-L26-Flag (Addgene; #19972) were from Moshe Oren. 6x-His Tag Antibody (clone HIS.H8) (1:1000, # MA1-21315) was from ThermoFisher Scientific. Antibodies for GST-HRP (1:5000, #sc-138), and Myc (1:3000, #sc-40) were obtained from Santa Cruz Biotechnology. mCherry antibody (1:1000, NBP2-25157) was from Novus Biologicals. Flag antibody (1:1000, F7425) was obtained from Sigma-Aldrich. HA.11 Epitope Tag Antibody (1:1000, #901513) was from BioLegend (previously Covance catalog #MMS-101R). V5-Tag (1:1000, #13,202), GFP (1:1000, #2956), mTOR (1:3000, #2983), Histone H3 (1:10,000, #4499), MEK1/2 (1:1000, #8727) and LDH (1:5000, #2012) were from Cell Signaling Technology, Inc. Rhes antibody (1:1000, RHES-101AP) was from Fabgennix.
Ni–NTA denaturing pull down for western blotting
Ni–NTA pull down of His-mSUMO3 conjugates was performed as previously described [12, 29]. Briefly, HEK293 cells (expressing transfected His-SUMO mutant and indicated constructs) were pretreated with proteosome inhibitor, MG132 (25 µM, 4 h), rinsed in PBS, scraped from 10cm2 dishes, and centrifuged at 750 × g for 5 min. Since a small fraction of proteins are SUMOylated, MG132 was added to stabilize SUMOylated proteins by preventing their proteasome degradation [28, 30, 31]. Cell pellets were then directly lysed in pull-downown buffer [6 M Guanidine hydrochloride, 10 mM Tris, 100 mM sodium phosphate] and sonicated. After collecting 50ul of sample for quantification, 40 mM imidazole and 5mM β-mercaptoethanol was added to the remaining lysates and remaining pull-down buffer. imidazole and β-mercaptoethanol interfere with BCA protein assay. Lysates were then clarified by centrifugation at 3,000 × g for 15 min. All subsequent wash steps were performed with 10 resin volumes of buffer followed by centrifugation at 800 × g for 2 min. Ni–NTA Agarose beads (#30,210; Qiagen) were pre-equilibrated by washing three times with pull-down buffer. After equilibration, beads were resuspended in pull-down buffer as a 50% slurry of beads to buffer. After protein quantification of cleared cell lysates, 1 mg of lysate was added to 40 µL of Ni–NTA TALON bead slurry to a total volume of 1 mL in microcentrifuge tubes. The beads were then incubated overnight at 4 °C mixing end over end. The following day, beads were centrifuged at 4000 RPMs for 2 minutes at 4 °C in a tabletop centrifuge and washed in the following sequence: once in pull-down buffer, once in pH 8.0 urea buffer (8 M Urea, 10 mM Tris, 100 mM sodium phosphate, 0.1% Triton X-100, 20 mM imidazole, 5 mM β-ME, pH 8.0), and three additional times in pH 6.3 urea buffer (8 M Urea, 10 mM Tris, 100 mM sodium phosphate, 0.1% Triton-X-100, 20 mM imidazole, 5 mM β-ME, pH 6.3). Elution was performed using 25 µL of Elution Buffer (pH 8.0 urea buffer containing 200 mM imidazole, 4X NuPAGE LDS loading dye, 720 mM β-ME). Samples were then heated at 95 °C for 5 min and directly used for Western Blotting. Inputs were loaded as 1% of the total cell lysate. The SUMOylation was quantified by normalizing the intensity of SUMOylation bands to the respective unmodified bands using Image-J software.
GFP-Rhes localization studies
Approximately 75,000 STHdhQ7/Q7 cells were seeded on 35 mm glass bottom dishes. After 24 h the cells were transfected with indicated plasmids. Cells were imaged live using a Zeiss 880 confocal microscope at 63X oil immersion Plan- apochromat objective (1.4 NA) as before (22).
Purification of SUMOylated Rhes interacting proteins
Briefly, GST + mSUMO3, and GST-Rhes + mSUMO3 (5 μg each) were transfected in 10 cm dish HEK293 cells, and after 48 h, cells were lysed in lysis/binding buffer [50 mM tris (pH 8.0), 150 mM NaCl, 10% glycerol, and 1.0% NP-40] with a protease inhibitor cocktail (Roche) and phosphatase inhibitor II (Sigma). For GST-affinity experiments, protein lysates were pretreated with glutathione beads for 1 h, glutathione beads were added, and the lysates were then rotated overnight at 4°C. The beads were washed three times in binding buffer without a protease inhibitor cocktail. The resulting purified material was subjected to denaturing Ni–NTA purification step to enrich for the SUMOylated interacting partners. The Ni–NTA bound resin underwent a thorough washing with 50 mM ammonium bicarbonate. Subsequently, the proteins were digested using trypsin for a duration of 16 h at 37 °C. The mSUMO3-modified peptides were immunoprecipitated using a custom anti-NQTGG antibody that targets the tryptic remnant generated on the lysine side chain of SUMO, followed by mass spectrometry identification as described before [30, 32, 33].
SUMO peptide enrichment in Rhes overexpressing cells
Identification of SUMO modification lysine sites were carried out as described before [30, 32, 33]. Briefly, HEK293 cells were transfected with myc + mSUMO3 or myc-Rhes + mSUMO3, followed by denaturating Ni–NTA pulldown as described above. The Ni–NTA resin was extensively washed with 50 mM ammonium bicarbonate and the proteins digested with trypsin directly on the Ni–NTA solid support for 16 h at 37 °C. The mSUMO3-modified peptides were immunoprecipitated with anti-NQTGG antibody, as described before [30, 32, 33].
Mass spectrometry
Samples were analyzed on the Q-Exactive HF instrument (ThermoFisher Scientific) and raw files processed using MaxQuant and Perseus, as described previously [30, 32, 33]. Briefly, samples were analyzed by LC–MS/MS on a Proxeon EASY-nLC system connected to Q-Exactive HF mass spectrometer (Thermo Fisher Scientific). Samples were loaded on a reverse-phase pre-column (5 mm length, 360 μm i.d.) and separated on a reverse-phase analytical column (18 cm length, 150 μm i.d.) (Phenomenex). Both columns were manually packed in-house. Separations were performed at flow rate of 0.6 μL/min using a linear gradient of 5–30% aqueous acetonitrile (0.2% formic acid) over 106 min. MS survey scans were performed at a resolution of 70,000 at m/z 200 with a mass window of m/z 350–1,500, maximum injection time of 200 ms and an automatic gain control of 1e6. MS/MS scans were acquired using a data dependent acquisition approach with a Top12 method for the proteome or Top speed of 3 s for SUMO peptides. The precursor isolation window was set to 2 m/z with a HCD normalized collision energy of 25, and a resolution of 17,500 at m/z 200. Automatic gain control (AGC) target values for MS/MS scans were set to 5e3 with a maximum fill time of 3 s. A dynamic exclusion of the previously acquired precursor ions was set to 15 s.
Generation of KR mutants of Rhes
EGFP hRASD2[NM_014310.4] was first used as the template to generate five lysine to arginine (K to R) mutations at positions 32, 110, 114, 120 and 245 (5KR). It was then subcloned into myc vector backbone using specific primers with Sal1 and Not1 restriction sites. Three additional K to R mutations were added at position 174, 175 and 191 on myc-Rhes_5KR template using a standard site-directed mutagenesis protocol with the respective primers containing the desired mutations. The desired mutations were confirmed by sequencing using CMV forward primer.
