Abstract
Background
Vascular endothelial dysfunction is regarded as an early event of hypertension. Galectin-3 (Gal-3) is known to participate in various pathological processes. Whilst previous studies showed that inhibition of Gal-3 effectively ameliorates angiotensin II (Ang II)-induced atherosclerosis or hypertension, it remains unclear whether Ang II regulates Gal-3 expression and actions in vascular endothelium.
Methods
Using techniques of molecular biology and myograph, we investigated Ang II-mediated changes in Gal-3 expression and activity in thoracic aortas and mesenteric arteries from wild-type and Gal-3 gene deleted (Gal-3−/−) mice and cultured endothelial cells.
Results
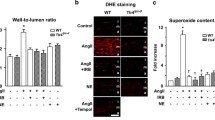
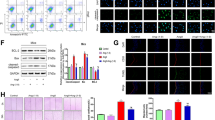
The serum level of Gal-3 was significantly higher in hypertensive patients or in mice with chronic Ang II-infusion. Ang II infusion to wild-type mice enhanced Gal-3 expression in the aortic and mesenteric arteries, elevated systolic blood pressure and impaired endothelium-dependent relaxation of the thoracic aortas and mesenteric arteries, changes that were abolished in Gal-3−/− mice. In human umbilical vein endothelial cells, Ang II significantly upregulated Gal-3 expression by promoting nuclear localization of Yes-associated protein (YAP) and its interaction with transcription factor Tead1 with enhanced YAP/Tead1 binding to Gal-3 gene promoter region. Furthermore, Gal-3 deletion augmented the bioavailability of nitric oxide, suppressed oxidative stress, and alleviated inflammation in the thoracic aorta of Ang II-infused mice or endothelial cells exposed to Ang II.
Conclusions
Our results demonstrate for the first time that Ang II upregulates Gal-3 expression via increment in YAP nuclear localization in vascular endothelium, and that Gal-3 mediates endothelial dysfunction contributing to the development of hypertension.









Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- Ang II:
-
Angiotensin II
- DHFR:
-
Dihydrofolate reductase
- eNOS:
-
Endothelial nitric oxide synthase
- Gal-3:
-
Galectin-3
- GPCRs:
-
G protein-coupled receptors
- HUVECs:
-
Human umbilical vein endothelial cells
- IL-6:
-
Interleukin-6
- MAECs:
-
Mouse thoracic aorta endothelial cells
- MCP-1:
-
Monocyte chemoattractant protein-1
- NO:
-
Nitric oxide
- NOX2:
-
Nicotinamide adenine dinucleotide phosphate oxidase 2
- ROS:
-
Reactive oxygen species
- Tead1:
-
TEA domain transcription factor 1
- VCAM-1:
-
Vascular cell adhesion molecule-1
- YAP:
-
Yes-associated protein
References
Liao JK (2013) Linking endothelial dysfunction with endothelial cell activation. J Clin Invest 123(2):540–541. https://doi.org/10.1172/JCI66843
Du XJ, Zhao WB, Nguyen MN, Lu Q, Kiriazis H (2019) β-Adrenoceptor activation affects galectin-3 as a biomarker and therapeutic target in heart disease. Br J Pharmacol 176(14):2449–2464. https://doi.org/10.1111/bph.14620
Anand IS, Rector TS, Kuskowski M, Adourian A, Muntendam P, Cohn JN (2013) Baseline and serial measurements of galectin-3 in patients with heart failure: relationship to prognosis and effect of treatment with valsartan in the Val-HeFT. Eur J Heart Fail 15(5):511–518. https://doi.org/10.1093/eurjhf/hfs205
Francia P, Adduci C, Semprini L, Borro M, Ricotta A, Sensini I, Santini D, Caprinozzi M, Balla C, Simmaco M, Volpe M (2014) Osteopontin and galectin-3 predict the risk of ventricular tachycardia and fibrillation in heart failure patients with implantable defibrillators. J Cardiovasc Electrophysiol 25(6):609–616. https://doi.org/10.1111/jce.12364
Maiolino G, Rossitto G, Pedon L, Cesari M, Frigo AC, Azzolini M, Plebani M, Rossi GP (2015) Galectin-3 predicts long-term cardiovascular death in high-risk patients with coronary artery disease. Arterioscler Thromb Vasc Biol 35(3):725–732. https://doi.org/10.1161/ATVBAHA.114.304964
González GE, Rhaleb NE, D’Ambrosio MA, Nakagawa P, Liao TD, Peterson EL, Leung P, Dai X, Janic B, Liu YH, Yang XP, Carretero OA (2016) Cardiac-deleterious role of galectin-3 in chronic angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 311(5):H1287–H1296. https://doi.org/10.1152/ajpheart.00096.2016
Calvier L, Miana M, Reboul P, Cachofeiro V, Martinez-Martinez E, de Boer RA, Poirier F, Lacolley P, Zannad F, Rossignol P, López-Andrés N (2013) Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol 33(1):67–75. https://doi.org/10.1161/ATVBAHA.112.300569
