Abstract
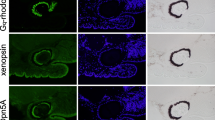
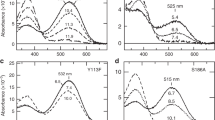
Opsins are universal photoreceptive proteins in animals. Vertebrate rhodopsin in ciliary photoreceptor cells photo-converts to a metastable active state to regulate cyclic nucleotide signaling. This active state cannot photo-convert back to the dark state, and thus vertebrate rhodopsin is categorized as a mono-stable opsin. By contrast, mollusk and arthropod rhodopsins in rhabdomeric photoreceptor cells photo-convert to a stable active state to stimulate IP3/calcium signaling. This active state can photo-convert back to the dark state, and thus these rhodopsins are categorized as bistable opsins. Moreover, the negatively charged counterion position crucial for the visible light sensitivity is different between vertebrate rhodopsin (Glu113) and mollusk and arthropod rhodopsins (Glu181). This can be explained by an evolutionary scenario where vertebrate rhodopsin newly acquired Glu113 as a counterion, which is thought to have led to higher signaling efficiency of vertebrate rhodopsin. However, the detailed evolutionary steps which led to the higher efficiency in vertebrate rhodopsin still remain unknown. Here, we analyzed the xenopsin group, which is phylogenetically distinct from vertebrate rhodopsin and functions in protostome ciliary cells. Xenopsins are blue-sensitive bistable opsins that regulate cAMP signaling. We found that a bistable xenopsin of Leptochiton asellus had Glu113 as a counterion but did not exhibit elevated signaling efficiency. Therefore, our results show that vertebrate rhodopsin and L. asellus xenopsin regulate cyclic nucleotide signaling in ciliary cells and displaced the counterion position from Glu181 to Glu113 via convergent evolution, whereas subsequently only vertebrate rhodopsin elevated its signaling efficiency by acquiring the mono-stable property.




Similar content being viewed by others
Data availability
All data are available in the main text or supplementary materials.
References
Arendt D (2003) Evolution of eyes and photoreceptor cell types. Int J Dev Biol 47:563–571
Shichida Y, Matsuyama T (2009) Evolution of opsins and phototransduction. Philos Trans R Soc Lond B Biol Sci 364:2881–2895
Yau KW, Hardie RC (2009) Phototransduction motifs and variations. Cell 139:246–264
Fain GL, Hardie R, Laughlin SB (2010) Phototransduction and the evolution of photoreceptors. Curr Biol 20:R114-124
Eakin RM (1979) Evolutionary significance of photoreceptors—retrospect. Am Zool 19:647–653
Lamb TD, Collin SP, Pugh EN Jr (2007) Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat Rev Neurosci 8:960–976
Koyanagi M, Terakita A (2014) Diversity of animal opsin-based pigments and their optogenetic potential. Biochim Biophys Acta 1837:710–716
Sakmar TP, Franke RR, Khorana HG (1989) Glutamic acid-113 serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proc Natl Acad Sci USA 86:8309–8313
Zhukovsky EA, Oprian DD (1989) Effect of carboxylic acid side chains on the absorption maximum of visual pigments. Science 246:928–930
Nathans J (1990) Determinants of visual pigment absorbance: identification of the retinylidene Schiff’s base counterion in bovine rhodopsin. Biochemistry 29:9746–9752
Terakita A, Koyanagi M, Tsukamoto H, Yamashita T, Miyata T, Shichida Y (2004) Counterion displacement in the molecular evolution of the rhodopsin family. Nat Struct Mol Biol 11:284–289
Murakami M, Kouyama T (2008) Crystal structure of squid rhodopsin. Nature 453:363–367
Varma N, Mutt E, Muhle J, Panneels V, Terakita A, Deupi X, Nogly P, Schertler GFX, Lesca E (2019) Crystal structure of jumping spider rhodopsin-1 as a light sensitive GPCR. Proc Natl Acad Sci USA 116:14547–14556
Ramirez MD, Pairett AN, Pankey MS, Serb JM, Speiser DI, Swafford AJ, Oakley TH (2016) The last common ancestor of most bilaterian animals possessed at least nine opsins. Genome Biol Evol 8:3640–3652
Kojima K, Yamashita T, Imamoto Y, Kusakabe TG, Tsuda M, Shichida Y (2017) Evolutionary steps involving counterion displacement in a tunicate opsin. Proc Natl Acad Sci USA 114:6028–6033
Lamb TD (2009) Evolution of vertebrate retinal photoreception. Philos Trans R Soc Lond B Biol Sci 364:2911–2924
Tsukamoto H, Farrens DL, Koyanagi M, Terakita A (2009) The magnitude of the light-induced conformational change in different rhodopsins correlates with their ability to activate G proteins. J Biol Chem 284:20676–20683
Sato K, Yamashita T, Ohuchi H, Shichida Y (2011) Vertebrate ancient-long opsin has molecular properties intermediate between those of vertebrate and invertebrate visual pigments. Biochemistry 50:10484–10490
Baldwin MW, Ko MC (2020) Functional evolution of vertebrate sensory receptors. Horm Behav 124:104771