SUMO1/2/3 knockout in striatal cells
Striatal STHdhQ7/Q7 cells deleted for SUMO1, 2 and 3 using CRISPR/Cas9 SUMO gRNAs as described [19]. Mouse gRNA sequences consisting of three oligo pools are as follows: SUMO1 CRISPR/Cas9 plasmid (SC-423588) (GGAGGCAAAACCTTCAACTG; GAGTTCCAATGAATTCACTC; GCCCGGTACCTGGTCAGACA), SUMO2 CRISPR/Cas9 plasmid (SC-431342. CCTCACCTGCCGTTCACAAT, GCTCACCTTGGGTTTCTCGT, CTTGTTAGGGTTTGTCAATG) and SUMO3 CRISPR/Cas9 (SC-423045, TTCCCCAGGGCTTGTCAATG, ACACACCTGCCTCTCACAGT, CCGTCGCTGCGCAACCATGT). CRISPR/Cas9 control plasmid (SC-418922) consisted of scrambled sequences.
Mice
For organelle seperation and RNA-seq experiments, we used Rhes KO (Rhes−/−) mice, and C57BL/6 J mice. Rhes KO mice were obtained from Alessandro Usiello and were backcrossed with C57BL/6 J mice at least 8 generations; homozygous Rhes KO were used for all the experiments [9, 34]. WT mice (C57BL/6) were obtained from Jackson Laboratory and maintained in our animal facility according to Institutional Animal Care and Use Committee (IACUC) at The UF Wertheim Scripps Research Institute. Mice were euthanized by cervical dislocation and striatal tissues dissected and rapidly frozen in liquid nitrogen.
Nuclear and cytoplasmic separation from striatum
The striatum from C57BL/6 J and Rhes KO mice was fractionated using the nuclear/cytosol fractionation kit according to the manufacturer’s instructions with minor modifications (BioVision). Briefly, striatum from C57BL/6 J and Rhes KO mice was rapidly dissected out and homogenized in CEB (cytosolic extraction buffer)-A with DTT and protease inhibitor, and incubating for 10 min on ice prior to addition of CEB-B. The lysates were vortexed for 5 s and incubated on ice for 1 min. The lysates were then centrifuged at 4 °C for 5 min at 16,000 × g in a microcentrifuge. And the supernatants were kept as the cytoplasmic fraction. The nuclear pellet was resuspended in NEB (nuclear extraction buffer). And vortexed the lysates for 15 s. This step was repeated 5 times every 10 min. The nuclear pellet was centrifuged at 4 °C for 10 min at 16,000 × g in a microcentrifuge. And the supernatants were kept as the nuclear fraction. The protein concentration was determined in the cytoplasmic and nuclear fractions using the BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Equivalent amounts of protein samples (50 µg) were resolved by SDS-PAGE followed by immunoblotting as described below.
Western blotting
Equal amounts of protein (20–50 µg) were loaded and were separated by electrophoresis in NuPAGE 4 to 12% bis–tris Gel (Thermo Fisher Scientific), transferred to polyvinylidene difluoride membranes, and probed with the indicated antibodies. HRP-conjugated secondary antibodies (Jackson ImmunoResearch Inc.) were probed to detect bound primary IgG with a chemiluminescence imager (Alpha Innotech) using enhanced chemiluminescence from WesternBright Quantum (Advansta). Where indicated the membranes were stained for ponceau.
qPCR validation of targets
Striatum of mice (WT or Rhes KO) lysed in Trizol reagent. 250 ng RNA was used to prepare cDNA using Takara primescript kit (Cat no. 6110A) using random hexamers. The qRT-PCR of genes was performed with SYBR green (Takara RR420A) reagents. Primers for all the genes were designed based on sequences available from the Harvard qPCR primer bank. The primer sequences are as follows:
Gapdh mouse (Forward primer) 5’ primer AGGTCGGTGTGAACGGATTTG
(Reverse primer) 3’ primer TGTAGACCATGTAGTTGAGGTCA
Nrp1 mouse (Forward primer) 5’ primer GACAAATGTGGCGGGACCATA
(Reverse primer) 3’ primer TGGATTAGCCATTCACACTTCTC
Bhlhe22 mouse (Forward primer) 5’ primer TGAACGACGCTCTGGATGAG
(Reverse primer) 3’ primer GGTTGAGGTAGGCGACTAAGC
Slit2 mouse (Forward primer) 5’ primer GGCAGACACTGTCCCTATCG
(Reverse primer) 3’ primer GTGTTGCGGGGGATATTCCT
Epha5 mouse (Forward primer) 5’ primer AAGGAACCCTGTGGCTATTGG
(Reverse primer) 3’ primer GCAAACATGCCCGTTTTAGAGAA
Dcx mouse (Forward primer) 5’ primer CATTTTGACGAACGAGACAAAGC
(Reverse primer) 3’ primer TGGAAGTCCATTCATCCGTGA
Ext1 mouse (Forward primer) 5’ primer TGGAGGCGTGCAGTTTAGG
(Reverse primer) 3’ primer GAAGCGGGGCCAGAAATGA
Egr2 mouse (Forward primer) 5’ primer GCCAAGGCCGTAGACAAAATC
(Reverse primer) 3’ primer CCACTCCGTTCATCTGGTCA
Mef2c mouse (Forward primer) 5’ primer ATCCCGATGCAGACGATTCAG
(Reverse primer) 3’ primer AACAGCACACAATCTTTGCCT
Plxna1 mouse (Forward primer) 5’ primer GGGTGTGTGGATAGCCATCAG
(Reverse primer) 3’ primer GCCAACATATACCTCTCCTGTCT
Met mouse (Forward primer) 5’ primer GTGAACATGAAGTATCAGCTCCC
(Reverse primer) 3’ primer TGTAGTTTGTGGCTCCGAGAT
Efna5 mouse (Forward primer) 5’ primer ACACGTCCAAAGGGTTCAAGA
(Reverse primer) 3’ primer GTACGGTGTCATTTGTTGGTCT
Global RNA-seq from WT and Rhes KO striatum
WT and Rhes KO mouse striatum (1 male and 1 female pooled per sample, 3 biological replicates per group) were lysed in Trizol and RNA extracted using the miRNeasy kit from Qiagen (cat. # 217,004). RNA was DNase-treated on column. Total RNA (500 ng) was depleted of ribosomal RNA using probes provided by the NEBNext rRNA depletion module (Cat. #: E6310L, NEB), according to manufacturer recommendations. The library preparation from the rRNA-depleted RNA was conducted according to NEBNext Ultra II Directional RNA kit (Cat. # E7760, NEB) guidelines. Briefly, the rRNA-depleted RNA samples were chemically fragmented and random hexamer primed for reverse transcription. First and second strand cDNA was generated with dUTP incorporation to the second strand. The ds cDNA was end repaired and adenylated at their 3’ ends. A corresponding ‘T’ nucleotide on the hairpin loop adaptors was utilized to ligate to the ds cDNA. The loop contains a dUTP that is removed along with all other incorporated U’s in the second strand by treatment with USER enzyme (Uracil-specific excision reagent). The degradation of the second strand in this step preserves directional sequencing of the intact first-strand thus preserving strand information of the RNA. The adaptor ligated DNA was PCR amplified with barcoded Illumina-compatible primers to generate the final libraries. The final libraries were sequenced on the NextSeq 500 with paired end 40 bp read lengths. Raw sequencing reads were quality and adapter-trimmed using Trimmomatic and mapped to the mouse genome (mouse-UCSC: M.musculus-UCSC-mm10: downloaded March 22, 2016) using STAR version 2.5.2a aligner and gene abundance was estimated with HTSeq version 0.8.0. Differential gene expressions were assessed with DESeq2. DESeq2 provides the greatest power (90%) for protein-coding genes with n = 3, while the power for long non-coding RNA is marginally lower. With three biological replicates per condition, DESeq2 has sufficient power to identify significant genes. In addition, these samples had a greater sequencing depth, with an average of 32 million reads per sample and a mapping rate of 55%, resulting in at least 17 million reads mapped to genes. With three biological replicates per condition and a high sequencing depth, the statistical power to detect significance in our reported genes should be sufficient. In addition, we only report genes whose p-values were lower than the FDR-adjusted p-value and should be adequately controlled for false positives.
