Abstract
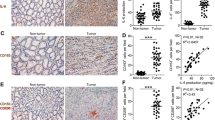
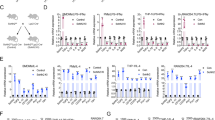
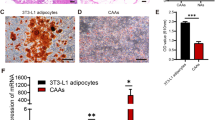
Glucose-6-phosphate dehydrogenase (G6PD) is involved in triple-negative breast cancer (TNBC) progression. Metabolic crosstalk between cancer cells and tumor-associated macrophages mediates tumor progression in TNBC. Molecular biological methods were applied to clarify the mechanism of the crosstalk between TNBC cells and M2 macrophages. In the present study, we verified that G6PD overexpression drives M2 macrophage polarization by directly combining with phospho-STAT1 and upregulating CCL2 and TGF-β1 secretion in TNBC cells. In turn, M2-like TAMs activated TNBC cells through IL-10 secretion, providing feedback to upregulate G6PD and promote TNBC cell migration and proliferation in vitro. Furthermore, we found that 6-AN (a specific inhibitor of G6PD) not only suppressed the cancer-driven polarization of macrophages toward the M2 phenotype but also inhibited the inherent M2 polarization of macrophages. Targeting the G6PD-regulated pentose phosphate pathway restrained TNBC progression and M2-type polarization of macrophages in vitro and in vivo.







Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. The raw data are available from the corresponding author upon reasonable request. Figure 1D data analyzed in this study were obtained from the Gene Expression Omnibus (GEO) at GSE81032.
Abbreviations
- TNBC:
-
Triple-negative breast cancer.
- PPP:
-
Pentose phosphate pathway.
- G6PD:
-
Glucose-6-phosphate dehydrogenase.
- 6-AN:
-
6-Aminonicotinamide.
- TAMs:
-
Tumor-associated macrophages.
- Mφ:
-
Macrophage.
- ELISA:
-
Enzyme-linked Immunosorbent Assay.
- CM:
-
Conditioned medium.
- q-PCR:
-
Quantitative polymerase chain reaction.
- MTT:
-
Thiazolyl blue.
- IHC:
-
Immunohistochemistry.
- H&E:
-
Hematoxylin and eosin.
- DAPI:
-
4′,6-Diamidino-2-phenylindole.
- CCL2:
-
Chemokine (C–C motif) ligand 2
- TGF-β1:
-
Transforming growth factor beta 1
- IL-10:
-
Interleukin-10
References
Siegel RL et al (2021) Cancer Statistics, 2021. CA Cancer J Clin 71(1):7–33
Yin L et al (2020) Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res 22(1):61
Robey IF et al (2005) Hypoxia-inducible factor-1alpha and the glycolytic phenotype in tumors. Neoplasia 7(4):324–330
Pu H et al (2015) Overexpression of G6PD is associated with high risks of recurrent metastasis and poor progression-free survival in primary breast carcinoma. World J Surg Oncol 13:323
Dong T et al (2016) Altered glycometabolism affects both clinical features and prognosis of triple-negative and neoadjuvant chemotherapy-treated breast cancer. Tumour Biol 37(6):8159–8168
Tu D et al (2021) M2 macrophages contribute to cell proliferation and migration of breast cancer. Cell Biol Int 45(4):831–838
Jayasingam SD et al (2019) Evaluating the polarization of tumor-associated macrophages into m1 and m2 phenotypes in human cancer tissue: technicalities and challenges in routine clinical practice. Front Oncol 9:1512
Chen P et al (2017) Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci USA 114(3):580–585
Chen F et al (2019) Extracellular vesicle-packaged HIF-1alpha-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol 21(4):498–510
Li Y et al. (2022) Targeting glucose-6-phosphate dehydrogenase by 6-AN induces ROS-mediated autophagic cell death in breast cancer. FEBS J.
Shi T et al. Conservation of protein abundance patterns reveals the regulatory architecture of the EGFR-MAPK pathway. Sci Signal, 2016. 9(436): p. rs6.
Dias AS et al (2019) Metabolic crosstalk in the breast cancer microenvironment. Eur J Cancer 121:154–171
Zheng H et al. (2021) Tumor Microenvironment: Key Players in Triple Negative Breast Cancer Immunomodulation. Cancers (Basel) 13(13).
