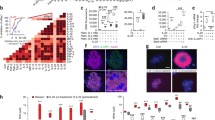
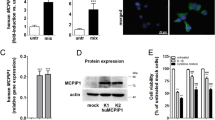
Abstract
Type 1 diabetes (T1D) is characterized by an immune-mediated progressive destruction of the insulin-producing β-cells. Proinflammatory cytokines trigger endoplasmic reticulum (ER) stress and subsequent insulin secretory deficiency in cultured β-cells, mimicking the islet microenvironment in T1D. β-cells undergo physiologic ER stress due to the high rate of insulin production and secretion under stimulated conditions. Severe and uncompensated ER stress in β-cells is induced by several pathological mechanisms before onset and during T1D. We previously described that the small drug Compound A (CpdA), a selective glucocorticoid receptor (GR/NR3C1, nuclear receptor subfamily 3, group C, member 1) ligand with demonstrated inflammation-suppressive activity in vivo, is an effective modulator of effector T and dendritic cells and of macrophages, yet, in a GR-independent manner. Here, we focus on CpdA’s therapeutic potential in T1D cellular and animal models. We demonstrate that CpdA improves the unfolded protein response (UPR) by attenuating ER stress and favoring the survival and function of β-cells exposed to an environment of proinflammatory cytokines. CpdA administration to NODscid mice adoptively transferred with diabetogenic splenocytes (from diabetic NOD mice) led to a delay of disease onset and reduction of diabetes incidence. Histological analysis of the pancreas showed a reduction in islet leukocyte infiltration (insulitis) and preservation of insulin expression in CpdA-treated normoglycemic mice in comparison with control group. These new findings together with our previous reports justify further studies on the administration of this small molecule as a novel therapeutic strategy with dual targets (effector immune and β-cells) during autoimmune diabetes.








Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CpdA:
-
Compound A
- eIF2α:
-
Eukaryotic translation initiation factor 2α
- ER:
-
Endoplasmic reticulum
- GR:
-
Glucocorticoid receptor
- GSIS:
-
Glucose-stimulated insulin secretion
- SEGRAM:
-
Selective glucocorticoid receptor agonists and modulators
- STZ:
-
Streptozotocin
- T1D:
-
Type 1 diabetes
- UPR:
-
Unfolded protein response
References
Clark AL, Urano F (2016) Endoplasmic reticulum stress in β-cells and autoimmune diabetes. Curr Opin Immunol 43:60–66. https://doi.org/10.1016/j.coi.2016.09.006
Tersey SA, Nishiki Y, Templin AT et al (2012) Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes 61(4):818–827. https://doi.org/10.2337/db11-1293
Cardozo AK, Ortis F, Storling J et al (2005) Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic b-cells. Diabetes 54:452–461. https://doi.org/10.2337/diabetes.54.2.452
Miani M, Colli ML, Ladrière L, Cnop M, Eizirik DL (2012) Mild endoplasmic reticulum stress augments the proinflammatory effect of IL-1β in pancreatic rat β-cells via the IRE1α/XBP1s pathway. Endocrinology 153(7):3017–3028. https://doi.org/10.1210/en.2011-2090
Mandrup-Poulsen T, Pickersgill L, Donath MY (2010) Blockade of interleukin 1 in type 1 diabetes mellitus. Nat Rev Endocrinol 6(3):158–166. https://doi.org/10.1038/nrendo.2009.271 (PMID: 20173777)
Engin F, Yermalovich A, Nguyen T et al (2013) Restoration of the unfolded protein response in pancreatic β cells protects mice against type 1 diabetes. Sci Transl Med 5(211):211ra156. https://doi.org/10.1126/scitranslmed.3006534
Fu S, Yalcin A, Lee GY et al. (2015) Phenotypic assays identify azoramide as a small-molecule modulator of the unfolded protein response with antidiabetic activity. Sci Transl Med 7(292):292ra98. https://doi.org/10.1126/scitranslmed.aaa9134
