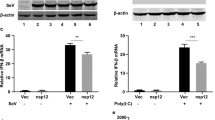
Abstract
Evasion and antagonism of host cellular immunity upon SARS-CoV-2 infection provide replication advantage to the virus and contribute to COVID-19 pathogenesis. We explored the ability of different SARS-CoV-2 proteins to antagonize the host’s innate immune system and found that the ORF6 protein mitigated type-I Interferon (IFN) induction and downstream IFN signaling. Our findings also corroborated previous reports that ORF6 blocks the nuclear import of IRF3 and STAT1 to inhibit IFN induction and signaling. Here we show that ORF6 directly interacts with RIG-I and blocks downstream type-I IFN induction and signaling by reducing the levels of K63-linked ubiquitinated RIG-I. This involves ORF6-mediated targeting of E3 ligase TRIM25 for proteasomal degradation, which was also observed during SARS-CoV-2 infection. The type-I IFN antagonistic activity of ORF6 was mapped to its C-terminal cytoplasmic tail, specifically to amino acid residues 52–61. Overall, we provide new insights into how SARS-CoV-2 inhibits type-I IFN induction and signaling through distinct actions of the viral ORF6 protein.








Similar content being viewed by others
Data availability
All primary data associated with this study have been included in the manuscript. Any additional information query can be directed to corresponding author.
References
Coronaviridae Study Group of the International Committee on Taxonomy of V (2020) The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 5:536–544. https://doi.org/10.1038/s41564-020-0695-z
Finkel Y, Mizrahi O, Nachshon A, Weingarten-Gabbay S, Morgenstern D, Yahalom-Ronen Y et al (2021) The coding capacity of SARS-CoV-2. Nature 589:125–130. https://doi.org/10.1038/s41586-020-2739-1
Li JY, Zhou ZJ, Wang Q, He QN, Zhao MY, Qiu Y et al (2021) Innate immunity evasion strategies of highly pathogenic coronaviruses: SARS-CoV, MERS-CoV, and SARS-CoV-2. Front Microbiol 12:770656. https://doi.org/10.3389/fmicb.2021.770656
Thorne LG, Reuschl AK, Zuliani-Alvarez L, Whelan MVX, Turner J, Noursadeghi M et al (2021) SARS-CoV-2 sensing by RIG-I and MDA5 links epithelial infection to macrophage inflammation. EMBO J 40:e107826. https://doi.org/10.15252/embj.2021107826
Yin X, Riva L, Pu Y, Martin-Sancho L, Kanamune J, Yamamoto Y et al (2021) MDA5 governs the innate immune response to SARS-CoV-2 in lung epithelial cells. Cell Rep 34:108628. https://doi.org/10.1016/j.celrep.2020.108628
Kouwaki T, Nishimura T, Wang G, Oshiumi H (2021) RIG-I-Like receptor-mediated recognition of viral genomic RNA of severe acute respiratory syndrome coronavirus-2 and viral escape from the host innate immune responses. Front Immunol 12:700926. https://doi.org/10.3389/fimmu.2021.700926
Hiscott J, Nguyen TL, Arguello M, Nakhaei P, Paz S (2006) Manipulation of the nuclear factor-kappaB pathway and the innate immune response by viruses. Oncogene 25:6844–6867. https://doi.org/10.1038/sj.onc.1209941
Platanias LC (2005) Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 5:375–386. https://doi.org/10.1038/nri1604
Darnell JE Jr, Kerr IM, Stark GR (1994) Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415–1421. https://doi.org/10.1126/science.8197455
Garcia-Sastre A (2017) Ten strategies of interferon evasion by viruses. Cell Host Microbe 22:176–184. https://doi.org/10.1016/j.chom.2017.07.012
Lei X, Dong X, Ma R, Wang W, Xiao X, Tian Z et al (2020) Activation and evasion of type I interferon responses by SARS-CoV-2. Nat Commun 11:3810. https://doi.org/10.1038/s41467-020-17665-9
Xia H, Cao Z, Xie X, Zhang X, Chen JY, Wang H et al (2020) Evasion of type I interferon by SARS-CoV-2. Cell Rep 33:108234. https://doi.org/10.1016/j.celrep.2020.108234
V’Kovski P, Kratzel A, Steiner S, Stalder H, Thiel V (2021) Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol 19:155–170. https://doi.org/10.1038/s41579-020-00468-6
Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P et al (2020) Genome composition and divergence of the novel coronavirus (2019-nCoV) Originating in China. Cell Host Microbe 27:325–328. https://doi.org/10.1016/j.chom.2020.02.001
Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L et al (2007) TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446:916–920. https://doi.org/10.1038/nature05732
Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM et al (2020) A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583:459–468. https://doi.org/10.1038/s41586-020-2286-9
