Abstract
Thymically-derived Foxp3+ regulatory T cells (Treg) critically control immunological tolerance. These cells are generated in the medulla through high affinity interactions with medullary thymic epithelial cells (mTEC) expressing the Autoimmune regulator (Aire). Recent advances have revealed that thymic Treg contain not only developing but also recirculating cells from the periphery. Although Aire is implicated in the generation of Foxp3+ Treg, its role in the biology of recirculating Treg remains elusive. Here, we show that Aire regulates the suppressive signature of recirculating Treg independently of the remodeling of the medullary 3D organization throughout life where Treg reside. Accordingly, the adoptive transfer of peripheral Foxp3+ Treg in AireKO recipients led to an impaired suppressive signature upon their entry into the thymus. Furthermore, recirculating Treg from AireKO mice failed to attenuate the severity of multiorgan autoimmunity, demonstrating that their suppressive function is altered. Using bone marrow chimeras, we reveal that mTEC-specific expression of Aire controls the suppressive signature of recirculating Treg. Finally, mature mTEC lacking Aire were inefficient in stimulating peripheral Treg both in polyclonal and antigen-specific co-culture assays. Overall, this study demonstrates that Aire confers to mTEC the ability to restimulate recirculating Treg, unravelling a novel function for this master regulator in Treg biology.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. The dataset generated in this study are available in the Gene Expression Omnibus (GEO) database under accession number GSE188419.
Code availability
Not applicable.
References
Owen DL, Mahmud SA, Sjaastad LE, Williams JB, Spanier JA, Simeonov DR et al (2019) Thymic regulatory T cells arise via two distinct developmental programs. Nat Immunol 20(2):195–205
Lio CW, Hsieh CS (2008) A two-step process for thymic regulatory T cell development. Immunity 28(1):100–111
Santamaria JC, Borelli A, Irla M (2021) Regulatory T cell heterogeneity in the thymus: impact on their functional activities. Front Immunol 12:643153
Marshall D, Sinclair C, Tung S, Seddon B (2014) Differential requirement for IL-2 and IL-15 during bifurcated development of thymic regulatory T cells. J Immunol 193(11):5525–5533
Thiault N, Darrigues J, Adoue V, Gros M, Binet B, Perals C et al (2015) Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat Immunol 16:628–634
Cowan JE, McCarthy NI, Anderson G (2016) CCR7 controls thymus recirculation, but not production and emigration, of Foxp3(+) T cells. Cell Rep 14(5):1041–1048
Yang E, Zou T, Leichner TM, Zhang SL, Kambayashi T (2014) Both retention and recirculation contribute to long-lived regulatory T-cell accumulation in the thymus. Eur J Immunol 44(9):2712–2720
Cowan JE, Baik S, McCarthy NI, Parnell SM, White AJ, Jenkinson WE et al (2018) Aire controls the recirculation of murine Foxp3(+) regulatory T-cells back to the thymus. Eur J Immunol 48(5):844–854
Yamano T, Nedjic J, Hinterberger M, Steinert M, Koser S, Pinto S et al (2015) Thymic B cells are licensed to present self antigens for central T cell tolerance induction. Immunity 42:1048–1061
Aricha R, Feferman T, Scott HS, Souroujon MC, Berrih-Aknin S, Fuchs S (2011) The susceptibility of Aire(-/-) mice to experimental myasthenia gravis involves alterations in regulatory T cells. J Autoimmun 36(1):16–24
Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT et al (2011) Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med 208(2):383–394
Malchow S, Leventhal DS, Lee V, Nishi S, Socci ND, Savage PA (2016) Aire enforces immune tolerance by directing autoreactive T cells into the regulatory T cell lineage. Immunity 44(5):1102–1113
Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D (2015) Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science 348(6234):589–594
Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ et al (2002) Projection of an immunological self shadow within the thymus by the aire protein. Science 298(5597):1395–1401
Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C (2005) Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med 202(6):805–815
Kuroda N, Mitani T, Takeda N, Ishimaru N, Arakaki R, Hayashi Y et al (2005) Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol 174(4):1862–1870
Ramsey C, Winqvist O, Puhakka L, Halonen M, Moro A, Kämpe O et al (2002) Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet 11(4):397–409
Consortium. F-GA (1997) An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet 17(4):399–403
Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M et al (1997) Positional cloning of the APECED gene. Nat Genet 17(4):393–398
Ryan KR, Lawson CA, Lorenzi AR, Arkwright PD, Isaacs JD, Lilic D (2005) CD4+CD25+ T-regulatory cells are decreased in patients with autoimmune polyendocrinopathy candidiasis ectodermal dystrophy. J Allergy Clin Immunol 116(5):1158–1159
Kekäläinen E, Tuovinen H, Joensuu J, Gylling M, Franssila R, Pöntynen N et al (2007) A defect of regulatory T cells in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Immunol 178(2):1208–1215
Laakso SM, Laurinolli TT, Rossi LH, Lehtoviita A, Sairanen H, Perheentupa J et al (2010) Regulatory T cell defect in APECED patients is associated with loss of naive FOXP3(+) precursors and impaired activated population. J Autoimmun 35(4):351–357
Sansom SN, Shikama-Dorn N, Zhanybekova S, Nusspaumer G, Macaulay IC, Deadman ME, et al (2014) Population and single-cell genomics reveal the aire dependency, relief from polycomb silencing, and distribution of self-antigen expression in thymic epithelia. Genome Res 24(12):1918–1931
Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M et al (1992) RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68(5):855–867
Wang Y, Kissenpfennig A, Mingueneau M, Richelme S, Perrin P, Chevrier S et al (2008) Th2 lymphoproliferative disorder of LatY136F mutant mice unfolds independently of TCR-MHC engagement and is insensitive to the action of Foxp3+ regulatory T cells. J Immunol 180(3):1565–1575
Barnden MJ, Allison J, Heath WR, Carbone FR (1998) Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol 76(1):34–40
Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H (1996) Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med 184(3):923–930
Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14(4):R36
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ et al (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28(5):511–515
Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L (2013) Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol 31(1):46–53
Pavlidis P, Noble WS (2003) Matrix2png: a utility for visualizing matrix data. Bioinformatics 19(2):295–296
Irla M, Guenot J, Sealy G, Reith W, Imhof BA, Serge A (2013) Three-dimensional visualization of the mouse thymus organization in health and immunodeficiency. J Immunol 190(2):586–596
Serge A, Bailly AL, Aurrand-Lions M, Imhof BA, Irla M (2015) For3D: full organ reconstruction in 3D, an automatized tool for deciphering the complexity of lymphoid organs. J Immunol Methods 424:32–42
Perniola R (2018) Twenty Years of AIRE Front Immunol 9:98
Klug DB, Carter C, Crouch E, Roop D, Conti CJ, Richie ER (1998) Interdependence of cortical thymic epithelial cell differentiation and T-lineage commitment. Proc Natl Acad Sci USA 95(20):11822–11827
Fontenot JD, Dooley JL, Farr AG, Rudensky AY (2005) Developmental regulation of Foxp3 expression during ontogeny. J Exp Med 202(7):901–906
Cheng G, Yuan X, Tsai MS, Podack ER, Yu A, Malek TR (2012) IL-2 receptor signaling is essential for the development of Klrg1+ terminally differentiated T regulatory cells. J Immunol 189(4):1780–1791
Cretney E, Kallies A, Nutt SL (2013) Differentiation and function of Foxp3(+) effector regulatory T cells. Trends Immunol 34(2):74–80
Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C et al (2014) Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 40(4):569–581
Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R et al (2003) Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol 4(12):1206–1212
Garín MI, Chu CC, Golshayan D, Cernuda-Morollón E, Wait R, Lechler RI (2007) Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood 109(5):2058–2065
Frias AB Jr, Hyzny EJ, Buechel HM, Beppu LY, Xie B, Jurczak MJ et al (2019) The transcriptional regulator Id2 is critical for adipose-resident regulatory T cell differentiation, survival, and function. J Immunol 203(3):658–664
Sawant DV, Vignali DA (2014) Once a Treg, always a Treg? Immunol Rev 259(1):173–191
Peligero-Cruz C, Givony T, Sebé-Pedrós A, Dobeš J, Kadouri N, Nevo S et al (2020) IL18 signaling promotes homing of mature Tregs into the thymus. Elife 9:e58213
Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D (2005) The cellular mechanism of Aire control of T cell tolerance. Immunity 23(2):227–239
Gray D, Abramson J, Benoist C, Mathis D (2007) Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med 204(11):2521–2528
Mahmud SA, Manlove LS, Schmitz HM, Xing Y, Wang Y, Owen DL et al (2014) Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat Immunol 15(5):473–481
Rodewald HR, Paul S, Haller C, Bluethmann H, Blum C (2001) Thymus medulla consisting of epithelial islets each derived from a single progenitor. Nature 414(6865):763–768
Irla M, Guerri L, Guenot J, Serge A, Lantz O, Liston A et al (2012) Antigen recognition by autoreactive cd4(+) thymocytes drives homeostasis of the thymic medulla. PLoS ONE 7(12):e52591
Lopes N, Serge A, Ferrier P, Irla M (2015) Thymic crosstalk coordinates medulla organization and T-cell tolerance induction. Front Immunol 6:365
Lopes N, Boucherit N, Santamaria JC, Provin N, Charaix J, Ferrier P, et al (2022) Thymocytes trigger self-antigen-controlling pathways in immature medullary thymic epithelial stages. Elife 11:e69982
Borelli A, Irla M (2021) Lymphotoxin: from the physiology to the regeneration of the thymic function. Cell Death Differ 28(8):2305–2314
Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC (2003) Aire regulates negative selection of organ-specific T cells. Nat Immunol 4(4):350–354
Cowan JE, Parnell SM, Nakamura K, Caamano JH, Lane PJ, Jenkinson EJ et al (2013) The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. J Exp Med 210(4):675–681
Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK et al (2007) Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol 8(4):351–358
Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP et al (2013) Aire-dependent thymic development of tumor-associated regulatory T cells. Science 339(6124):1219–1224
Weist BM, Kurd N, Boussier J, Chan SW, Robey EA (2015) Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat Immunol 16(6):635–641
Acknowledgements
We are grateful to Georg Holländer (University of Basel, Switzerland) and Bernard Malissen (CIML, Marseille, France) for providing us AireKO and Foxp3eGFP mice, respectively. We thank the CIML flow cytometry, histology, PICSL imaging facility of the CIML (ImagImm) and animal facility platforms for technical support. We thank Cloé Zamit (CIML, France) for help with mouse genotyping.
Funding
This work was supported by institutional grants from INSERM, CNRS and Aix-Marseille Université. The Immune Tolerance and T-Cell Differentiation laboratory received funding from the ARC Foundation (PJA20171206491 to M.I.), CoPoC-proof of concept (MAT-PI-17326-A-01 to M.I.), a prematuration grant from A*MIDEX, a French “Investissements d'avenir” program (LTalpha-Treg to M.I.) and Agence Nationale de la Recherche (grant ANR-19-CE18-0021–01, RANKLthym to M.I.). We also acknowledge financial support from France Bio Imaging (ANR-10-INBS-04–01) and France Génomique national infrastructure, funded as part of the "Investissements d'Avenir" program managed by the ANR (ANR-10-INBS-0009). J.C. and A.B. were supported by a PhD fellowship from the Ministère de l’Enseignement Supérieur et de la Recherche et de l’Innovation (MESRI).