De Bosscher K, Vanden Berghe W, Beck IM et al (2005) A fully dissociated compound of plant origin for inflammatory gene repression. Proc Natl Acad Sci U S A 102(44):15827–15832. https://doi.org/10.1073/pnas.0505554102
Liberman AC, Antunica-Noguerol M, Ferraz-de-Paula V et al (2012) Compound A, a dissociated glucocorticoid receptor modulator, inhibits T-bet (Th1) and induces GATA-3 (Th2) activity in immune cells. PLoS ONE 7(4):e35155. https://doi.org/10.1371/journal.pone.0035155
Barcala Tabarrozzi AE, Andreone L, Deckers J et al (2016) GR-independent down-modulation on GM-CSF bone marrow-derived dendritic cells by the selective glucocorticoid receptor modulator Compound A. Sci Rep 6:36646. https://doi.org/10.1038/srep36646
Louw A, Swart P, de Kock SS, van der Merwe KJ (1997) Mechanism for the stabilization in vivo of the aziridine precursor 2-(4-acetoxyphenyl)-2-chloro-N-methyl-ethylammonium chloride by serum proteins. Biochem Pharmacol 53(2):189–197. https://doi.org/10.1016/s0006-2952(96)00661-2
Ricordi and Rastellini C (2000) Methods in pancreatic islet separation. In: Ricordi C, (Ed.) Methods in cell transplantation. Austin, TX: RG Landes 2000; 433–438
Ricordi C, Lacy PE, Scharp DW (1989) Automated islet isolation from human pancreas. Diabetes 38(1):140–142. https://doi.org/10.2337/diab.38.1.s140
Lakey JR, Warnock GL, Shapiro AM, Korbutt GS, Ao Z, Kneteman NM, Rajotte RV (1999) Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell Transplant 8(3):285–292. https://doi.org/10.1177/096368979900800309
Bottino R, Balamurugan AN, Bertera S, Pietropaolo M, Trucco M, Piganelli JD (2002) Preservation of human islet cell functional mass by anti-oxidative action of a novel SOD mimic compound. Diabetes 51(8):2561–2567. https://doi.org/10.2337/diabetes.51.8.2561
Castro CN, Barcala Tabarrozzi AE, Winnewisser J et al (2014) Curcumin ameliorates autoimmune diabetes. Evidences in accelerated murine models of type 1 diabetes. Clin Exp Immunol 177(1):149–160. https://doi.org/10.1111/cei.12322
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Atorrasagasti C, Onorato A, Gimeno ML et al (2019) SPARC is required for the maintenance of glucose homeostasis and insulin secretion in mice. Clin Sci (Lond) 133(2):351–365. https://doi.org/10.1042/CS20180714
Perone MJ, Larregina AT, Shufesky WJ et al (2006) Transgenic galectin-1 induces maturation of dendritic cells that elicit contrasting responses on naïve and activated T cells. J Immunol 176(12):7207–7220. https://doi.org/10.4049/jimmunol.176.12.7207
Gurzov EN, Ortis F, Cunha DA et al (2009) Signaling by IL-1beta+IFN-gamma and ER stress converge on DP5/Hrk activation: a novel mechanism for pancreatic beta-cell apoptosis. Cell Death Differ 16(11):1539–1550. https://doi.org/10.1038/cdd.2009.99
Burke SJ, Lu D, Sparer TE, Karlstad MD, Collier JJ (2014) Transcription of the gene encoding TNF-α is increased by IL-1β in rat and human islets and β-cell lines. Mol Immunol 62(1):54–62. https://doi.org/10.1016/j.molimm.2014.05.019
Igoillo-Esteve M, Marselli L, Cunha DA et al (2010) Palmitate induces a pro-inflammatory response in human pancreatic islets that mimics CCL2 expression by beta cells in type 2 diabetes. Diabetologia 53(7):1395–1405. https://doi.org/10.1007/s00125-010-1707-y
Eguchi K, Nagai R (2017) Islet inflammation in type 2 diabetes and physiology. J Clin Invest 127(1):14–23. https://doi.org/10.1172/JCI88877
Donath MY, Størling J, Berchtold LA, Billestrup N, Mandrup-Poulsen T (2008) Cytokines and beta-cell biology: from concept to clinical translation. Endocr Rev 29(3):334–350. https://doi.org/10.1210/er.2007-0033
Størling J, Binzer J, Andersson AK et al Nitric oxide contributes to cytokine-induced apoptosis in pancreatic beta cells via potentiation of JNK activity and inhibition of Akt. Diabetologia 48(10):2039–50. https://doi.org/10.1007/s00125-005-1912-2.