Normalized gene counts were averaged and log10 transformed for WT and Rhes KO samples and were plotted against each other where Rhes KO log10 mean values were on x-axis and WT log10 mean values were on y-axis. Differentially regulated genes were identified using padj < 0.05 cut off and up (higher in Rhes KO) and down (higher in WT) regulated genes were marked with green and red respectively. The graph was generated using JMP®, Version 13.2.1. SAS Institute Inc., Cary, NC. The Top Diseased and Functions were generated through the use of QIAGEN Ingenuity Pathway Analysis (QIAGEN IPA) [35].
Bioinformatic analysis with MaxQuant
Peptide identification from the raw files are searched using MaxQuant (version 1.6.2.10) [36]. MS/MS spectra are searched against Uniprot/SwissProt database that include Isoforms (released on November 6, 2020). The first search peptide tolerance is set to 20 ppm, the main search to 4.5 ppm, and fragment ion tolerance to 7.5 ppm. The maximum allowed number of missed cleavages by trypsin is set to 3 with a maximum of 5 modifications per peptide. Carbamidomethylation of cysteine residues is set as a fixed modification, while methionine oxidation, asparagine and glutamine deamidation, phosphorylation (STY), lysine SUMO3 (NQTGG) and protein N-acetylation are set as variable modifications. The false discovery rate (FDR) for peptide and protein is set to 1%, and the minimum peptide length is set to 6. Additional MaxQuant search parameters are listed in supplementary Data file S6.
Statistical analysis
Data were expressed as means ± SEM. All the experiments were performed at least in triplicate and repeated twice at minimum. Statistical analysis was performed using Student's t test or one-Way ANOVA followed by multiple comparison test (GraphPad Prism 7).
Data availability
Sequencing data have been submitted to the Gene Expression Omnibus (GEO) data repository, under the accession number GSE150990.The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD023394. The account details for PRIDE: Username: reviewer_pxd023394@ebi.ac.uk. Password: iM1zNSnE.
Results
Rhes interacts with SUMOylated proteins
Like ubiquitin, SUMO (which has 5 paralogues in vertebrates: SUMO1-5) is a conserved ~ 10.5 kDa protein modification that is covalently attached to lysine residues on multiple substrate proteins in a dynamic and reversible manner [37, 38]. We identified SUMO substrates of Rhes using an mSUMO3 construct in combination with a large-scale unbiased proteomics approach for SUMOylation site identification. The mSUMO3 structure has a mutation in the C-terminal that can be cleaved by trypsin, resulting in a C-terminus penta-peptide, which is compatible for mass-spectrometry (MS) detection [33, 39,40,41]. We employed mSUMO3 in this study because its transient expression produced more abundant protein than was produced by mSUMO1 or mSUMO2, suggesting that its use may aid in SUMO substrate detection in HEK293 cells (Fig. 1A).
Rhes interacts with SUMOylated proteins. A Western blot of HEK293 cells expressing His-mSUMO1, His-mSUMO2, or His-mSUMO3. B Western blot for indicated proteins after glutathione-affinity pulldown in HEK293 cells expressing GST + His-mSUMO3 (control) or GST-Rhes + His-mSUMO3 and corresponding input (5%). C Volcano plot of proteins bound to affinity purified GST-Rhes (Rhes) co-expressing mSUMO3, compared to affinity purified GST co-expressing mSUMO3 (control), identified ~ 300 significant interactors by LC–MS/MS in biological triplicate. D Volcano plot of SUMOylated proteins that are bound to GST-Rhes (Rhes) + His-mSUMO3, compared to GST + His-mSUMO3 (control) identified by LC–MS/MS in biological triplicate. There was no substantial cut off in any of the proteins
First, we hypothesized that the SUMO proteins that bind to Rhes are the potential SUMO targets of Rhes. To examine this hypothesis, we co-expressed GST-Rhes or GST (control) with His-tagged mSUMO3 in HEK293 cells and affinity purified Rhes using a GST-glutathione-affinity column. We found that GST-Rhes readily bound to proteins that had been modified by mSUMO3 (Fig. 1B). The samples purified using the GST-Rhes affinity column was subjected to MS analysis to identify and quantify the interacting partners. We discovered several proteins that exhibit a strong and statistically significant binding affinity to Rhes, namely RAC1, TSC2, VDAC3, PLEC, DDX3X, and mTOR (Fig. 1C, Data file S1). These proteins have been previously recognized as interactors of Rhes [9, 10].
Attempts at identifying the interacting SUMOylated partners from the GST pulldown were futile. Although several targets, such as RPL3 (K399), histone H4 (K6, K9, and K13) and RANGAP1 (K524), were found SUMOylated, the log10-transformed p value for all of the targets all exceeded 0.05 (Fig. 1D, Data file S2). We reasoned that the SUMOylated proteins that are bound to Rhes are either very few in number or below the threshold needed for the detection by MS. Moreover, GST pulldown experiments are conducted under native conditions, which are also conditions under which the SUMO specific proteases (SENPs) are highly active and could deconjugate the SUMO moiety from the target proteins [42].
Mass spectrometry reveals nuclear SUMO targets and SUMO modified lysines of Rhes
We then enriched the SUMOylated proteins using a denaturing purification protocol. We co-expressed myc-Rhes or myc (control) with His-tagged mSUMO3 in HEK293 cells and lysed the cells in guanidine-hydrochloride/urea denaturing buffer, followed by purification using a Ni–NTA column. We found that the myc-Rhes expression significantly enhanced the overall SUMOylation of proteins (Fig. 2A, B) and that Rhes was itself SUMOylated (S* Rhes, Fig. 2A), consistent with our previous report [12]. However, whether the increased SUMOylation seen in total lysates can be attributed to overexpressed Rhes SUMOylation remained unclear.
Interactome and SUMO proteome identifies putative SUMO substrates of Rhes. A Western blot of His-tagged SUMO enrichment using Ni–NTA TALON beads in HEK293 cells expressing myc + His- mSUMO3 or myc-Rhes + His- mSUMO3. S*Rhes represents SUMOylated Rhes. B Quantification of overall SUMOylation in Ni–NTA enriched, myc + His- mSUMO3 or myc-Rhes + His-mSUMO3. Data represents means ± SEM, (n = 3), ***P < 0.001, Student’s t test. C Volcano plot of SUMOylation site changes in myc-Rhes + His-mSUMO3 compared to myc + His-mSUMO3 (control), identified by LC–MS/MS in biological triplicate. D High and low confidence SUMO substrates of Rhes identified in (C) . E Representation of Rhes domains with mapping of the SUMO sites identified. F Structure prediction of Rhes from AlphaFold with MS-identified SUMO sites. G Western blot of His-tagged SUMO enrichment using Ni–NTA TALON beads in HEK293 cells expressing myc + His- mSUMO3 or myc-Rhes + His- mSUMO3, myc-Rhes 5KR + His- mSUMO3 or myc-Rhes 8KR + His- mSUMO3. S*Rhes represents SUMOylated Rhes
We identified the SUMO targets of Rhes by subjecting Ni–NTA-enriched myc and myc-Rhes samples (Fig. 2C) to SUMO peptide immunopurification using a custom antibody that recognizes the NQTGG remnant created on the modified lysine residue during trypsin digestion. Identification and quantification of the SUMOylation site by MS analysis identified several SUMOylated proteins showing a > fourfold change increase in SUMOylation, including 13 targets in myc-Rhes–expressing cells, compared to the myc control (Fig. 2D, Data file S3). We identified SUMO-regulated sites using a fold change in abundance > 4 between replicate analyses with p values < 0.05 (high confidence), while low confidence sites displayed p values < 0.05 and a fold change in abundance > 0 but < 4.