Versteeg GA, Rajsbaum R, Sanchez-Aparicio MT, Maestre AM, Valdiviezo J, Shi M et al (2013) The E3-ligase TRIM family of proteins regulates signaling pathways triggered by innate immune pattern-recognition receptors. Immunity 38:384–398. https://doi.org/10.1016/j.immuni.2012.11.013
Edelheit O, Hanukoglu A, Hanukoglu I (2009) Simple and efficient site-directed mutagenesis using two single-primer reactions in parallel to generate mutants for protein structure-function studies. BMC Biotechnol 9:61. https://doi.org/10.1186/1472-6750-9-61
Case JB, Bailey AL, Kim AS, Chen RE, Diamond MS (2020) Growth, detection, quantification, and inactivation of SARS-CoV-2. Virology 548:39–48. https://doi.org/10.1016/j.virol.2020.05.015
Liu G, Lee JH, Parker ZM, Acharya D, Chiang JJ, van Gent M et al (2021) ISG15-dependent activation of the sensor MDA5 is antagonized by the SARS-CoV-2 papain-like protease to evade host innate immunity. Nat Microbiol 6:467–478. https://doi.org/10.1038/s41564-021-00884-1
Moustaqil M, Ollivier E, Chiu HP, Van Tol S, Rudolffi-Soto P, Stevens C et al (2021) SARS-CoV-2 proteases PLpro and 3CLpro cleave IRF3 and critical modulators of inflammatory pathways (NLRP12 and TAB1): implications for disease presentation across species. Emerg Microbes Infect 10:178–195. https://doi.org/10.1080/22221751.2020.1870414
Fu YZ, Wang SY, Zheng ZQ, Yi H, Li WW, Xu ZS et al (2021) SARS-CoV-2 membrane glycoprotein M antagonizes the MAVS-mediated innate antiviral response. Cell Mol Immunol 18:613–620. https://doi.org/10.1038/s41423-020-00571-x
Hayn M, Hirschenberger M, Koepke L, Nchioua R, Straub JH, Klute S et al (2021) Systematic functional analysis of SARS-CoV-2 proteins uncovers viral innate immune antagonists and remaining vulnerabilities. Cell Rep 35:109126. https://doi.org/10.1016/j.celrep.2021.109126
Li JY, Liao CH, Wang Q, Tan YJ, Luo R, Qiu Y et al (2020) The ORF6, ORF8 and nucleocapsid proteins of SARS-CoV-2 inhibit type I interferon signaling pathway. Virus Res 286:198074. https://doi.org/10.1016/j.virusres.2020.198074
Shemesh M, Aktepe TE, Deerain JM, McAuley JL, Audsley MD, David CT et al (2021) Correction: SARS-CoV-2 suppresses IFNbeta production mediated by NSP1, 5, 6, 15, ORF6 and ORF7b but does not suppress the effects of added interferon. PLoS Pathog 17:e1010146. https://doi.org/10.1371/journal.ppat.1010146
Stukalov A, Girault V, Grass V, Karayel O, Bergant V, Urban C et al (2021) Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature 594:246–252. https://doi.org/10.1038/s41586-021-03493-4
Yuen CK, Lam JY, Wong WM, Mak LF, Wang X, Chu H et al (2020) SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg Microbes Infect 9:1418–1428. https://doi.org/10.1080/22221751.2020.1780953
Zhang Q, Chen Z, Huang C, Sun J, Xue M, Feng T et al (2021) Severe Acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Membrane (M) and Spike (S) proteins antagonize host type I interferon response. Front Cell Infect Microbiol 11:766922. https://doi.org/10.3389/fcimb.2021.766922
Vazquez C, Swanson SE, Negatu SG, Dittmar M, Miller J, Ramage HR et al (2021) SARS-CoV-2 viral proteins NSP1 and NSP13 inhibit interferon activation through distinct mechanisms. PLoS ONE 16:e0253089. https://doi.org/10.1371/journal.pone.0253089
Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S et al (2020) Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect 9:221–236. https://doi.org/10.1080/22221751.2020.1719902
Frieman M, Yount B, Heise M, Kopecky-Bromberg SA, Palese P, Baric RS (2007) Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J Virol 81:9812–9824. https://doi.org/10.1128/JVI.01012-07
Riojas MA, Frank AM, Puthuveetil NP, Flores B, Parker M, King SP et al (2020) A rare deletion in SARS-CoV-2 ORF6 dramatically alters the predicted three-dimensional structure of the resultant protein. bioRxiv. https://doi.org/10.1101/2020.06.09.134460
Rehwinkel J, Gack MU (2020) RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol 20:537–551. https://doi.org/10.1038/s41577-020-0288-3
Choi Y, Bowman JW, Jung JU (2018) Autophagy during viral infection - a double-edged sword. Nat Rev Microbiol 16:341–354. https://doi.org/10.1038/s41579-018-0003-6
Gao G, Luo H (2006) The ubiquitin-proteasome pathway in viral infections. Can J Physiol Pharmacol 84:5–14. https://doi.org/10.1139/y05-144
Min YQ, Huang M, Sun X, Deng F, Wang H, Ning YJ (2021) Immune evasion of SARS-CoV-2 from interferon antiviral system. Comput Struct Biotechnol J 19:4217–4225. https://doi.org/10.1016/j.csbj.2021.07.023
