Abstract
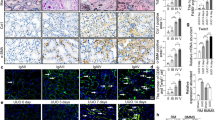
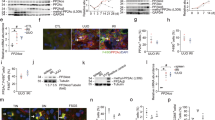
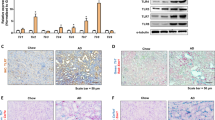
The crosstalk between macrophages and tubular epithelial cells (TECs) actively regulates the progression of renal fibrosis. In the present study, we revealed the significance of circular RNA ACTR2 (circACTR2) in regulating macrophage inflammation, epithelial–mesenchymal transition (EMT) of TECs, and the development of renal fibrosis. Our results showed UUO-induced renal fibrosis was associated with increased inflammation and EMT, hypertrophy of contralateral kidney, up-regulations of circACTR2 and NLRP3, and the down-regulation of miR-561. CircACTR2 sufficiently and essentially promoted the activation of NLRP3 inflammasome, pyroptosis, and inflammation in macrophages, and through paracrine effect, stimulated EMT and fibrosis of TECs. Mechanistically, circACTR2 sponged miR-561 and up-regulated NLRP3 expression level to induce the secretion of IL-1β. In TECs, IL-1β induced renal fibrosis via up-regulating fascin-1. Knocking down circACTR2 or elevating miR-561 potently alleviated renal fibrosis in vivo. In summary, circACTR2, by sponging miR-561, activated NLRP3 inflammasome, promoted macrophage inflammation, and stimulated macrophage-induced EMT and fibrosis of TECs. Knocking down circACTR2 and overexpressing miR-561 may, thus, benefit the treatment of renal fibrosis.
Graphical abstract










Similar content being viewed by others
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Nogueira A, Pires MJ, Oliveira PA (2017) Pathophysiological mechanisms of renal fibrosis: a review of animal models and therapeutic strategies. In Vivo 31(1):1–22
Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C (2019) A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int 96(5):1048–1050
Humphreys BD (2018) Mechanisms of renal fibrosis. Annu Rev Physiol 80:309–326
Zhong J, Yang HC, Fogo AB (2017) A perspective on chronic kidney disease progression. Am J Physiol Renal Physiol 312(3):F375–F384
Grande MT, Sanchez-Laorden B, Lopez-Blau C, De Frutos CA, Boutet A, Arevalo M et al (2015) Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med 21(9):989–997
Lovisa S, LeBleu VS, Tampe B, Sugimoto H, Vadnagara K, Carstens JL et al (2015) Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med 21(9):998–1009
Kalluri R (2009) EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest 119(6):1417–1419
Lopez-Novoa JM, Nieto MA (2009) Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med 1(6–7):303–314
Stone RC, Pastar I, Ojeh N, Chen V, Liu S, Garzon KI et al (2016) Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res 365(3):495–506
Masola V, Carraro A, Granata S, Signorini L, Bellin G, Violi P et al (2019) In vitro effects of interleukin (IL)-1 beta inhibition on the epithelial-to-mesenchymal transition (EMT) of renal tubular and hepatic stellate cells. J Transl Med 17(1):12
Meng XM (2019) Inflammatory mediators and renal fibrosis. Adv Exp Med Biol 1165:381–406
Tan TK, Zheng G, Hsu TT, Lee SR, Zhang J, Zhao Y et al (2013) Matrix metalloproteinase-9 of tubular and macrophage origin contributes to the pathogenesis of renal fibrosis via macrophage recruitment through osteopontin cleavage. Lab Invest 93(4):434–449
Tan TK, Zheng G, Hsu TT, Wang Y, Lee VW, Tian X et al (2010) Macrophage matrix metalloproteinase-9 mediates epithelial-mesenchymal transition in vitro in murine renal tubular cells. Am J Pathol 176(3):1256–1270
