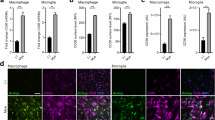
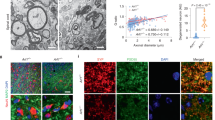
Abstract
Foamy macrophages and microglia containing lipid droplets (LDs) are a pathological hallmark of demyelinating disorders affecting the central nervous system (CNS). We and others showed that excessive accumulation of intracellular lipids drives these phagocytes towards a more inflammatory phenotype, thereby limiting CNS repair. To date, however, the mechanisms underlying LD biogenesis and breakdown in lipid-engorged phagocytes in the CNS, as well as their impact on foamy phagocyte biology and lesion progression, remain poorly understood. Here, we provide evidence that LD-associated protein perilipin-2 (PLIN2) controls LD metabolism in myelin-containing phagocytes. We show that PLIN2 protects LDs from lipolysis-mediated degradation, thereby impairing intracellular processing of myelin-derived lipids in phagocytes. Accordingly, loss of Plin2 stimulates LD turnover in foamy phagocytes, driving them towards a less inflammatory phenotype. Importantly, Plin2-deficiency markedly improves remyelination in the ex vivo brain slice model and in the in vivo cuprizone-induced demyelination model. In summary, we identify PLIN2 as a novel therapeutic target to prevent the pathogenic accumulation of LDs in foamy phagocytes and to stimulate remyelination.






Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Grajchen E, Hendriks JJA, Bogie JFJ (2018) The physiology of foamy phagocytes in multiple sclerosis. Acta Neuropathol Commun 6(1):124
Bogie J et al (2011) Myelin-phagocytosing macrophages modulate autoreactive T cell proliferation. J Neuroinflamm 8(1):85
Bogie JF et al (2012) Myelin-derived lipids modulate macrophage activity by liver X receptor activation. PLoS ONE 7(9):e44998
Bogie JF et al (2013) Myelin alters the inflammatory phenotype of macrophages by activating PPARs. Acta Neuropathol Commun 1:43
Bogie JFJ et al (2020) Stearoyl-CoA desaturase-1 impairs the reparative properties of macrophages and microglia in the brain. J Exp Med 217(5):e20191660
Cantuti-Castelvetri L et al (2018) Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 359(6376):684–688
Haidar M et al (2022) Targeting lipophagy in macrophages improves repair in multiple sclerosis. Autophagy. https://doi.org/10.1080/15548627.2022.2047343
Olzmann JA, Carvalho P (2019) Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol 20(3):137–155
Plakkal-Ayyappan J, Paul A, Goo YH (2016) Lipid droplet-associated proteins in atherosclerosis (Review). Mol Med Rep 13(6):4527–4534
Walther TC, Chung J, Farese RV Jr (2017) Lipid droplet biogenesis. Annu Rev Cell Dev Biol 33:491–510
Shiffman D et al (2000) Large scale gene expression analysis of cholesterol-loaded macrophages. J Biol Chem 275(48):37324–37332
Bickel PE, Tansey JT, Welte MA (2009) PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. BBA-Mol Cell Biol L 1791(6):419–440
Buechler C et al (2001) Adipophilin is a sensitive marker for lipid loading in human blood monocytes. Biochim Biophys Acta 1532(1–2):97–104
Imamura M et al (2002) ADRP stimulates lipid accumulation and lipid droplet formation in murine fibroblasts. Am J Physiol Endocrinol Metab 283(4):E775–E783
Xu S et al (2019) Perilipin 2 and lipid droplets provide reciprocal stabilization. Biophys Rep 5(3):145–160
Paul A et al (2008) Deficiency of adipose differentiation-related protein impairs foam cell formation and protects against atherosclerosis. Circ Res 102(12):1492–1501
Larigauderie G et al (2006) Adipophilin increases triglyceride storage in human macrophages by stimulation of biosynthesis and inhibition of beta-oxidation. FEBS J 273(15):3498–3510
Larigauderie G et al (2004) Adipophilin enhances lipid accumulation and prevents lipid efflux from THP-1 macrophages: potential role in atherogenesis. Arterioscler Thromb Vasc Biol 24(3):504–510
McManaman JL et al (2013) Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J Lipid Res 54(5):1346–1359
Erwig MS et al (2019) Myelin: methods for purification and proteome analysis. Methods Mol Biol 1936:37–63
Jorissen W et al (2017) Relapsing-remitting multiple sclerosis patients display an altered lipoprotein profile with dysfunctional HDL. Sci Rep 7:43410
Cohen S (2018) Chapter three - lipid droplets as organelles. In: Galluzzi L (ed) International review of cell and molecular biology. Academic Press, pp 83–110
Fan B et al (2013) High glucose, insulin and free fatty acid concentrations synergistically enhance perilipin 3 expression and lipid accumulation in macrophages. Metabolism 62(8):1168–1179
Grajchen E et al (2020) CD36-mediated uptake of myelin debris by macrophages and microglia reduces neuroinflammation. J Neuroinflamm 17(1):224
Wouters E et al (2020) Altered PPARγ expression promotes myelin-induced foam cell formation in macrophages in multiple sclerosis. Int J Mol Sci 21(23):9329
Bildirici I et al (2003) The lipid droplet-associated protein adipophilin is expressed in human trophoblasts and is regulated by peroxisomal proliferator-activated receptor-gamma/retinoid X receptor. J Clin Endocrinol Metab 88(12):6056–6062
Schadinger SE et al (2005) PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am J Physiol Endocrinol Metab 288(6):E1195–E1205