Nishida-Aoki N, Gujral TS (2019) Emerging approaches to study cell-cell interactions in tumor microenvironment. Oncotarget 10(7):785–797
Tymoszuk P et al (2014) High STAT1 mRNA levels but not its tyrosine phosphorylation are associated with macrophage infiltration and bad prognosis in breast cancer. BMC Cancer 14:257
Tilborghs S et al (2017) The role of Nuclear Factor-kappa B signaling in human cervical cancer. Crit Rev Oncol Hematol 120:141–150
Ma X et al (2017) Polo-like kinase 1 coordinates biosynthesis during cell cycle progression by directly activating pentose phosphate pathway. Nat Commun 8(1):1506
Ni Y et al (2021) Silent information regulator 2 promotes clear cell renal cell carcinoma progression through deacetylation and small ubiquitin-related modifier 1 modification of glucose 6-phosphate dehydrogenase. Cancer Sci 112(10):4075–4086
Butturini E, Carcereri de Prati A, Mariotto S (2020) Redox Regulation of STAT1 and STAT3 Signaling. Int J Mol Sci. 21(19).
Verhoeven Y et al (2020) The potential and controversy of targeting STAT family members in cancer. Semin Cancer Biol 60:41–56
Ch'ng ES, Jaafar H (2011) Tuan Sharif SE Breast Tumor Angiogenesis and Tumor-Associated Macrophages: Histopathologist's Perspective. Patholog Res Int. 2011: 572706.
Wang H, et al. (2021) The impact of the tumor microenvironment on macrophage polarization in cancer metastatic progression. Int J Mol Sci. 22(12).
Owen KL, Brockwell NK, Parker BS (2019) JAK-STAT Signaling: A Double-Edged Sword of Immune Regulation and Cancer Progression. Cancers (Basel). 11(12).
Sun SC (2017) The non-canonical NF-kappaB pathway in immunity and inflammation. Nat Rev Immunol 17(9):545–558
Sun L et al (2018) Metabolic reprogramming for cancer cells and their microenvironment: beyond the Warburg Effect. Biochim Biophys Acta Rev Cancer 1870(1):51–66
Jiang P, Du W, Wu M (2014) Regulation of the pentose phosphate pathway in cancer. Protein Cell 5(8):592–602
More TH et al (2018) Metabolomic alterations in invasive ductal carcinoma of breast: a comprehensive metabolomic study using tissue and serum samples. Oncotarget 9(2):2678–2696
Tayyari F et al (2018) Metabolic profiles of triple-negative and luminal A breast cancer subtypes in African-American identify key metabolic differences. Oncotarget 9(14):11677–11690
Budczies J et al (2012) Remodeling of central metabolism in invasive breast cancer compared to normal breast tissue—a GC-TOFMS based metabolomics study. BMC Genomics 13:334
Willmann L et al (2015) Metabolic profiling of breast cancer: differences in central metabolism between subtypes of breast cancer cell lines. J Chromatogr B Analyt Technol Biomed Life Sci 1000:95–104
Tang X et al (2014) A joint analysis of metabolomics and genetics of breast cancer. Breast Cancer Res 16(4):415
Willmann L et al (2016) Alterations of the exo- and endometabolite profiles in breast cancer cell lines: a mass spectrometry-based metabolomics approach. Anal Chim Acta 925:34–42
Patra KC, Hay N (2014) The pentose phosphate pathway and cancer. Trends Biochem Sci 39(8):347–354
Yin X et al (2017) ID1 promotes hepatocellular carcinoma proliferation and confers chemoresistance to oxaliplatin by activating pentose phosphate pathway. J Exp Clin Cancer Res 36(1):166
Barajas JM et al (2018) The role of miR-122 in the dysregulation of glucose-6-phosphate dehydrogenase (G6PD) expression in hepatocellular cancer. Sci Rep 8(1):9105
Wu S et al (2018) Transcription factor YY1 promotes cell proliferation by directly activating the pentose phosphate pathway. Cancer Res 78(16):4549–4562
Massari F et al (2016) Metabolic phenotype of bladder cancer. Cancer Treat Rev 45:46–57
Pathria P, Louis TL, Varner JA (2019) Targeting tumor-associated macrophages in cancer. Trends Immunol 40(4):310–327
Mantovani A et al (2017) Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14(7):399–416
Cook J, Hagemann T (2013) Tumour-associated macrophages and cancer. Curr Opin Pharmacol 13(4):595–601
Pitt JM et al (2016) Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol 27(8):1482–1492
Castellaro AM et al. (2019) Tumor-associated macrophages induce endocrine therapy resistance in ER+ breast cancer cells. Cancers (Basel). 11(2).