He J, Bao Q, Zhang Y, Liu M, Lv H, Liu Y, Yao L, Li B, Zhang C, He S, Zhai G, Zhu Y, Liu X, Zhang K, Wang XJ, Zou MH, Zhu Y, Ai D (2018) Yes-associated protein promotes angiogenesis via signal transducer and activator of transcription 3 in endothelial cells. Circ Res 122(4):591–605. https://doi.org/10.1161/CIRCRESAHA.117.311950
Wang L, Luo JY, Li B, Tian XY, Chen LJ, Huang Y, Liu J, Deng D, Lau CW, Wan S, Ai D, Mak KK, Tong KK, Kwan KM, Wang N, Chiu JJ, Zhu Y, Huang Y (2016) Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 540(7634):579–582. https://doi.org/10.1038/nature20602
Luo J, Yu FX (2019) GPCR-Hippo Signaling in Cancer Cells 8(5):426. https://doi.org/10.3390/cells8050426
Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL (2012) Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150(4):780–791. https://doi.org/10.1016/j.cell.2012.06.037
Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, Zhao L, Peyman G, Ouyang H, Jiang W, Zhao J, Chen X, Zhang L, Wang CY, Bastian BC, Zhang K, Guan KL (2014) Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell 25(6):822–830. https://doi.org/10.1016/j.ccr.2014.04.017
Zindel D, Mensat P, Vol C, Homayed Z, Charrier-Savournin F, Trinquet E, Banères JL, Pin JP, Pannequin J, Roux T, Dupuis E, Prézeau L (2021) G protein-coupled receptors can control the Hippo/YAP pathway through Gq signaling. FASEB J 35(7):e21668. https://doi.org/10.1096/fj.202002159R
Lin M, Yuan W, Su Z, Lin C, Huang T, Chen Y, Wang J (2018) Yes-associated protein mediates angiotensin II-induced vascular smooth muscle cell phenotypic modulation and hypertensive vascular remodelling. Cell Prolif 51(6):e12517. https://doi.org/10.1111/cpr.12517
Li B, He J, Lv H, Liu Y, Lv X, Zhang C, Zhu Y, Ai D (2019) c-Abl regulates YAPY357 phosphorylation to activate endothelial atherogenic responses to disturbed flow. J Clin Invest 129(3):1167–1179. https://doi.org/10.1172/JCI122440
She G, Ren YJ, Wang Y, Hou MC, Wang HF, Gou W, Lai BC, Lei T, Du XJ, Deng XL (2019) KCa3.1 channels promote cardiac fibrosis through mediating inflammation and differentiation of monocyte into myofibroblast in angiotensin II treated rats. J Am Heart Assoc. 8(1):e010418. https://doi.org/10.1161/JAHA.118.010418
Kobayashi M, Inoue K, Warabi E, Minami T, Kodama T (2005) A simple method of isolating mouse aortic endothelial cells. J Atheroscler Thromb 12(3):138–142. https://doi.org/10.5551/jat.12.138
Shalhout SZ, Yang PY, Grzelak EM, Nutsch K, Shao S, Zambaldo C, Iaconelli J, Ibrahim L, Stanton C, Chadwick SR, Chen E, DeRan M, Li S, Hull M, Wu X, Chatterjee AK, Shen W, Camargo FD, Schultz PG, Bollong MJ (2021) YAP-dependent proliferation by a small molecule targeting annexin A2. Nat Chem Biol 17(7):767–775. https://doi.org/10.1038/s41589-021-00755-0
Mount PF, Kemp BE, Power DA (2007) Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 42(2):271–279. https://doi.org/10.1016/j.yjmcc.2006.05.023.。
Chalupsky K, Cai H (2005) Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci USA 102(25):9056–9061. https://doi.org/10.1073/pnas.0409594102
Levy D, Adamovich Y, Reuven N, Shaul Y (2008) Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol Cell 29(3):350–361. https://doi.org/10.1016/j.molcel.2007.12.022
Ikeda S, Sadoshima J (2016) Regulation of Myocardial Cell Growth and Death by the Hippo Pathway. Circ J 80(7):1511–1519. https://doi.org/10.1253/circj.CJ-16-0476
Zhang Q, Li W, Zhu Y, Wang Q, Zhai C, Shi W, Feng W, Wang J, Yan X, Chai L, Chen Y, Li C, Liu P, Li M (2021) Activation of AMPK inhibits Galectin-3-induced pulmonary artery smooth muscle cells proliferation by upregulating hippo signaling effector YAP. Mol Cell Biochem 476(8):3037–3049. https://doi.org/10.1007/s11010-021-04131-3
Ajani JA, Estrella JS, Chen Q, Correa AM, Ma L, Scott AW, Jin J, Liu B, Xie M, Sudo K, Shiozaki H, Badgwell B, Weston B, Lee JH, Bhutani MS, Onodera H, Suzuki K, Suzuki A, Ding S, Hofstetter WL, Johnson RL, Bresalier RS, Song S (2018) Galectin-3 expression is prognostic in diffuse type gastric adenocarcinoma, confers aggressive phenotype, and can be targeted by YAP1/BET inhibitors. Br J Cancer 118(1):52–61. https://doi.org/10.1038/bjc.2017.388
Konukoglu D, Uzun H (2017) Endothelial dysfunction and hypertension. Adv Exp Med Biol 956:511–540. https://doi.org/10.1007/5584_2016_90