Among the high-confidence SUMO substrates, we found that Rhes increased the RapGEF5 SUMOylation at the lysine 422 and lysine 428 residues that are part of catalytic domain [40, 43]. RapGEF5 is a direct target of 3'-5'-cyclic adenosine monophosphate (cAMP) and is involved in cAMP-mediated signal transduction through activation of the Ras-like small GTPase RAPs: RAP1A, RAP1B, and RAP2A [41, 44]. We found that Rhes increased the SUMOylation at lysine 803 of diacylglycerol kinase eta (DGKH), an enzyme that generates phosphatidic acid (PA) and activates the Ras/B-Raf/C-Raf/MEK/ERK pathway. Rhes also increased the SUMOylation of CCDC54 (the coiled coil domain containing protein 54), a protein of unknown function, at lysine 218 (Fig. 2D).
Among the low-confidence SUMO targets, we found that Rhes promoted the SUMOylation of the SUMO-3 ligases PIASy (PIAS4) (K59) and RanBP2 (K1605), as well as the proteins polypbromo-1 (PBRM1, K1398, K1642), heterogenous nuclear ribonucleoprotein M (HNRNPM, K145, K698), histone-lysine N-methyltransferase (SETDB1, K1032), RNA polymerase II-associated factor 1 homolog (PAF1, K133), mothers against decapentaplegic homolog 4 (SMAD4, K113), and unconventional myosin 1b (K287) (Fig. 2D). Interestingly, Rhes was also one of the 4 high-confidence SUMOylated targets. Rhes was SUMOylated at six lysine residues (lys 32, 110, 114, 120, 124, 245 and 248) that spanned across the protein.
The MS/MS spectra of SUMOylated Rhes are presented in Supplementary Fig. 1. The GTPase domain of Rhes contains 5 SUMO modification sites, while the C-terminal SUMO E3-like region harbors a SUMOylation site (Fig. 2E); these sites are conserved across species (Supplementary Fig. 2). The Rhes AlphaFold structure also showed that K32, K120, and K124 are entrenched deeply in the structure's three-dimensional space, where they interact with other residues, whereas K110, K114, and K245 extend from the surface (Fig. 2F).
We then mutated these lysines to determine possible effects on the SUMOylation of Rhes. Surprisingly, the mutation of lysine at 32, 110, 114, 120, and 245 (5KR) or at 32, 110, 114, 120, 245, 174, 175, and 191 (8KR) did not diminish the SUMOylation of Rhes. Rhes contains up to 20 lysine residues (Supplementary Fig. 2), and the substitution of 8 of these lysines did not abolish SUMOylation, indicating that Rhes can also be modified by SUMOylation at multiple lysine residues. This phenomenon has also been observed for other SUMOylated proteins, such as H4, Cav-3, and α-synuclein [43,44,45].
Taken together, these data indicate that (a) Rhes modulates SUMOylation of substrates involved in cellular signaling, (b) Rhes is SUMOylated at multiple lysine residues and (c) the attachment of SUMO to a lysine residue is likely promiscuous within the Rhes protein.
Comparison of the Rhes interactome and SUMOylation targets for further refinement of potential new SUMO targets of Rhes
While the quantitative SUMO proteome analysis (Fig. 2) identified new potential SUMO targets of Rhes, their abundance is limited. These results are not entirely unexpected because of the hurdles that are inherent in SUMOylation studies, such as the low abundance of the SUMO modified targets and the highly dynamic nature of SUMOylation. Indeed, at any given time, only < 1% of the total proteins are modified, further complicating their identification [46]. Finally, SUMOylation of certain substrates in native tissues, such as the striatum, may also be under the strict control of extracellular signaling.
Keeping all these limitations in perspective, we sought to further identify the potential SUMO targets using an unbiased approach. Hypothesizing that the potential SUMO substrates of Rhes must interact with it, we selected the common proteins by comparing endogenous Rhes interactors in the mouse striatum [8] (Fig. 3A, (a)) with all the SUMOylated proteins in Rhes expressing HEK293 cells (Fig. 3C, (b)) (Data file S4). We have also included the high- and low-confidence SUMO substrates of Rhes in HEK293 cells (Fig. 2D). This cross-species protein comparison approach resulted in the identification of 40 novel substrates that are both SUMOylated and shown to interact with Rhes (Fig. 3B). The STRING analysis revealed strong protein–protein association networks and functional enrichment that included SUMO ligase activity, DNA binding activity, rRNA binding, 3’UTR binding, and translation initiation. Many of the targets, such as histone deacetylase 1 (HDAC1), PIASy, histone-2B, (H2B), HNRNPM, and PBRM1, have roles in gene regulation, indicating a potential nuclear function for Rhes.
Flow chart to identify potential SUMOylated protein targets of Rhes. A Proteins that interacts with endogenous Rhes in striatum Shahani, 2016 [9] (a) are compared with all the SUMOylated proteins in HEK293 cells (b) and obtained 40 novel targets that are SUMOylated as well as binds to Rhes. B The STRING database analysis of protein–protein interactions of 40 potential targets of Rhes, and C Shows the significant evidence for molecular and cellular functions. Interaction score at STRING was set as medium confidence (0.400)
Rhes regulates SUMOylation of nuclear targets in cells
We then investigated whether Rhes can directly modulate the SUMOylation of the nuclear targets identified in Fig. 4. We performed this using a Ni–NTA denaturing protocol, (Fig. 4) for the selected nuclear targets by expressing them in HEK293 cells.
Rhes regulates the SUMOylation of nuclear targets. A Western blot of Ni–NTA enrichment of SUMOylated proteins (S*) from the lysates obtained from HEK293 cells expressing myc + His-mSUMO3 or myc-Rhes + His-mSUMO3 constructs either with GFP-HDAC1, m-cherry H2B or GFP-H2A1.2. B. Bar graphs indicate quantification of SUMOylation (%) from (A). Data represents mean ± SEM, (n = 3), *P < 0.05, **P < 0.01, Student’s t test. n.s not significant. (C) Western blot of Ni–NTA enrichment of SUMOylated proteins from the lysates obtained from HEK293 cells expressing myc + His-mSUMO3 or myc-Rhes + His-mSUMO3 constructs either with V5-HNRNPM1, GFP-PBRM1 or Flag-PIASy. D Bar graphs indicate quantification of SUMOylation (%) from (C). Data represents mean ± SEM, (n = 3), **P < 0.01, ****P < 0.0001, Student’s t test
We found that Rhes increased the SUMOylation of HDAC1 and H2B but had no effect on the SUMOylation of histone H2A.1 (Fig. 4A, B). Surprisingly, Rhes decreased the overall SUMOylation of HNRNPM, PBRM1, and PIASy SUMOylation (Fig. 4B, C). We could not detect SUMOylation of other targets, including nucleophosmin (NPM), diacylglycerol kinase beta (DGKb), and ribosomal protein L26 (RPL26) (Supplementary Fig. 3). As expected, Rhes was readily SUMOylated under these experimental conditions (Fig. 4A–D). Taken together, these data indicated that Rhes differentially modulates the SUMOylation of substrates that are involved in nuclear functions, especially the regulation of gene expression.