Chan JY, Cooney GJ, Biden TJ, Laybutt DR (2011) Differential regulation of adaptive and apoptotic unfolded protein response signalling by cytokine-induced nitric oxide production in mouse pancreatic beta cells. Diabetologia 54(7):1766–1776. https://doi.org/10.1007/s00125-011-2139-z
Brozzi F, Nardelli TR, Lopes M et al (2015) Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia 58:2307–2316. https://doi.org/10.1007/s00125-015-3669-6
Mandrup-Poulsen T (2001) beta-cell apoptosis: stimuli and signaling. Diabetes 50(Suppl 1):S58-63. https://doi.org/10.2337/diabetes.50.2007.s58
Takamura T, Kato I, Kimura N, Nakazawa T, Yonekura H, Takasawa S, Okamoto H (1998) Transgenic mice overexpressing type 2 nitric-oxide synthase in pancreatic beta cells develop insulin-dependent diabetes without insulitis. J Biol Chem 273(5):2493–2496. https://doi.org/10.1074/jbc.273.5.2493
Flodström M, Tyrberg B, Eizirik DL, Sandler S (1999) Reduced sensitivity of inducible nitric oxide synthase-deficient mice to multiple low-dose streptozotocin-induced diabetes. Diabetes 48(4):706–713. https://doi.org/10.2337/diabetes.48.4.706
Jiang X, Zhou Y, Wu KK, Chen Z, Xu A, Cheng KK (2017) APPL1 prevents pancreatic beta cell death and inflammation by dampening NFκB activation in a mouse model of type 1 diabetes. Diabetologia 60(3):464–474. https://doi.org/10.1007/s00125-016-4185-z
Patel S, Santani D (2009) Role of NF-kappa B in the pathogenesis of diabetes and its associated complications. Pharmacol Rep 61:595–603
Grey ST, Arvelo MB, Hasenkamp W, Bach FH, Ferran C (1999) A20 inhibits cytokine-induced apoptosis and nuclear factor kappaB-dependent gene activation in islets. J Exp Med 190(8):1135–1146. https://doi.org/10.1084/jem.190.8.1135
Cardozo AK, Heimberg H, Heremans Y et al (2001) A comprehensive analysis of cytokine-induced and nuclear factor-kappa B-dependent genes in primary rat pancreatic beta-cells. J Biol Chem 276(52):48879–48886. https://doi.org/10.1074/jbc.M108658200
Harding HP, Zeng H, Zhang Y et al (2001) Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell 7(6):1153–1163. https://doi.org/10.1016/s1097-2765(01)00264-7
Back SH, Kaufman RJ (2012) Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem 81:767–793. https://doi.org/10.1146/annurev-biochem-072909-095555
Brozzi F, Eizirik DL (2016) ER stress and the decline and fall of pancreatic beta cells in type 1 diabetes. Ups J Med Sci 121(2):133–139. https://doi.org/10.3109/03009734.2015.1135217
Marhfour I, Lopez XM, Lefkaditis D et al (2012) Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia 55(9):2417–2420. https://doi.org/10.1007/s00125-012-2604-3
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529. https://doi.org/10.1038/nrm2199
Tiedge M, Lortz S, Drinkgern J, Lenzen S (1997) Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 46(11):1733–1742. https://doi.org/10.2337/diab.46.11.1733
Dillon CP, Green DR (2016) Molecular cell biology of apoptosis and necroptosis in cancer. Adv Exp Med Biol 930:1–23. https://doi.org/10.1007/978-3-319-39406-0_1
Baker RG, Hayden MS, Ghosh S (2011) NF-κB, inflammation, and metabolic disease. Cell Metab 13(1):11–22. https://doi.org/10.1016/j.cmet.2010.12.008
Rauner M, Thiele S, Sinningen K et al (2013) Effects of the selective glucocorticoid receptor modulator compound A on bone metabolism and inflammation in male mice with collagen-induced arthritis. Endocrinology 154(10):3719–3728. https://doi.org/10.1210/en.2012-2221
Dewint P, Gossye V, De Bosscher K et al (2008) A plant-derived ligand favoring monomeric glucocorticoid receptor conformation with impaired transactivation potential attenuates collagen-induced arthritis. J Immunol 180(4):2608–2615. https://doi.org/10.4049/jimmunol.180.4.2608