Passamaneck YJ, Furchheim N, Hejnol A, Martindale MQ, Luter C (2011) Ciliary photoreceptors in the cerebral eyes of a protostome larva. EvoDevo 2:6
Vocking O, Kourtesis I, Tumu SC, Hausen H (2017) Co-expression of xenopsin and rhabdomeric opsin in photoreceptors bearing microvilli and cilia. Elife 6:e23435. https://doi.org/10.7554/eLife.23435
Rawlinson KA, Lapraz F, Ballister ER, Terasaki M, Rodgers J, McDowell RJ, Girstmair J, Criswell KE, Boldogkoi M, Simpson F, Goulding D, Cormie C, Hall B, Lucas RJ, Telford MJ (2019) Extraocular, rod-like photoreceptors in a flatworm express xenopsin photopigment. Elife 8:e45465. https://doi.org/10.7554/eLife.45465
Doring CC, Kumar S, Tumu SC, Kourtesis I, Hausen H (2020) The visual pigment xenopsin is widespread in protostome eyes and impacts the view on eye evolution. Elife 9:e55193. https://doi.org/10.7554/eLife.55193
Niwa H, Yamamura K, Miyazaki J (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199
Motohashi K (2017) Seamless ligation cloning extract (SLiCE) method using cell lysates from laboratory Escherichia coli strains and its application to SLiP site-directed mutagenesis. Methods Mol Biol 1498:349–357
Lamb TD (1995) Photoreceptor spectral sensitivities: common shape in the long-wavelength region. Vision Res 35:3083–3091
Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K (2000) In search of the visual pigment template. Vis Neurosci 17:509–528
Tsutsui K, Imai H, Shichida Y (2007) Photoisomerization efficiency in UV-absorbing visual pigments: protein-directed isomerization of an unprotonated retinal Schiff base. Biochemistry 46:6437–6445
Bailes HJ, Lucas RJ (2013) Human melanopsin forms a pigment maximally sensitive to blue light (lambdamax approximately 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades. Proc Biol Sci 280:20122987
Yamashita T, Terakita A, Shichida Y (2000) Distinct roles of the second and third cytoplasmic loops of bovine rhodopsin in G protein activation. J Biol Chem 275:34272–34279
Yamashita T, Ohuchi H, Tomonari S, Ikeda K, Sakai K, Shichida Y (2010) Opn5 is a UV-sensitive bistable pigment that couples with Gi subtype of G protein. Proc Natl Acad Sci USA 107:22084–22089
Lee E, Linder ME, Gilman AG (1994) Expression of G-protein alpha subunits in Escherichia coli. Methods Enzymol 237:146–164
Tachibanaki S, Imai H, Mizukami T, Okada T, Imamoto Y, Matsuda T, Fukada Y, Terakita A, Shichida Y (1997) Presence of two rhodopsin intermediates responsible for transducin activation. Biochemistry 36:14173–14180
Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V (2004) The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J Mol Biol 342:571–583
Yan EC, Kazmi MA, Ganim Z, Hou JM, Pan D, Chang BS, Sakmar TP, Mathies RA (2003) Retinal counterion switch in the photoactivation of the G protein-coupled receptor rhodopsin. Proc Natl Acad Sci USA 100:9262–9267
Ludeke S, Beck M, Yan EC, Sakmar TP, Siebert F, Vogel R (2005) The role of Glu181 in the photoactivation of rhodopsin. J Mol Biol 353:345–356
Tsutsui K, Shichida Y (2010) Multiple functions of Schiff base counterion in rhodopsins. Photochem Photobiol Sci 9:1426–1434
Terakita A, Yamashita T, Nimbari N, Kojima D, Shichida Y (2002) Functional interaction between bovine rhodopsin and G protein transducin. J Biol Chem 277:40–46
Matsuo R, Koyanagi M, Nagata A, Matsuo Y (2019) Co-expression of opsins in the eye photoreceptor cells of the terrestrial slug Limax valentianus. J Comp Neurol 527:3073–3086
Choe HW, Kim YJ, Park JH, Morizumi T, Pai EF, Krauss N, Hofmann KP, Scheerer P, Ernst OP (2011) Crystal structure of metarhodopsin II. Nature 471:651–655
Acknowledgements
We thank Dr. Elizabeth Nakajima for a critical reading of the manuscript and Dr. Keita Sato for technical advice for the cAMP assay and the Ca2+ assay. We also thank Prof. Robert. S. Molday for the generous gift of a Rho1D4-producing hybridoma.
Funding
This work was supported in part by CREST, JST JPMJCR1753 (T.Y.), a grant from the Kyoto University Foundation (T.Y.) and a grant from the Takeda Science Foundation (T.Y.).
Author information
Authors and Affiliations
Contributions
TY designed research; KS, HI, CF and TY performed research; KS, HI, CF, YI and TY analyzed data; TY wrote the manuscript with editing by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no competing interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sakai, K., Ikeuchi, H., Fujiyabu, C. et al. Convergent evolutionary counterion displacement of bilaterian opsins in ciliary cells. Cell. Mol. Life Sci. 79, 493 (2022). https://doi.org/10.1007/s00018-022-04525-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04525-6