Munoz-Garcia J et al (2021) The twin cytokines interleukin-34 and CSF-1: masterful conductors of macrophage homeostasis. Theranostics 11(4):1568–1593
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122(3):787–795
Shapouri-Moghaddam A et al (2018) Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 233(9):6425–6440
Hu T et al (2013) Variant G6PD levels promote tumor cell proliferation or apoptosis via the STAT3/5 pathway in the human melanoma xenograft mouse model. BMC Cancer 13:251
Yang HC et al (2015) Glucose 6-phosphate dehydrogenase knockdown enhances IL-8 expression in HepG2 cells via oxidative stress and NF-kappaB signaling pathway. J Inflamm (Lond) 12:34
Dai L et al (2019) SARI attenuates colon inflammation by promoting STAT1 degradation in intestinal epithelial cells. Mucosal Immunol 12(5):1130–1140
Jia P et al. (2022) Chemokine CCL2 from proximal tubular epithelial cells contributes to sepsis-induced acute kidney injury. Am J Physiol Renal Physiol.
Cao J et al (2021) NR4A1 knockdown confers hepatoprotection against ischaemia-reperfusion injury by suppressing TGFbeta1 via inhibition of CYR61/NF-kappaB in mouse hepatocytes. J Cell Mol Med 25(11):5099–5112
Duke KS et al (2017) STAT1-dependent and -independent pulmonary allergic and fibrogenic responses in mice after exposure to tangled versus rod-like multi-walled carbon nanotubes. Part Fibre Toxicol 14(1):26
Zhang F et al (2016) TGF-beta induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 7(32):52294–52306
Van den Bossche J, O’Neill LA, Menon D (2017) Macrophage Immunometabolism: Where Are We (Going)? Trends Immunol 38(6):395–406
Noy R, Pollard JW (2014) Tumor-associated macrophages: from mechanisms to therapy. Immunity 41(1):49–61
Ungefroren H et al. (2021) Autocrine TGFbeta1 opposes exogenous tgfbeta1-induced cell migration and growth arrest through sustainment of a feed-forward loop involving MEK-ERK signaling. Cancers (Basel), 13(6).
David CJ et al (2016) TGF-beta tumor suppression through a Lethal EMT. Cell 164(5):1015–1030
Chen RH et al (2020) Tumor cell-secreted ISG15 promotes tumor cell migration and immune suppression by inducing the macrophage M2-like phenotype. Front Immunol 11:594775
Su B et al (2021) Let-7d inhibits intratumoral macrophage M2 polarization and subsequent tumor angiogenesis by targeting IL-13 and IL-10. Cancer Immunol Immunother 70(6):1619–1634
Acknowledgements
We would like to thank Gang Zhao (Department of Pathology, Tianjin Cancer Hospital) and the breast surgery department of Peking Union Medical College Hospital for supplying breast cancer tissue sections, Miaomiao Sheng (Kunming University of Science and Technology) for providing breast cancer cell lines, and Xinying Wu (Animal Center of Nankai University) for technical support of in vivo imaging.
Funding
This study was partially funded by “State Key Laboratory of Medicinal Chemical Biology (NanKai University, No. 2019014)” to C W and “The Fundamental Research Funds for the Central Universities (Nankai University, #ZB19100128)” to C L.
Author information
Authors and Affiliations
Contributions
Y L, X H and Z F designed, performed and analyzed the in vivo experiments. Y L, X H and Z F performed and analyzed G6PD knockdown experiments in vitro and in vivo. Y L, X H and Z L provided guidance on data processing and writing. C L, Y L, C W, Q S and Z F contributed to the study design, implementation and supervision of the study. Z L, Z F, C W, Q S and C L contributed to the study design, supervised the study, and reviewed the manuscript. Y L and C L wrote the manuscript. All authors had full access to the data and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Animal experiments were performed according to the Guidelines on Laboratory Animals of Nankai University and were approved by the Institute Research Ethics Committee at Nankai University (No: 2021-SYDWLL-000464). Human experiments were approved by the human experimentation committee, and informed consent was obtained from all subjects (No: NKUIRB2021105).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Han, X., Lin, Z. et al. G6PD activation in TNBC cells induces macrophage recruitment and M2 polarization to promote tumor progression. Cell. Mol. Life Sci. 80, 165 (2023). https://doi.org/10.1007/s00018-023-04810-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04810-y