Rhes regulates the expression of genes involved in neuronal morphogenesis in the striatum
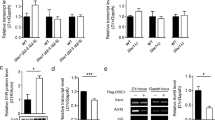
The finding that Rhes differentially regulates the SUMOylation of nuclear targets implicated in gene expression led us to hypothesize that Rhes may alter gene expression in the striatum in vivo. We addressed this possibility by isolating mRNA from WT and Rhes KO (Rhes–/– ) mice (RNA from 1 male and 1 female mouse was pooled per sample, with triplicate samples prepared for each group WT or Rhes−/−) and conducting high-throughput RNA-seq analysis. We found that the absence of Rhes significantly altered the gene expression profile in the striatum (Fig. 5A). Of the ~ 15,000 sequenced genes, 155 genes were significantly downregulated and 52 genes were significantly upregulated in the Rhes−/− mouse striatum compared to WT control mouse striatum (Fig. 5A, Data file S5). Ingenuity pathway analysis (IPA) showed the major hits to be the molecular and cellular functions involved in cell morphology, cellular development, growth and proliferation, and molecular transport (Fig. 5B). For example, genes involved in cell morphogenesis, including early growth response 2 (Egr2), necdin (Ndn), serum- and glucocorticoid-regulated kinase 1 (Sgk1), and netrin-1 (Ntn1), were upregulated [47,48,49,50,51], and neuropilin1 (Nrp1), basic-helix-loop helix 22 (Bhlhe22), and myocyte enhancer factor 2 (Mef2c) were downregulated [52,53,54,55,56] (Fig. 5A). Most genes, including ephrin type-A receptor 5 (Epha5) and slit guidance ligand 2 (Slit2), were unaffected. We also used qPCR for further validation of selected targets. We confirmed, for example, that Nrp1, Bhlhe22, Mef2c were downregulated, Egr2 was upregulated, and exostosin glycosyltransferase 1 (Ext1) showed a decreased trend) in the striatum of Rhes–/– mice compared to WT striatum (Fig. 5C). These data indicate that Rhes regulates the expression of genes involved in a variety of biological processes and has a prominent role in determining cellular morphology in the striatum.
Rhes regulates genes involved in cellular differentiation and morphogenesis via SUMOylation. A Mean normalized counts of gene expression based on RNA seq data from Rhes KO (Rhes−/−) vs. WT (Rhes+/+) striatum. B Functional analysis of the molecular and cellular functions altered in striatum of Rhes KO mice compared to WT mice from (A) in Ingenuity Pathway Analysis. C Expression of indicated genes (normalized to Gapdh), involved in cell morphology and cellular development were validated by qPCR in Rhes KO vs. WT mice striatum. Error bar represents mean ± SEM, (n = 3), *P < 0.05, **p < 0.01 by Student t test. D Gene expression data for the indicated genes (normalized to Gapdh) in WT or SUMO 1, 2, 3 KO (Δ) cells to assess the effect of SUMOylation in presence of GFP or GFP-Rhes. Error bar represents mean ± SEM, (n = 3), *P < 0.05 compared to GFP in WT or SUMO 1, 2, 3 Δ cells ##P < 0.01 between Rhes in WT and Rhes in SUMO1,2,3 Δ cells by One-Way ANOVA followed by Newman-Keuls multiple comparison test
Rhes regulates a selected gene expression via SUMO
Because Rhes regulates the SUMOylation of several proteins that are involved in gene expression such as HDAC1, HNRNPM, PBRM1 and PIASy [57,58,59,60,61,62,63,64,65], we investigated whether Rhes alters gene expression via SUMO. We compared CRISPR/Cas-9–control (WT cells) and CRISPR/Cas-9 SUMO1/2/3–depleted striatal neuronal cells that show ~ 60–70% of loss of SUMO (SUMO Δ 1, 2, 3 cells) [19]. We analyzed the effect of Rhes on the expression of Ext1, Mef2c, Slit2, Epha5, and Bhlhe22 in WT and SUMO Δ 1, 2, 3 cells by transfecting the cells with GFP or GFP-Rhes and sorting using flow cytometry to obtain enriched population of cells expressing GFP or GFP-Rhes. While Rhes increased the expression of Mef2c and Bhlhe22 in WT cells, it failed to do so in the SUMO Δ cells (Fig. 5D). This result indicated that Rhes positively modulated the gene expression of Bhlhe22 and Mef2c via SUMO. Rhes increased Epha5 and Ext1 in both the control and the SUMO Δ 1, 2, 3 cells, indicating that Rhes promoted Epha5 and Ext1 expression through a mechanism that was independent of SUMO (Fig. 5D). Furthermore, while Slit2 was induced by Rhes in control cells, a high basal Slit2 expression was found in SUMO Δ cells that was not affected by Rhes expression (Fig. 5D). Collectively, these results indicate that Rhes regulates gene expression via SUMO-dependent and SUMO-independent mechanisms.
Rhes is enriched in the perinuclear membrane fractions
Our finding that Rhes mostly regulated the gene expression of selected nuclear targets prompted further investigation of whether Rhes is localized in the nucleus. Using biochemical tools, we isolated and separated cytoplasmic and nuclear fractions of the striatum from Rhes+/+ (WT) and Rhes−/− (KO) mice (Fig. 6A). Using western blotting, we confirmed that Rhes was highly enriched in the nuclear fractions that were positive for histone H3 (Fig. 6A). Rhes was also observed in the cytoplasmic fractions that were enriched for the cytoplasmic markers mTOR, mitogen-activated protein kinase kinase (MEK), and lactate dehydrogenase (LDH) (Fig. 6A).
Rhes is preferentially localized around the perinuclear membrane. A Subcellular localization of Rhes was assessed in cytosolic and nuclear fractions from Rhes KO (Rhes−/−) vs. WT (Rhes+/+) mice striatum. The cytosolic markers MEK and LDH and the nuclear marker Histone H3 were probed to validate proper cellular fractionation. B Representative confocal and brightfield (DIC) image of striatal neuronal cell expressing GFP-Rhes, indicating the localization of Rhes on nuclear membrane and perinuclear space. Inset shows the boxed area at higher magnification. Arrow indicate perinuclear localization of Rhes, and arrowhead indicates the localization of Rhes on plasma membrane. C Model depicting that Rhes can SUMOylate its targets, including HDAC1, in the cytoplasm and promotes its entry into the nucleus to regulate gene expression
Because biochemically isolated subcellular fractions do not unmistakably report the distribution of subcellular proteins, we also verified the localization of Rhes using confocal fluorescence microscopy. Imaging analysis revealed that GFP-tagged-Rhes was predominantly associated with perinuclear membranes, with a negligible presence in the nucleus (Fig. 6B, arrows). In addition to its perinuclear localization, Rhes was also enriched in the plasma membrane (Fig. 6B, arrowhead) and in membranous vesicles, consistent with our previous reports [19, 63]. Taken together, our results indicate that Rhes is predominantly localized on the perinuclear membranes, indicating that Rhes may affect the SUMOylation of nuclear targets located in the perinuclear region (Fig. 6C).
Discussion
The data presented here demonstrate the following: (a) Rhes is SUMOylated on multiple lysine sites; (b) Rhes regulates the SUMOylation of nuclear proteins, and (c) Rhes regulates gene expression in the striatum at least partly via SUMO-mediated mechanisms. Nevertheless, Rhes is well known to undergo post-translational modification in vivo [9, 64], although the nature of this modification remains unknown. This findings from the present study indicate that SUMO may contribute to this in vivo Rhes modification.