Thoms M, Buschauer R, Ameismeier M, Koepke L, Denk T, Hirschenberger M et al (2020) Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science 369:1249–1255. https://doi.org/10.1126/science.abc8665
Guo G, Gao M, Gao X, Zhu B, Huang J, Luo K et al (2021) SARS-CoV-2 non-structural protein 13 (nsp13) hijacks host deubiquitinase USP13 and counteracts host antiviral immune response. Signal Transduct Target Ther 6:119. https://doi.org/10.1038/s41392-021-00509-3
Hsu JC, Laurent-Rolle M, Pawlak JB, Wilen CB, Cresswell P (2021) Translational shutdown and evasion of the innate immune response by SARS-CoV-2 NSP14 protein. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.2101161118
Miorin L, Kehrer T, Sanchez-Aparicio MT, Zhang K, Cohen P, Patel RS et al (2020) SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling. Proc Natl Acad Sci USA 117:28344–28354. https://doi.org/10.1073/pnas.2016650117
Addetia A, Lieberman NAP, Phung Q, Hsiang TY, Xie H, Roychoudhury P et al (2021) SARS-CoV-2 ORF6 Disrupts Bidirectional Nucleocytoplasmic Transport through Interactions with Rae1 and Nup98. mBio. https://doi.org/10.1128/mBio.00065-21
Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I to promote interferon-beta induction during the early phase of viral infection
Liu Y, Olagnier D, Lin R (2016) Host and viral modulation of RIG-I-mediated antiviral immunity. Front Immunol 7:662. https://doi.org/10.3389/fimmu.2016.00662
Wu Y, Ma L, Zhuang Z, Cai S, Zhao Z, Zhou L et al (2020) Main protease of SARS-CoV-2 serves as a bifunctional molecule in restricting type I interferon antiviral signaling. Signal Transduct Target Ther 5:221. https://doi.org/10.1038/s41392-020-00332-2
Zhao Y, Sui L, Wu P, Wang W, Wang Z, Yu Y et al (2021) A dual-role of SARS-CoV-2 nucleocapsid protein in regulating innate immune response. Signal Transduct Target Ther 6:331. https://doi.org/10.1038/s41392-021-00742-w
Inn KS, Gack MU, Tokunaga F, Shi M, Wong LY, Iwai K et al (2011) Linear ubiquitin assembly complex negatively regulates RIG-I- and TRIM25-mediated type I interferon induction. Mol Cell 41:354–365. https://doi.org/10.1016/j.molcel.2010.12.029
Queromes G, Destras G, Bal A, Regue H, Burfin G, Brun S et al (2021) Characterization of SARS-CoV-2 ORF6 deletion variants detected in a nosocomial cluster during routine genomic surveillance, Lyon, France. Emerg Microbes Infect 10:167–177. https://doi.org/10.1080/22221751.2021.1872351
Kehrer T, Cupic A, Ye C, Yildiz S, Bouhhadou M, Crossland NA et al (2022) Impact of SARS-CoV-2 ORF6 and its variant polymorphisms on host responses and viral pathogenesis. bioRxiv. https://doi.org/10.1101/2022.10.18.512708
Acknowledgements
We are grateful to Prof. Adolfo Garcia Sastre, Microbiology Department, Icahn School of Medicine, New York, and Prof. Nevan J. Krogan, Cellular Molecular Pharmacology, University of California, San Francisco, for providing plasmids for studying IFN response and SARS-CoV-2 protein expression, respectively. We thank Rajesh Thangavel Yadav for his help with creating the 3D structure model of ORF6.
Funding
We acknowledge research funding to ST Lab and infrastructure support to IISc from the DBT-IISc partnership, DBT-BIRAC, Crypto Relief Fund, L & T Trust, and DST-FIST program to IISc.
Author information
Authors and Affiliations
Contributions
Conceptualization, funding acquisition, project administration, supervision, resources: ST. Methodology, data curation, formal analysis, validation, visualization: OK, MS, RN, ST. Manuscript writing—review and editing: OK, MS, RN, ST.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no competing interest to declare.
Ethical approval and consent to participate
This study was conducted in compliance with institutional biosafety guidelines, (IBSC/IISc/ST/17/2020; IBSC/IISc/ST/18/2021). All experiments involving SARS-CoV-2 virus were performed in Viral BSL3 facility, following the Indian Council of Medical Research and Department of Biotechnology recommendations.
Consent for publication
All the authors have provided consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khatun, O., Sharma, M., Narayan, R. et al. SARS-CoV-2 ORF6 protein targets TRIM25 for proteasomal degradation to diminish K63-linked RIG-I ubiquitination and type-I interferon induction. Cell. Mol. Life Sci. 80, 364 (2023). https://doi.org/10.1007/s00018-023-05011-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-05011-3