Yu CC, Chien CT, Chang TC (2016) M2 macrophage polarization modulates epithelial-mesenchymal transition in cisplatin-induced tubulointerstitial fibrosis. Biomedicine (Taipei) 6(1):5
Kelley N, Jeltema D, Duan Y, He Y (2019) The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. https://doi.org/10.3390/ijms20133328
Zhang H, Wang Z (2019) Effect and regulation of the NLRP3 inflammasome during renal fibrosis. Front Cell Dev Biol 7:379
Wen S, Li S, Li L, Fan Q (2020) circACTR2: a novel mechanism regulating high glucose-induced fibrosis in renal tubular cells via pyroptosis. Biol Pharm Bull 43(3):558–564
Yun J, Ren J, Liu Y, Dai L, Song L, Ma X et al (2021) Circ-ACTR2 aggravates the high glucose-induced cell dysfunction of human renal mesangial cells through mediating the miR-205-5p/HMGA2 axis in diabetic nephropathy. Diabetol Metab Syndr 13(1):72
Chen LL (2020) The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol 21(8):475–490
Soji K, Doi S, Nakashima A, Sasaki K, Doi T, Masaki T (2018) Deubiquitinase inhibitor PR-619 reduces Smad4 expression and suppresses renal fibrosis in mice with unilateral ureteral obstruction. PLoS ONE 13(8):e0202409
Panda AC (2018) Circular RNAs act as miRNA sponges. Adv Exp Med Biol 1087:67–79
Lee MK, Park JH, Gi SH, Hwang YS (2018) IL-1beta induces fascin expression and increases cancer invasion. Anticancer Res 38(11):6127–6132
Zhang X, Cho IH, Park JH, Lee MK, Hwang YS (2019) Fascin is involved in cancer cell invasion and is regulated by stromal factors. Oncol Rep 41(1):465–474
Fu H, Gu YH, Yang YN, Liao S, Wang GH (2020) MiR-200b/c family inhibits renal fibrosis through modulating epithelial-to-mesenchymal transition via targeting fascin-1/CD44 axis. Life Sci 252:117589
Gewin LS (2018) Renal fibrosis: primacy of the proximal tubule. Matrix Biol 68–69:248–262
Cao Q, Harris DC, Wang Y (2015) Macrophages in kidney injury, inflammation, and fibrosis. Physiology (Bethesda) 30(3):183–194
Song S, Qiu D, Luo F, Wei J, Wu M, Wu H et al (2018) Knockdown of NLRP3 alleviates high glucose or TGFB1-induced EMT in human renal tubular cells. J Mol Endocrinol 61(3):101–113
Zhuang Y, Ding G, Zhao M, Bai M, Yang L, Ni J et al (2014) NLRP3 inflammasome mediates albumin-induced renal tubular injury through impaired mitochondrial function. J Biol Chem 289(36):25101–25111
Anders HJ, Suarez-Alvarez B, Grigorescu M, Foresto-Neto O, Steiger S, Desai J et al (2018) The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1-mediated tissue injury. Kidney Int 93(3):656–669
Jin J, Sun H, Shi C, Yang H, Wu Y, Li W et al (2020) Circular RNA in renal diseases. J Cell Mol Med 24(12):6523–6533
Lin J, Jiang Z, Liu C, Zhou D, Song J, Liao Y et al (2020) Emerging roles of long non-coding RNAs in renal fibrosis. Life (Basel). https://doi.org/10.3390/life10080131
Ge X, Xi L, Wang Q, Li H, Xia L, Cang Z et al (2020) Circular RNA Circ_0000064 promotes the proliferation and fibrosis of mesangial cells via miR-143 in diabetic nephropathy. Gene 758:144952
Hu W, Han Q, Zhao L, Wang L (2019) Circular RNA circRNA_15698 aggravates the extracellular matrix of diabetic nephropathy mesangial cells via miR-185/TGF-beta1. J Cell Physiol 234(2):1469–1476
Liu R, Zhang M, Ge Y (2021) Circular RNA HIPK3 exacerbates diabetic nephropathy and promotes proliferation by sponging miR-185. Gene 765:145065
Wei M, Wang L, Wu T, Xi J, Han Y, Yang X et al (2016) NLRP3 activation was regulated by DNA methylation modification during Mycobacterium tuberculosis infection. Biomed Res Int 2016:4323281
Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D et al (2009) Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183(2):787–791