Interestingly, the presence of multiple closely spaced SUMO modification sites on Rhes at K110, K114, K120, and K124 indicates that these residues may act as anchors that can associate with SUMO-interacting motifs (SIMs), which have known involvements in protein–protein interactions [65, 66]. These closely spaced SUMOylation events are also found on other SUMO E3 ligases, such as the PIAS1 lysines K40, K46, K56, and K58; the PIASy lysines K59, K69, K128, K135; and the RanBP2 lysines K1596,d K1605, K2513, K2531, K2571, and K2592 [67, 68]. Thus, multiple lysine SUMO modifications appear to be a characteristic feature in many SUMO E3-like proteins. However, further study is needed to identify the role of each lysine modification and whether Rhes SUMOylates itself or if other potential SUMO E3 ligases SUMOylate Rhes. Other unanswered questions include, for instance, whether SUMOylation of Rhes regulates its activity toward mTORC1 kinase [10], TNT formation [19], or gene expression (Fig. 5). SUMOylation site mutants continue to attach to targets such as HDAC1 or H2b (Supplementary Fig. 4), indicating that alternative SUMO sites on Rhes may still connect to targets and presumably influence Rhes activity. These studies suggest that SUMOylation is flexible and can occur beyond a specified lysine, perhaps providing an evolutionary benefit for SUMO posttranslational modifications in molecular and cellular processes, while the exact cause of this flexibility is unknown.
Intriguingly, we report that Rhes differentially alters the SUMOylation of SUMO E3 ligases. Rhes decreased the overall SUMOylation on PIASy in the cells (Fig. 4B), while promoting the specific SUMOylation of PIASy on lysine 59 (Fig. 2D). Similarly, MS analysis revealed that Rhes significantly increased the SUMOylation of RanBP2 on lysine 1605, but not on lysine 2592 (Fig. 2D, Data file S3). These results indicate that Rhes may differentially affect the SUMOylation of SUMO E3 ligase(s) on specific lysine targets. An interesting future strategy will be to test whether PIASy or RanBP2 can act as a SUMO E3 ligase for Rhes. Even though SUMOylation is known to modify SUMO E3 ligases, the biological significance of the resulting modifications remains poorly understood. Previously, we reported that Rhes promoted cross-SUMOylation between the SUMO E1 (Aos/Uba9) and E2 (Ubc9) ligases and predicted that this regulation might work as a form of “symbiotic” regulation between two evolutionarily conserved SUMO E1 and E2 enzymes [25]. Based on the data presented here, we propose that Rhes may also promote reciprocal and symbiotic regulations between SUMO E3 ligases through SUMO modification and may therefore have a significant impact on the regulation of complex cellular and behavioral functions of the striatum via protein–protein interactions [9].
The results presented here clearly indicate that Rhes is involved in the regulation of gene expression in the striatum and that, intriguingly, it can both increase and decrease striatal gene expression. A previous independent study also reported that Rhes might inhibit gene expression by acting as a cis modulator [69], but the mechanisms were unclear. SUMOylation is a well-established participant in cis-regulation and is involved in both transcriptional repression and activation functions [46, 70, 71]. As Rhes KO cells show an overall diminishment of SUMOylation in the striatum [25], as well as altered gene expressions, we predict that the Rhes-SUMO pathway may regulate gene expression by functioning as a cis-modulator, possibly via the SUMOylation of transcription factors. Consistent with this notion, although our proteomics analysis did not reveal a significant alteration of HDAC1 SUMOylation on lysine 89 or lysine 476 (Data file S3), presumably due to low stoichiometry, our biochemical experiments showed that Rhes enhanced the SUMOylation of HDAC1 (Fig. 2B). Thus, Rhes may alter HDAC1 activity via SUMOylation at lysine 89 and at lysine 476, which is a catalytically essential residue involved in gene repression [72,73,74].
Previous studies have indicated an involvement of SUMOylation of H2B in the repression of gene expression [45]. Because we found that Rhes increases the SUMOylation of H2B (Fig. 3), which is SUMOylated at multiple lysine residues (Data file S4), including the previously reported lysine 6 [45], we propose that Rhes-SUMO pathway may affect gene expression via more than one nuclear target. However, the cellular compartment in which Rhes regulates the SUMOylation of nuclear proteins remains to be elucidated. Our finding that Rhes is enriched in the perinuclear space (Fig. 6B) leads us to predict that this perinuclear location may serve as the prime site for protein SUMOylation (Fig. 6C). In support of this notion, previous studies have shown that the SUMO E3 ligase RanBP2 is localized on the perinuclear location associated with the cytoplasmic side of the nuclear pore complex (NPC)—a complex that regulates the import and export of proteins and mediates global gene expression in cell models [75, 76]. RanBP2 also enhances the SUMOylation of HDAC4 and HNRNPM proteins, which are involved in mRNA splicing and transport [77, 78]. Although localization of Rhes on the NPC is not currently documented, we found that striatal Rhes coimmunoprecipitates with RanBP2 and Sec13 [9], two known components of the NPC. Rhes has also shown interactions with NPC-associated proteins, including HNRNP (L1 and H2 isoforms), during motor stimulation in the striatum [9]. Thus, we predict that Rhes may associate with the NPC to regulate the SUMOylation of targets involved in gene regulation, as well as mRNA splicing, another process shown to require SUMOylation [79]. In addition, Rhes may alter SUMO-independent signaling, perhaps by modulating mTOR and PKA signaling pathways that are linked to gene expression [7, 10, 80,81,82].
Aberrant SUMOylation has been linked to a variety of diseases, including cancer and neurodegenerative diseases. Blocking Rhes-mediated mHTT SUMOylation or the Rhes-E2 enzyme Ubc9 may therefore provide protection against HD [12, 25, 83]. Recent research has shown that HD is associated with significant changes in the nuclear envelope shape, aberrant localization of Ran guanosine triphosphatase (GTPase)-activating protein (RanGAP1), and improper nuclear export [84,85,86]. Since Rhes boosts the SUMOylation of RanGAP1 and binds to its SUMO E3 ligase, RanBP2, we anticipate that interfering with Rhes SUMOylation activities will likely benefit HD. Furthermore, the RanBP2/RanGAP1/Ubc9 SUMO E3 ligase pathway functions as a disassembler for nuclear pore export complexes [87]. Thus, Rhes, together with the RanBP2/RanGAP1/Ubc9 complexes, may aberrantly influence nuclear export pathways in HD, thereby acting as an attractive target for therapeutic interference.
Our new findings identify potential regulators of Rhes-mediated TNT-like membranous communication [19, 20]. We predict that Rhes may mediate the formation of TNT-like protrusions and the cell-to-cell transport of cargoes by a SUMO-dependent regulation of the expression of transcription factors, such as Mecf2, Egr2, Bhlhe22, which are known regulators of neuronal morphology and differentiation. Consistent with this notion, depletion of SUMO diminishes both the formation of TNT-like protrusions [19] and cell-to-cell transport, while also altering the Rhes-mediated expression of Mef2c and Bhlhe22 (Fig. 5).
Collectively, our results, obtained by a combination of high throughput interactomics, SUMO proteomics, gene expression analysis, and biochemical tools, demonstrate that Rhes promotes the SUMOylation of nuclear substrates involved in gene expression. Thus, Rhes may impact striatal function and dysfunction associated with neurological and neurodegenerative diseases via the SUMO-mediated regulation of gene expression.
Data availability
Enquiries about data availability should be directed to the authors.