van Loo G, Sze M, Bougarne N (2010) Antiinflammatory properties of a plant-derived nonsteroidal, dissociated glucocorticoid receptor modulator in experimental autoimmune encephalomyelitis. Mol Endocrinol 24(2):310–322. https://doi.org/10.1210/me.2009-0236
Gavrila A, Chachi L, Tliba O, Brightling C, Amrani Y (2015) Effect of the plant derivative Compound A on the production of corticosteroid-resistant chemokines in airway smooth muscle cells. Am J Respir Cell Mol Biol 53(5):728–737. https://doi.org/10.1165/rcmb.2014-0477OC
Gossye V, Elewaut D, Bougarne N et al (2009) Differential mechanism of NF-kappaB inhibition by two glucocorticoid receptor modulators in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 60(11):3241–3250. https://doi.org/10.1002/art.24963
Mylka V, Deckers J, Ratman D et al (2018) The autophagy receptor SQSTM1/p62 mediates anti-inflammatory actions of the selective NR3C1/glucocorticoid receptor modulator compound A (CpdA) in macrophages. Autophagy 14(12):2049–2064. https://doi.org/10.1080/15548627.2018.1495681
Saksida T, Vujicic M, Nikolic I, Stojanovic I, Haegeman G, Stosic-Grujicic S (2014) Compound A, a selective glucocorticoid receptor agonist, inhibits immunoinflammatory diabetes, induced by multiple low doses of streptozotocin in mice. Br J Pharmacol 171(24):5898–5909. https://doi.org/10.1111/bph.12892
Like AA, Rossini AA (1976) Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science 193(4251):415–417. https://doi.org/10.1126/science.180605
Zhang Z, Zhang ZY, Schluesener HJ (2009) Compound A, a plant origin ligand of glucocorticoid receptors, increases regulatory T cells and M2 macrophages to attenuate experimental autoimmune neuritis with reduced side effects. J Immunol 183(5):3081–3091. https://doi.org/10.4049/jimmunol.0901088
Lesovaya E, Yemelyanov A, Swart AC, Swart P, Haegeman G, Budunova I (2015) Discovery of Compound A-a selective activator of the glucocorticoid receptor with anti-inflammatory and anti-cancer activity. Oncotarget 6(31):30730–44. https://doi.org/10.18632/oncotarget.5078.
Acknowledgements
The 5xATF6-LUC and XBP1u-LUC plasmids were a kind gift from Prof. Dr. Sarah Gerlo (Univ. Ghent, Belgium).
Funding
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT #2018–1577 to MJP and #2018–719 to LA); from Universidad Austral (#2020 to MJP and LA); and from Sociedad Argentina de Diabetes (#2018 to MJP and #2019 to LA). Also, we thank the support of Fundación Marjorie para la Investigación en Diabetes (www.fumdiab.org.ar).
Author information
Authors and Affiliations
Contributions
LA and MJP conceived and designed the work. LA, FF and CS performed the in vitro experiments and acquired data. LA, MJP and AEBT performed the animal experiments and acquired data. RB provided de human islets. MSO contributed to sample preparation and data acquisition. LA and MJP interpreted the data and wrote the manuscript. RAD made important contributions in the interpretation of the results. KDB provided CpdA and research tools supported by FWO-Vlaanderen (G044217N) and contributed to the interpretation of data. All authors gave final approval of the version to be published. MJP is the guarantor of this work, has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All experimental procedures were conducted in accordance with the Guide for the care and use of laboratory animals, Eighth edition (2011) and were approved by the Animal Research and Care Committee (#0001 & #0069), FCEyN, University of Buenos Aires.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andreone, L., Fuertes, F., Sétula, C. et al. Compound A attenuates proinflammatory cytokine-induced endoplasmic reticulum stress in beta cells and displays beneficial therapeutic effects in a mouse model of autoimmune diabetes. Cell. Mol. Life Sci. 79, 587 (2022). https://doi.org/10.1007/s00018-022-04615-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04615-5