Abstract
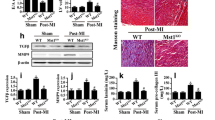
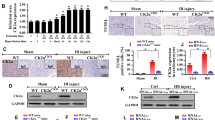
Mitochondrial dynamics are critical for maintaining mitochondrial morphology and function during cardiac ischemia and reperfusion (I/R). The immunoproteasome complex is an inducible isoform of the proteasome that plays a key role in modulating inflammation and some cardiovascular diseases, but the importance of immunoproteasome catalytic subunit β2i (also known as LMP10 or MECL1) in regulating mitochondrial dynamics and cardiac I/R injury is largely unknown. Here, using β2i-knockout (KO) mice and rAAV9-β2i-injected mice, we discovered that β2i expression and its trypsin-like activity were significantly attenuated in the mouse I/R myocardium and in patients with myocardial infarction (MI). Moreover, β2i-KO mice exhibited greatly enhanced I/R-mediated cardiac dysfunction, infarct size, myocyte apoptosis and oxidative stress accompanied by excessive mitochondrial fission due to Mfn1/2 and Drp1 imbalance. Conversely, cardiac overexpression of β2i in mice injected with recombinant adeno-associated virus 9 (rAAV9)-β2i ameliorated cardiac I/R injury. Mechanistically, I/R injury reduced β2i expression and activity, which increased the expression of the E3 ligase Parkin protein and promoted the degradation of mitofusin 1/2 (Mfn1/2), leading to excessive mitochondrial fission. In conclusion, our data suggest for the first time that β2i exerts a protective role against cardiac I/R injury and that increasing β2i expression may be a new therapeutic option for cardiac ischemic disease in clinical practice.
Graphical Abstract
Graphical abstract showing how the immunoproteasome subunit β2i ameliorates myocardial I/R injury by regulating Parkin-Mfn1/2-mediated mitochondrial fusion.








Similar content being viewed by others
Availability of data and materials
Dataset used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Zhou M et al (2021) myocardial ischemia-reperfusion injury: therapeutics from a mitochondria-centric perspective. Cardiology 146(6):781–792. https://doi.org/10.1159/000518879
Cadenas S (2018) ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med 117:76–89. https://doi.org/10.1016/j.freeradbiomed.2018.01.024
Bou-Teen D et al (2021) Mitochondrial ROS and mitochondria-targeted antioxidants in the aged heart. Free Radic Biol Med 167:109–124. https://doi.org/10.1016/j.freeradbiomed.2021.02.043
Liu Y et al (2023) Non-coding RNA-mediated modulation of ferroptosis in cardiovascular diseases. Biomed Pharmacother 164:114993. https://doi.org/10.1016/j.biopha.2023.114993
Kulek AR et al (2020) Mitochondrial quality control: role in cardiac models of lethal ischemia-reperfusion. Injury Cells 9(1):214. https://doi.org/10.3390/cells9010214
Marin W et al (2021) Mitochondria as a therapeutic target for cardiac ischemia-reperfusion injury (review). Int J Mol Med 47(2):485–499. https://doi.org/10.3892/ijmm.2020.4823
Basler M, Groettrup M (2021) On the role of the immunoproteasome in protein homeostasis. Cells 10(11):3216. https://doi.org/10.3390/cells10113216
Angeles A, Fung G, Luo H (2012) Immune and non-immune functions of the immunoproteasome. Front Biosci (Landmark Ed) 17(5):1904–1916. https://doi.org/10.2741/4027
Basler M et al (2015) The immunoproteasome: a novel drug target for autoimmune diseases. Clin Exp Rheumatol 33(4 Suppl 92):S749
Yan W et al (2017) Knockout of immunoproteasome subunit beta2i ameliorates cardiac fibrosis and inflammation in DOCA/Salt hypertensive mice. Biochem Biophys Res Commun 490(2):84–90. https://doi.org/10.1016/j.bbrc.2017.05.011
Xie X et al (2019) The immunoproteasome catalytic beta5i subunit regulates cardiac hypertrophy by targeting the autophagy protein ATG5 for degradation. Sci Adv 5(5):eaau0495. https://doi.org/10.1126/sciadv.aau0495
Yan X et al (2020) Gallic acid attenuates angiotensin II-induced hypertension and vascular dysfunction by inhibiting the degradation of endothelial nitric oxide synthase. Front Pharmacol 11:1121. https://doi.org/10.3389/fphar.2020.01121
Li J et al (2018) Novel role for the immunoproteasome subunit PSMB10 in angiotensin II-induced atrial. Fibrillation in mice. Hypertension 71(5):866–876. https://doi.org/10.1161/HYPERTENSIONAHA.117.10390
Li J et al (2019) Immunoproteasome subunit beta5i promotes Ang II (Angiotensin II)-induced atrial fibrillation by targeting ATRAP (Ang II Type I Receptor-Associated Protein) degradation in mice. Hypertension 73(1):92–101. https://doi.org/10.1161/HYPERTENSIONAHA.118.11813
Li FD et al (2019) Ablation and inhibition of the immunoproteasome catalytic subunit LMP7 attenuate experimental abdominal aortic aneurysm formation in mice. J Immunol 202(4):1176–1185. https://doi.org/10.4049/jimmunol.1800197
Wang S et al (2018) The immunoproteasome subunit LMP10 mediates angiotensin II-induced retinopathy in mice. Redox Biol 16:129–138. https://doi.org/10.1016/j.redox.2018.02.022