Author information
Authors and Affiliations
Contributions
JC, AB, JCS, LC and MI conducted the experiments, analyzed and interpreted the data. MG and AS analyzed the data. JC, AB, JCS and MI wrote the manuscript. MI initiated, supervised and conceived the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments were done in accordance with National and European laws for laboratory animal welfare (EEC Council Directive 2010/63/UE) and the Marseille Ethical Committee for Animal experimentation.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Movie S1. 3D rotation of AireWT thymic lobe of 9-day-old mouse, with medullary compartment colored according to their volume. AireWT thymic lobe (DAPI, blue) of a 9-day-old mouse is rendered in 3D with medullary compartments (Keratin 14, pseudo-colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Movie S2. 3D rotation of AireKO thymic lobe of 9-day-old mouse, with medullary compartment colored according to their volume. AireKO thymic lobe (DAPI, blue) of a 9-day-old mouse is rendered in 3D with medullary compartments (Keratin 14, pseudo-colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Movie S3. 3D rotation of AireWT thymic lobe of 6-week-old mouse, with medullary compartment colored according to their volume. AireWT thymic lobe (DAPI, blue) of a 6-week-old mouse is rendered in 3D with medullary compartments (Keratin 14, pseudo-colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Movie S4. 3D rotation of AireKO thymic lobe of 6-week-old mouse, with medullary compartment colored according to their volume. AireKO thymic lobe (DAPI, blue) of a 6-week-old mouse is rendered in 3D with medullary compartments (Keratin 14, pseudo-colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Movie S5. 3D rotation of AireWT thymic lobe of 1-year-old mouse, with medullary compartment colored according to their volume. AireWT thymic lobe (DAPI, blue) of a 1-year-old mouse is rendered in 3D with medullary compartments (Keratin 14, pseudo-colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
Movie S6. 3D rotation of AireKO thymic lobe of 1-year-old mouse, with medullary compartment colored according to their volume. AireKO thymic lobe (DAPI, blue) of a 1-year-old mouse is rendered in 3D with medullary compartments (keratin 14, pseudo colors) encoded according to medullary volumes from cyan (smallest medullae) to magenta (largest medullae). All axes are graduated with 500-µm grid spacing
18_2022_4328_MOESM7_ESM.pdf
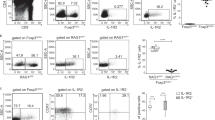
Fig. S1 Gating strategy used to purify recirculating CCR6+ Treg in the thymus. CD4+CD25+ cells were identified in CCR6+CD4+ T cells and analyzed for Foxp3 expression by flow cytometry.
Fig. S2 The suppressive signature of thymic CCR6+ Treg from 1-year-old AireKO mice is altered. The expression level of Foxp3, Klrg1, Il10, Gzmb, Fasl, Entpd1 and Nt5e was measured by qPCR in thymic CCR6+ Treg from 1-year-old AireWT (n=5-6) and AireKO (n=9) mice. Bar graphs show mean ± SEM, ns>0.05, ***p<0.001, ****p<0.0001 using two-tailed Mann–Whitney test.
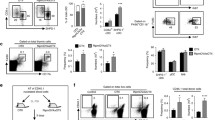
Fig. S3 Splenic AireKO Treg show a normal suppressive signature throughout life. A, B Flow cytometry profiles, frequencies and numbers of CD4+Foxp3+ Treg in the blood of 6-week- (A) and 1-year-old (B) AireWT and AireKO mice. C, D Flow cytometry profiles, frequencies and numbers of CD4+Foxp3+ Treg in the spleen of 6-week- (C) and 1-year-old (D) AireWT or AireKO mice. Data are derived from 2 independent experiments (n=2-5 mice per group and per experiment). E, F The expression level of Foxp3, Il10, Tgfb1, Gzmb, Fasl, Lag3, Entpd1 and Nt5e was measured by qPCR in splenic Treg from 6-week- (E) and 1-year- (F) old AireWT (n=4-9 for 6 wk and n=8-13 for 1 yr) and AireKO (n=5-9 for 6 wk and n=8-13 for 1 yr) mice. Bar graphs show mean ± SEM, *p<0.05 and **p<0.01 using unpaired Student’s t test for C, D.