References
Harrison LM (2012) Rhes: a GTP-binding protein integral to striatal physiology and pathology. Cell Mol Neurobiol 32(6):907–918
Usui H et al (1994) Isolation of clones of rat striatum-specific mRNAs by directional tag PCR subtraction. J Neurosci 14(8):4915–4926
Sciamanna G et al (2015) Rhes regulates dopamine D2 receptor transmission in striatal cholinergic interneurons. Neurobiol Dis 78:146–161
Subramaniam S (2022) Striatal Induction and Spread of the Huntington's Disease Protein: A Novel Rhes Route. J Huntingtons Dis
Vargiu P et al (2004) The small GTP-binding protein, Rhes, regulates signal transduction from G protein-coupled receptors. Oncogene 23(2):559–568
Errico F et al (2008) The GTP-binding protein Rhes modulates dopamine signalling in striatal medium spiny neurons. Mol Cell Neurosci 37(2):335–345
Ghiglieri V et al (2015) Rhes influences striatal cAMP/PKA-dependent signaling and synaptic plasticity in a gender-sensitive fashion. Sci Rep 5:10933
Harrison LM, He Y (2011) Rhes and AGS1/Dexras1 affect signaling by dopamine D1 receptors through adenylyl cyclase. J Neurosci Res 89(6):874–882
Shahani N et al (2016) RasGRP1 promotes amphetamine-induced motor behavior through a Rhes interaction network (“Rhesactome”) in the striatum. Sci Signal 9(454):ra111
Subramaniam S et al (2011) Rhes, a striatal-enriched small G protein, mediates mTOR signaling and L-DOPA-induced dyskinesia. Nat Neurosci 15(2):191–193
Eshragi M et al. (2019) RasGRP1 is a Causal Factor in the Development of L-DOPA-induced dyskinesia in Parkinson Disease. Sci Adv In press
Subramaniam S et al (2009) Rhes, a striatal specific protein, mediates mutant-huntingtin cytotoxicity. Science 324(5932):1327–1330
Ramirez-Jarquin UN et al (2022) Deletion of SUMO1 attenuates behavioral and anatomical deficits by regulating autophagic activities in Huntington disease. Proc Natl Acad Sci U S A 119:5
Ehrenberg AJ et al (2021) Patterns of neuronal Rhes as a novel hallmark of tauopathies. Acta Neuropathol 141(5):651–666
Hernandez I et al (2019) A farnesyltransferase inhibitor activates lysosomes and reduces tau pathology in mice with tauopathy. Sci Transl Med 11:485
Liu YL et al (2008) RASD2, MYH9, and CACNG2 genes at chromosome 22q12 associated with the subgroup of schizophrenia with non-deficit in sustained attention and executive function. Biol Psychiatry 64(9):789–796
Vitucci D et al (2016) Rasd2 Modulates Prefronto-Striatal Phenotypes in Humans and “Schizophrenia-Like Behaviors” in Mice. Neuropsychopharmacology 41(3):916–927
Vadgama N et al (2019) De novo single-nucleotide and copy number variation in discordant monozygotic twins reveals disease-related genes. Eur J Hum Genet 27(7):1121–1133
Sharma M, Subramaniam S (2019) Rhes travels from cell to cell and transports Huntington disease protein via TNT-like protrusion. J Cell Biol 218(6):1972–1993
Ramirez-Jarquin UN et al (2022) Rhes protein transits from neuron to neuron and facilitates mutant huntingtin spreading in the brain. Sci Adv 8(12):eabm3877
Subramaniam S (2020) Rhes Tunnels: A Radical New Way of Communication in the Brain’s Striatum? BioEssays 42(6):e1900231
Chapple CE et al (2015) Extreme multifunctional proteins identified from a human protein interaction network. Nat Commun 6:7412
Issaeva N (2019) p53 Signaling in Cancers. Cancers (Basel) 11:3
Santucci R et al (2019) Cytochrome c: An extreme multifunctional protein with a key role in cell fate. Int J Biol Macromol 136:1237–1246
Subramaniam S et al (2010) Rhes, a physiologic regulator of sumoylation, enhances cross-sumoylation between the basic sumoylation enzymes E1 and Ubc9. J Biol Chem 285(27):20428–20432
Mealer RG et al (2014) Rhes, a striatal-selective protein implicated in Huntington disease, binds beclin-1 and activates autophagy. J Biol Chem 289(6):3547–3554
Galisson F et al (2011) A novel proteomics approach to identify SUMOylated proteins and their modification sites in human cells. Mol Cell Proteomics 10(2):M110-004796
Lamoliatte F et al (2013) Targeted identification of SUMOylation sites in human proteins using affinity enrichment and paralog-specific reporter ions. Mol Cell Proteomics 12(9):2536–2550
Tammsalu T et al (2015) Proteome-wide identification of SUMO modification sites by mass spectrometry. Nat Protoc 10(9):1374–1388
Lamoliatte F et al (2014) Large-scale analysis of lysine SUMOylation by SUMO remnant immunoaffinity profiling. Nat Commun 5:5409
Lamoliatte F et al (2017) Uncovering the SUMOylation and ubiquitylation crosstalk in human cells using sequential peptide immunopurification. Nat Commun 8:14109
Li C et al (2020) Quantitative SUMO proteomics identifies PIAS1 substrates involved in cell migration and motility. Nat Commun 11(1):834
McManus FP et al (2018) Quantitative SUMO proteomics reveals the modulation of several PML nuclear body associated proteins and an anti-senescence function of UBC9. Sci Rep 8(1):7754
Swarnkar S et al (2015) Ectopic expression of the striatal-enriched GTPase Rhes elicits cerebellar degeneration and an ataxia phenotype in Huntington’s disease. Neurobiol Dis 82:66–77
Krämer A et al (2014) Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30(4):523–530
Cox J, Mann M (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26(12):1367–1372
Rabellino A, Andreani C, Scaglioni PP (2017) The Role of PIAS SUMO E3-Ligases in Cancer. Cancer Res 77(7):1542–1547
Bouchard D et al (2021) SUMO paralogue-specific functions revealed through systematic analysis of human knockout cell lines and gene expression data. Mol Biol Cell 32(19):1849–1866
McManus FP, Lamoliatte F, Thibault P (2017) Identification of cross talk between SUMOylation and ubiquitylation using a sequential peptide immunopurification approach. Nat Protoc 12(11):2342–2358
Nguyen HB, QLA (2012) Encyclopedia of Signaling Molecules. Springer, New York, NY
Caron E (2003) Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J Cell Sci 116(Pt 3):435–440
Jia Y et al (2019) Chemical Tools and Biochemical Assays for SUMO Specific Proteases (SENPs). ACS Chem Biol 14(11):2389–2395
Fuhs SR, Insel PA (2011) Caveolin-3 undergoes SUMOylation by the SUMO E3 ligase PIASy: sumoylation affects G-protein-coupled receptor desensitization. J Biol Chem 286(17):14830–14841
Krumova P et al (2011) Sumoylation inhibits alpha-synuclein aggregation and toxicity. J Cell Biol 194(1):49–60
Nathan D et al (2006) Histone sumoylation is a negative regulator in Saccharomyces cerevisiae and shows dynamic interplay with positive-acting histone modifications. Genes Dev 20(8):966–976
Geiss-Friedlander R, Melchior F (2007) Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol 8(12):947–956
Quach DH et al (2013) A sympathetic neuron autonomous role for Egr3-mediated gene regulation in dendrite morphogenesis and target tissue innervation. J Neurosci 33(10):4570–4583
Kuwajima T, Nishimura I, Yoshikawa K (2006) Necdin promotes GABAergic neuron differentiation in cooperation with Dlx homeodomain proteins. J Neurosci 26(20):5383–5392
Heikamp EB et al (2014) The AGC kinase SGK1 regulates TH1 and TH2 differentiation downstream of the mTORC2 complex. Nat Immunol 15(5):457–464