Martínez-Martínez E, Ibarrola J, Fernández-Celis A, Calvier L, Leroy C, Cachofeiro V, Rossignol P, López-Andrés N (2018) Galectin-3 pharmacological inhibition attenuates early renal damage in spontaneously hypertensive rats. J Hypertens 36(2):368–376. https://doi.org/10.1097/HJH.0000000000001545
Alp NJ, Channon KM (2004) Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24(3):413–420. https://doi.org/10.1161/01.ATV.0000110785.96039.f6
Kolluru GK, Siamwala JH, Chatterjee S (2010) eNOS phophorylation in health and disease. Biochimie 92:1186–1198
Jin YJ, Chennupati R, Li R, Liang G, Wang S, Iring A, Graumann J, Wettschureck N, Offermanns S (2021) Protein kinase N2 mediates flow-induced endothelial NOS activation and vascular tone regulation. J Clin Invest 131(21):e145734. https://doi.org/10.1172/JCI145734
Crabtree MJ, Brixey R, Batchelor H, Hale AB, Channon KM (2013) Integrated redox sensor and effector functions for tetrahydrobiopterin- and glutathionylation-dependent endothelial nitric-oxide synthase uncoupling. J Biol Chem 288(1):561–569. https://doi.org/10.1074/jbc.M112.415992
Mollnau H, Wendt M, Szöcs K, Lassègue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Förstermann U, Meinertz T, Griendling K, Münzel T (2002) Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 90(4):E58-65. https://doi.org/10.1161/01.res.0000012569.55432.02
Noguchi K, Hamadate N, Matsuzaki T, Sakanashi M, Nakasone J, Sakanashi M, Tsutsui M, Sakanashi M (2010) Improvement of impaired endothelial function by tetrahydrobiopterin in stroke-prone spontaneously hypertensive rats. Eur J Pharmacol 631(1–3):28–35. https://doi.org/10.1016/j.ejphar.2010.01.003
Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM (2011) Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med 208(3):519–533. https://doi.org/10.1084/jem.20102049
Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469(7329):221–225. https://doi.org/10.1038/nature09663
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB (2014) Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20(7):1126–1167. https://doi.org/10.1089/ars.2012.5149
Thomas SR, Witting PK, Drummond GR (2008) Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal 10(10):1713–1765. https://doi.org/10.1089/ars.2008.2027
Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AH, Janowitz D, Neff C, Shah AM, Wingler K, Schmidt HH (2010) Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension. 56(3):490–497. https://doi.org/10.1161/HYPERTENSIONAHA.109.149187
Loot AE, Schreiber JG, Fisslthaler B, Fleming I (2009) Angiotensin II impairs endothelial function via tyrosine phosphorylation of the endothelial nitric oxide synthase. J Exp Med 206(13):2889–2896. https://doi.org/10.1084/jem.20090449
Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG (2002) Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40(4):511–515. https://doi.org/10.1161/01.hyp.0000032100.23772.98
Dandona P, Dhindsa S, Ghanim H, Chaudhuri A (2007) Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens 21(1):20–27. https://doi.org/10.1038/sj.jhh.1002101
Acknowledgements
None.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81870223 and 82170298), Natural Science Foundation of Shannxi province (grant number 2022JM-557). Yulin Science and Technology Project (grant number CXY-2020-048).
Author information
Authors and Affiliations
Contributions
ZDP, XS, XJD and XLD contributed to the study conception and design. Material preparation, data collection and analysis were performed by ZDP, XS, RYB, MZH, YJZ, WW, YZ, BCL, YIZ, YW, XJD and XLD. The first draft of the manuscript was written by ZDP, XJD and XLD. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The animal study was approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, USA. The human study was performed in accordance with the guidelines in the Declaration of Helsinki and was approved by the Ethics Committee of Xi’an Jiaotong University Health Science Center (No. 2020-607).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pang, ZD., Sun, X., Bai, RY. et al. YAP-galectin-3 signaling mediates endothelial dysfunction in angiotensin II-induced hypertension in mice. Cell. Mol. Life Sci. 80, 38 (2023). https://doi.org/10.1007/s00018-022-04623-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04623-5