Cao B, Wang T, Qu Q, Kang T, Yang Q (2018) Long noncoding RNA SNHG1 promotes neuroinflammation in Parkinson’s disease via regulating miR-7/NLRP3 pathway. Neuroscience 388:118–127
Cong J, Gong J, Yang C, Xia Z, Zhang H (2020) miR-22 suppresses tumor invasion and metastasis in colorectal cancer by targeting NLRP3. Cancer Manag Res 12:5419–5429
Wu X, Ji H, Wang Y, Gu C, Gu W, Hu L et al (2019) Melatonin alleviates radiation-induced lung injury via regulation of miR-30e/NLRP3 axis. Oxid Med Cell Longev 2019:4087298
Xu W, Wang Y, Ma Y, Yang J (2020) MiR-223 plays a protecting role in neutrophilic asthmatic mice through the inhibition of NLRP3 inflammasome. Respir Res 21(1):116
Fernandes-Alnemri T, Kang S, Anderson C, Sagara J, Fitzgerald KA, Alnemri ES (2013) Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J Immunol 191(8):3995–3999
Lin KM, Hu W, Troutman TD, Jennings M, Brewer T, Li X et al (2014) IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc Natl Acad Sci U S A 111(2):775–780
Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J (2013) Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell 49(2):331–338
Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J et al (2017) NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol Cell 68(1):185–97.e6
Spalinger MR, Kasper S, Gottier C, Lang S, Atrott K, Vavricka SR et al (2016) NLRP3 tyrosine phosphorylation is controlled by protein tyrosine phosphatase PTPN22. J Clin Invest 126(5):1783–1800
Yang Y, Wang Y, He Z, Liu Y, Chen C, Wang Y et al (2020) Trimetazidine inhibits renal tubular epithelial cells to mesenchymal transition in diabetic rats via upregulation of Sirt1. Front Pharmacol 11:1136
Lv ZM, Wang Q, Wan Q, Lin JG, Hu MS, Liu YX et al (2011) The role of the p38 MAPK signaling pathway in high glucose-induced epithelial-mesenchymal transition of cultured human renal tubular epithelial cells. PLoS ONE 6(7):e22806
Bai Y, Lu H, Lin C, Xu Y, Hu D, Liang Y et al (2016) Sonic hedgehog-mediated epithelial-mesenchymal transition in renal tubulointerstitial fibrosis. Int J Mol Med 37(5):1317–1327
Hayashi Y, Osanai M, Lee GH (2011) Fascin-1 expression correlates with repression of E-cadherin expression in hepatocellular carcinoma cells and augments their invasiveness in combination with matrix metalloproteinases. Cancer Sci 102(6):1228–1235
Li A, Morton JP, Ma Y, Karim SA, Zhou Y, Faller WJ et al (2014) Fascin is regulated by slug, promotes progression of pancreatic cancer in mice, and is associated with patient outcomes. Gastroenterology 146(5):1386-96 e1–17
Mao X, Duan X, Jiang B (2016) Fascin induces epithelial-mesenchymal transition of cholangiocarcinoma cells by regulating wnt/beta-catenin signaling. Med Sci Monit 22:3479–3485
Funding
This work was supported by General Project of Health Committee of Hunan Province (202103050649) and Natural Science Funds for Youth Fund of Hunan Province (2018JJ3781).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
All animal procedures received approval from the Animal Care and Use Committee of the third Xiangya Hospital, Central South University (Changsha, China).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, H., Gu, YH., Tan, J. et al. CircACTR2 in macrophages promotes renal fibrosis by activating macrophage inflammation and epithelial–mesenchymal transition of renal tubular epithelial cells. Cell. Mol. Life Sci. 79, 253 (2022). https://doi.org/10.1007/s00018-022-04247-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04247-9