Wang S et al (2020) Ablation of immunoproteasome beta5i subunit suppresses hypertensive retinopathy by blocking ATRAP degradation in mice. Mol Ther 28(1):279–292. https://doi.org/10.1016/j.redox.2018.02.022
Zhang YL et al (2022) Blockage of fibronectin 1 ameliorates myocardial ischemia/reperfusion injury in association with activation of AMP-LKB1-AMPK signaling pathway. Oxid Med Cell Longev 2022:6196173. https://doi.org/10.1155/2022/6196173
Wang JX et al (2011) miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med 17(1):71–78. https://doi.org/10.1038/nm.2282
Shi KN et al (2023) MK-886 protects against cardiac ischaemia/reperfusion injury by activating proteasome-Keap1-NRF2 signalling. Redox Biol 62:102706. https://doi.org/10.1016/j.redox.2023.102706
Xu LL et al (2023) Ursolic acid ameliorates myocardial ischaemia/reperfusion injury by improving mitochondrial function via immunoproteasome-PP2A-AMPK signalling. Nutrients 15(4):1049. https://doi.org/10.3390/nu15041049
Ao X et al (2023) Non-coding RNAs regulating mitochondrial function in cardiovascular diseases. J Mol Med (Berl) 101(5):501–526. https://doi.org/10.1007/s00109-023-02305-8
Yonashiro R et al (2006) A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. EMBO J 25(15):3618–3626. https://doi.org/10.1038/sj.emboj.7601249
Hall AR et al (2021) Correction: hearts deficient in both Mfn1 and Mfn2 are protected against acute myocardial infarction. Cell Death Dis 12(7):660. https://doi.org/10.1038/s41419-021-03946-8
Cribbs JT, Strack S (2007) Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 8(10):939–944. https://doi.org/10.1038/sj.embor.7401062
Cereghetti GM et al (2008) Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A 105(41):15803–15808. https://doi.org/10.1073/pnas.0808249105
Jin JY et al (2021) Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol Sin 42(5):655–664. https://doi.org/10.1038/s41401-020-00518-y
Wang H et al (2011) Parkin ubiquitinates Drp1 for proteasome-dependent degradation: implication of dysregulated mitochondrial dynamics in Parkinson disease. J Biol Chem 286(13):11649–11658. https://doi.org/10.1074/jbc.M110.144238
Lutz AK et al (2009) Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J Biol Chem 284(34):22938–22951. https://doi.org/10.1074/jbc.M109.035774
Karbowski M, Neutzner A, Youle RJ (2007) The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol 178(1):71–84. https://doi.org/10.1083/jcb.200611064
Rakovic A et al (2011) Mutations in PINK1 and Parkin impair ubiquitination of mitofusins in human fibroblasts. PLoS One 6(3):e16746. https://doi.org/10.1371/journal.pone.0016746
Turkieh A et al (2022) Mitophagy regulation following myocardial infarction. Cells 11(2):199. https://doi.org/10.3390/cells11020199
Abdrakhmanov A, Gogvadze V, Zhivotovsky B (2020) To eat or to die: deciphering selective forms of autophagy. Trends Biochem Sci 45(4):347–364. https://doi.org/10.1016/j.tibs.2019.11.006
Tang L et al (2021) Dexpramipexole attenuates myocardial ischemia/reperfusion injury through upregulation of mitophagy. Eur J Pharmacol 899:173962. https://doi.org/10.1016/j.ejphar.2021.173962
Liu W et al (2021) AM1241 alleviates myocardial ischemia-reperfusion injury in rats by enhancing Pink1/Parkin-mediated autophagy. Life Sci 272:119228. https://doi.org/10.1016/j.lfs.2021.119228
Kubli DA et al (2013) Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem 288(2):915–926. https://doi.org/10.1074/jbc.M112.411363
Song M et al (2015) Interdependence of Parkin-mediated mitophagy and mitochondrial fission in adult mouse hearts. Circ Res 117(4):346–351. https://doi.org/10.1161/CIRCRESAHA.117.306859
Ji W et al (2016) Aldehyde dehydrogenase 2 has cardioprotective effects on myocardial ischaemia/reperfusion injury via suppressing mitophagy. Front Pharmacol 7:101. https://doi.org/10.3389/fphar.2016.00101
Zhou H et al (2017) Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res 63(1):e12413. https://doi.org/10.1111/jpi.12413
Funding
This work was supported by a grant from the National Natural Science Foundation of China (No. 82030009).
Author information
Authors and Affiliations
Contributions
HXS and PBL contributed to the experiments and data analyses. KNS and JG performed animal surgery and infarct staining. HJZ and HHL designed the study and supervised the research, funding acquisition, and writing. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Ethics approval
Animal experiments were approved by the Ethics Committee of Beijing Chao-Yang Hospital of Capital Medical University (2020-animal-164). The clinical study was approved by the Ethics Committee of Beijing Chao-Yang Hospital of Capital Medical University (2023-Science-3).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, HX., Li, PB., Shi, KN. et al. The immunoproteasome subunit β2i ameliorates myocardial ischemia/reperfusion injury by regulating Parkin-Mfn1/2-mediated mitochondrial fusion. Cell. Mol. Life Sci. 80, 231 (2023). https://doi.org/10.1007/s00018-023-04867-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04867-9