Fig. S4 The adoptive transfer of thymic CCR6+ Treg from AireKO mice fail to attenuate peripheral tissue infiltration. A Gating strategy used to sort CCR6+CD4+CD8-CD25+ cells, corresponding to CCR6+ Treg from the thymus of 6-week-old AireWT and AireKO mice. B Flow cytometry profiles and numbers of CD45.2 donor Treg in inguinal lymph nodes. C Flow cytometry profiles, frequencies and numbers of CD45.1 infiltrating cells in the pancreas, eyes and salivary glands. D Flow cytometry profiles and numbers of CD4+ and CD8+ T cells of CD45.1 origin infiltrating the pancreas, eyes and salivary glands. Data are derived from 3 independent experiments (n=2-5 mice per group and per experiment). Bar graphs show mean ± SEM, *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001 using two-tailed Mann–Whitney test for B or using unpaired Student’s t test for C,D.
Fig. S5 Aire expression in hematopoietic cells does not control the recirculation and suppressive signature of thymic CCR6+ Treg. A Experimental setup: Lethally irradiated CD45.1/2 WT recipients were reconstituted with CD45.2 AireWT or AireKO BM cells. Six weeks later, the recirculation and the suppressive signature of thymic CCR6+ Treg of CD45.2 origin were analyzed by flow cytometry and qPCR, respectively. B,C Flow cytometry profiles, frequencies and numbers of total B220+CD19+ B cells (B) and of IgD- or IgD+ cells in B220+CD19+ B cells (C). D,E Flow cytometry profiles, frequencies and numbers of CD25+ TregP, Foxp3lo TregP and CD25+Foxp3+ Treg (D) as well as CCR6- and CCR6+ cells in total CD25+Foxp3+ Treg (E). F The expression level of Foxp3, Klrg1, Il10, Gzmb, Fasl, Lag3, Entpd1 and Nt5e was measured by qPCR in CCR6+ Treg of CD45.2 origin purified from the thymus of AireWT (n=8) and AireKO (n=8) BM chimeric mice. Data are derived from 2 independent experiments (n=4 mice per group and per experiment). Bar graphs show mean ± SEM, *p<0.05 using two-tailed Mann–Whitney test for B, D.
Fig. S6 Gating strategy used to purify Aire+ mTEChi. Aire+ mTEChi were identified as EpCAM+Ly51-/loCD80+AireeGFP cells and purified from Airehet (AireeGFP/WT) and AireKO (AireeGFP/eGFP) mice.
Fig. S7 AireKO mTEC express reduced levels of OX40L and GITRL. A Representative flow cytometry profiles of MHCII in mTEC from AireWT and AireKO mice. The histogram shows the frequency of MCHII+ mTEC. Bar graphs show mean ± SEM, **p<0.001 using two-tailed Mann–Whitney. B-C AireKO mTEC express reduced levels of OX40L and GITRL. Expression levels of Tnfsf4 (OX40L) and Tnfsf18 (GITRL) measured by RNA-seq (B) and flow cytometry (C) in AireWT and AireKO mTEChi. Bar graphs show mean ± SEM, **p<0.01 using two-tailed Mann–Whitney test for A.
Table S1. FPKM values of RNA-seq data derived from thymic CCR6+ Treg from AireWT and AireKO mice.
Table S2. List of antibodies used for flow cytometry.
Table S3. List of primers used for RT-qPCR.
Rights and permissions
About this article
Cite this article
Charaix, J., Borelli, A., Santamaria, J.C. et al. Recirculating Foxp3+ regulatory T cells are restimulated in the thymus under Aire control. Cell. Mol. Life Sci. 79, 355 (2022). https://doi.org/10.1007/s00018-022-04328-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04328-9