Targett-Adams P et al (2005) A PPAR response element regulates transcription of the gene for human adipose differentiation-related protein. Biochim Biophys Acta 1728(1–2):95–104
Bickel PE, Tansey JT, Welte MA (2009) PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta 1791(6):419–440
Kimmel AR et al (2010) Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res 51(3):468–471
Fukushima M et al (2005) Adipose differentiation related protein induces lipid accumulation and lipid droplet formation in hepatic stellate cells. In Vitro Cell Dev Biol Anim 41(10):321–324
Bogie JF, Stinissen P, Hendriks JJ (2014) Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathol 128(2):191–213
Chen FL et al (2010) Adipophilin affects the expression of TNF-alpha, MCP-1, and IL-6 in THP-1 macrophages. Mol Cell Biochem 337(1–2):193–199
Klionsky DJ et al (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12(1):1–222
Singh R et al (2009) Autophagy regulates lipid metabolism. Nature 458(7242):1131–1135
Lampron A et al (2015) Inefficient clearance of myelin debris by microglia impairs remyelinating processes. J Exp Med 212(4):481–495
Becker L et al (2010) A macrophage sterol-responsive network linked to atherogenesis. Cell Metab 11(2):125–135
Son SH et al (2012) Perilipin 2 (PLIN2)-deficiency does not increase cholesterol-induced toxicity in macrophages. PLoS ONE 7(3):e33063
Nocetti D et al (2020) Lipid droplets are both highly oxidized and Plin2-covered in hepatocytes of diet-induced obese mice. Appl Physiol Nutr Metab 45(12):1368–1376
Crunk AE et al (2013) Dynamic regulation of hepatic lipid droplet properties by diet. PLoS ONE 8(7):e67631
Bildirici I et al (2018) PLIN2 is essential for trophoblastic lipid droplet accumulation and cell survival during hypoxia. Endocrinology 159(12):3937–3949
Saher G, Stumpf SK (2015) Cholesterol in myelin biogenesis and hypomyelinating disorders. Biochim Biophys Acta 1851(8):1083–1094
Gouna G et al (2021) TREM2-dependent lipid droplet biogenesis in phagocytes is required for remyelination. J Exp Med 218(10):e20210227
Matsumoto T, Kobayashi T, Kamata K (2007) Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr Med Chem 14(30):3209–3220
Mardani I et al (2019) Plin2-deficiency reduces lipophagy and results in increased lipid accumulation in the heart. Sci Rep 9(1):6909
Tsai TH et al (2017) The constitutive lipid droplet protein PLIN2 regulates autophagy in liver. Autophagy 13(7):1130–1144
Saliba-Gustafsson P et al (2019) Subclinical atherosclerosis and its progression are modulated by PLIN2 through a feed-forward loop between LXR and autophagy. J Intern Med 286(6):660–675
Feng X et al (2014) Autophagy involved in lipopolysaccharide-induced foam cell formation is mediated by adipose differentiation-related protein. Lipids Health Dis 13:10
Zechner R, Madeo F, Kratky D (2017) Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat Rev Mol Cell Biol 18(11):671–684
Listenberger LL et al (2007) Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J Lipid Res 48(12):2751–2761
Russell TD et al (2011) Adipophilin regulates maturation of cytoplasmic lipid droplets and alveolae in differentiating mammary glands. J Cell Sci 124(Pt 19):3247–3253
Ishii T et al (2004) Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res 94(5):609–616
Nagy L et al (1998) Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 93(2):229–240
Bogie JF et al (2017) Scavenger receptor collectin placenta 1 is a novel receptor involved in the uptake of myelin by phagocytes. Sci Rep 7:44794
Haidar M et al (2021) Lipophagy: a new player in CNS disorders. Trends Endocrinol Metab 32(11):941–951
Cingolani F, Czaja MJ (2016) Regulation and functions of autophagic lipolysis. Trends Endocrinol Metab 27(10):696–705
Ouimet M, Marcel YL (2012) Regulation of lipid droplet cholesterol efflux from macrophage foam cells. Arterioscler Thromb Vasc Biol 32(3):575–581
Boven LA et al (2006) Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain 129(Pt 2):517–526
Hikawa N, Takenaka T (1996) Myelin-stimulated macrophages release neurotrophic factors for adult dorsal root ganglion neurons in culture. Cell Mol Neurobiol 16(4):517–528
Acknowledgements
We thank M.P. Tulleners and L. Timmermans for excellent technical assistance.
Funding
The work has been supported by the Flemish Fund for Scientific Research (FWO Vlaanderen; 1141920N, 1S15519N), and the special research fund UHasselt (BOF).
Author information
Authors and Affiliations
Contributions
ML, EW, MH, JFJB, and JJAH conceived experiments. ML, EW, SV, JD, and MH performed experiments. ML, EW, SV, JD and MH analysed data. ML, EW, SV, HK, MH, JFJB, and JJAH discussed results. JLM provided the animals. ML, EW, and JFJB wrote the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests exist.
Ethics statement
Animal experiments in this study were carried out in accordance with the recommendations of the institutional animal care and use committee of Hasselt University. The protocol was approved by the institutional animal care and use committee of Hasselt University (protocol numbers: 201840, 201920, 201953).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Loix, M., Wouters, E., Vanherle, S. et al. Perilipin-2 limits remyelination by preventing lipid droplet degradation. Cell. Mol. Life Sci. 79, 515 (2022). https://doi.org/10.1007/s00018-022-04547-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04547-0