Lejard V et al (2011) EGR1 and EGR2 involvement in vertebrate tendon differentiation. J Biol Chem 286(7):5855–5867
Yamauchi K et al (2017) Netrin-1 Derived from the Ventricular Zone, but not the Floor Plate, Directs Hindbrain Commissural Axons to the Ventral Midline. Sci Rep 7(1):11992
Fantin A, Maden CH, Ruhrberg C (2009) Neuropilin ligands in vascular and neuronal patterning. Biochem Soc Trans 37(Pt 6):1228–1232
Das G et al (2016) EphA5 and EphA6: regulation of neuronal and spine morphology. Cell Biosci 6:48
Dennis DJ, Han S, Schuurmans C (2019) bHLH transcription factors in neural development, disease, and reprogramming. Brain Res 1705:48–65
Li H et al (2008) Transcription factor MEF2C influences neural stem/progenitor cell differentiation and maturation in vivo. Proc Natl Acad Sci U S A 105(27):9397–9402
Ozdinler PH, Erzurumlu RS (2002) Slit2, a branching-arborization factor for sensory axons in the Mammalian CNS. J Neurosci 22(11):4540–4549
Sun L et al (2013) PIASy mediates hypoxia-induced SIRT1 transcriptional repression and epithelial-to-mesenchymal transition in ovarian cancer cells. J Cell Sci 126(Pt 17):3939–3947
Yan C et al (2016) Protein Inhibitor of Activated STAT Y (PIASy) Regulates Insulin Secretion by Interacting with LIM Homeodomain Transcription Factor Isl1. Sci Rep 6:39308
Chowdhury B et al (2016) PBRM1 Regulates the Expression of Genes Involved in Metabolism and Cell Adhesion in Renal Clear Cell Carcinoma. PLoS ONE 11(4):e0153718
West KO et al (2019) The Splicing Factor hnRNP M Is a Critical Regulator of Innate Immune Gene Expression in Macrophages. Cell Rep 29(6):1594–1609
Zupkovitz G et al (2006) Negative and positive regulation of gene expression by mouse histone deacetylase 1. Mol Cell Biol 26(21):7913–7928
Greer CB et al (2015) Histone Deacetylases Positively Regulate Transcription through the Elongation Machinery. Cell Rep 13(7):1444–1455
Sharma M et al (2019) Rhes, a striatal-enriched protein, promotes mitophagy via Nix. Proc Natl Acad Sci U S A 116(47):23760–23771
Spano D et al (2004) Rhes is involved in striatal function. Mol Cell Biol 24(13):5788–5796
Hickey CM, Wilson NR, Hochstrasser M (2012) Function and regulation of SUMO proteases. Nat Rev Mol Cell Biol 13(12):755–766
Hecker CM et al (2006) Specification of SUMO1- and SUMO2-interacting motifs. J Biol Chem 281(23):16117–16127
Kumar R et al (2017) The STUbL RNF4 regulates protein group SUMOylation by targeting the SUMO conjugation machinery. Nat Commun 8(1):1809
Hendriks IA et al (2014) Uncovering global SUMOylation signaling networks in a site-specific manner. Nat Struct Mol Biol 21(10):927–936
Ciobanu DC et al (2010) Detection, validation, and downstream analysis of allelic variation in gene expression. Genetics 184(1):119–128
Liu HW et al (2012) Chromatin modification by SUMO-1 stimulates the promoters of translation machinery genes. Nucleic Acids Res 40(20):10172–10186
Rosonina E et al (2017) Regulation of transcription factors by sumoylation. Transcription 8(4):220–231
David G, Neptune MA, DePinho RA (2002) SUMO-1 modification of histone deacetylase 1 (HDAC1) modulates its biological activities. J Biol Chem 277(26):23658–23663
Joung H et al (2018) Sumoylation of histone deacetylase 1 regulates MyoD signaling during myogenesis. Exp Mol Med 50(1):e427
Tao CC et al (2017) Epigenetic regulation of HDAC1 SUMOylation as an endogenous neuroprotection against Abeta toxicity in a mouse model of Alzheimer’s disease. Cell Death Differ 24(4):597–614
Zhang R, Mehla R, Chauhan A (2010) Perturbation of host nuclear membrane component RanBP2 impairs the nuclear import of human immunodeficiency virus -1 preintegration complex (DNA). PLoS ONE 5(12):e15620
Turpin P, Ossareh-Nazari B, Dargemont C (1999) Nuclear transport and transcriptional regulation. FEBS Lett 452(1–2):82–86
Vassileva MT, Matunis MJ (2004) SUMO modification of heterogeneous nuclear ribonucleoproteins. Mol Cell Biol 24(9):3623–3632
Kirsh O et al (2002) The SUMO E3 ligase RanBP2 promotes modification of the HDAC4 deacetylase. EMBO J 21(11):2682–2691
Pozzi B et al (2017) SUMO conjugation to spliceosomal proteins is required for efficient pre-mRNA splicing. Nucleic Acids Res 45(11):6729–6745
Laplante M, Sabatini DM (2013) Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci 126(Pt 8):1713–1719
Kunkel J, Luo X, Capaldi AP (2019) Integrated TORC1 and PKA signaling control the temporal activation of glucose-induced gene expression in yeast. Nat Commun 10(1):3558
Yapo C et al (2018) Switch-like PKA responses in the nucleus of striatal neurons. J Cell Sci 131:14
Carbo M et al (2019) Bioinformatics analysis of Ras homologue enriched in the striatum, a potential target for Huntington’s disease therapy. Int J Mol Med 44(6):2223–2233
Gasset-Rosa F et al (2017) Polyglutamine-Expanded Huntingtin Exacerbates Age-Related Disruption of Nuclear Integrity and Nucleocytoplasmic Transport. Neuron 94(1):48–57
Grima JC et al (2017) Mutant Huntingtin Disrupts the Nuclear Pore Complex. Neuron 94(1):93–107
Cornett J et al (2005) Polyglutamine expansion of huntingtin impairs its nuclear export. Nat Genet 37(2):198–204
Ritterhoff T et al (2016) The RanBP2/RanGAP1*SUMO1/Ubc9 SUMO E3 ligase is a disassembly machine for Crm1-dependent nuclear export complexes. Nat Commun 7:11482
Acknowledgements
We would like to thank Trish Macdonald and Niki Mabie for their administrative help and the members of the lab for their continuous support and collaborative atmosphere.
Funding
This research was supported by funding from National Institutes of Health/National Institute of Neurological Disorders and Stroke grant R01-NS087019-01A1, National Institutes of Health/National Institute of Neurological Disorders and Stroke grant R01-NS094577-01A1, and a grant from Cure Huntington Disease Initiative (CHDI) foundation, a grant from NIH/NINDS 3R01NS128225-02S1 and a grant from NIH/NINDS 1R21NS128564-01.
Author information
Authors and Affiliations
Contributions
S.S conceptualized and designed the project. O.R carried out all the biochemical SUMO work. M.S carried out mRNA seq, qPCR, SUMO-KO cell generation and confocal microscopy. S.D generated lysine mutants of Rhes and assisted in SUMOylation experiments. N.S carried out cell culture and Rhes compartmentalization experiments. UNRJ assisted in mRNA seq and cell culture experiments. F.M and T.P designed the mass spec (MS) compatible his-mSUMO3 construct and carried out MS analysis of Rhes interactors and identification of SUMO. G.C assisted in bioinformatics. E.B participated in the SUMO bioinformatics study. P.K generated mRNA seq library and sequencing. S.S wrote the paper with input from the co-authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rivera, O., Sharma, M., Dagar, S. et al. Rhes, a striatal enriched protein, regulates post-translational small-ubiquitin-like-modifier (SUMO) modification of nuclear proteins and alters gene expression. Cell. Mol. Life Sci. 81, 169 (2024). https://doi.org/10.1007/s00018-024-05181-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-024-05181-8