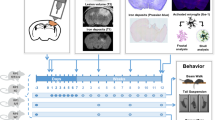
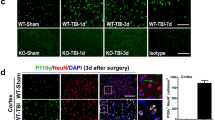
Abstract
Traumatic brain injury (TBI), often induced by sports, car accidents, falls, or other daily occurrences, is a primary non-genetically related risk factor for the development of subsequent neurodegeneration and neuronal cell death. However, the molecular mechanisms underlying neurodegeneration, cell death, and neurobehavioral dysfunction following TBI remain unclear. Here, we found that poly(ADP-ribose) polymerase-1 (PARP-1) was hyperactivated following TBI and its inhibition reduced TBI-induced brain injury. Macrophage migration inhibitory factor (MIF), a newly identified nuclease involved in PARP-1-dependent cell death, was translocated from the cytosol to the nucleus in cortical neurons following TBI and promoted neuronal cell death in vivo. Genetic deletion of MIF protected neurons from TBI-induced dendritic spine loss, morphological complexity degeneration, and subsequent neuronal cell death in mice. Moreover, MIF knockout reduced the brain injury volume and improved long-term animal behavioral rehabilitation. These neuroprotective effects in MIF knockout mice were reversed by the expression of wild-type MIF but not nuclease-deficient MIF mutant. In contrast, genetic deletion of MIF did not alter TBI-induced neuroinflammation. These findings reveal that MIF mediates TBI-induced neurodegeneration, neuronal cell death and neurobehavioral dysfunction through its nuclease activity, but not its pro-inflammatory role. Targeting MIF’s nuclease activity may offer a novel strategy to protect neurons from TBI.








Similar content being viewed by others
Availability of supporting data
The datasets used and/or analyzed during the current study are included in this article.
References
Fernandez-Gajardo R, Matamala JM, Carrasco R, Gutierrez R, Melo R, Rodrigo R (2014) Novel therapeutic strategies for traumatic brain injury: acute antioxidant reinforcement. CNS Drugs 28(3):229–248. https://doi.org/10.1007/s40263-013-0138-y
Meaney DF, Morrison B, Dale Bass C (2014) The mechanics of traumatic brain injury: a review of what we know and what we need to know for reducing its societal burden. J Biomech Eng 136(2):021008. https://doi.org/10.1115/1.4026364
Graham NS, Sharp DJ (2019) Understanding neurodegeneration after traumatic brain injury: from mechanisms to clinical trials in dementia. J Neurol Neurosurg Psychiatry 90(11):1221–1233. https://doi.org/10.1136/jnnp-2017-317557
Gupta R, Sen N (2016) Traumatic brain injury: a risk factor for neurodegenerative diseases. Rev Neurosci 27(1):93–100. https://doi.org/10.1515/revneuro-2015-0017
Barr TL, Conley YP (2007) Poly(ADP-ribose) polymerase-1 and its clinical applications in brain injury. J Neurosci Nurs 39(5):278–284. https://doi.org/10.1097/01376517-200710000-00004
Komjati K, Besson VC, Szabo C (2005) Poly (adp-ribose) polymerase inhibitors as potential therapeutic agents in stroke and neurotrauma. Curr Drug Targets CNS Neurol Disord 4(2):179–194. https://doi.org/10.2174/1568007053544138
Wang Y, An R, Umanah GK, Park H, Nambiar K, Eacker SM, Kim B, Bao L, Harraz MM, Chang C, Chen R, Wang JE, Kam TI, Jeong JS, Xie Z, Neifert S, Qian J, Andrabi SA, Blackshaw S, Zhu H, Song H, Ming GL, Dawson VL, Dawson TM (2016) A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science. https://doi.org/10.1126/science.aad6872
Wang Y, Dawson VL, Dawson TM (2009) Poly(ADP-ribose) signals to mitochondrial AIF: a key event in parthanatos. Exp Neurol 218(2):193–202. https://doi.org/10.1016/j.expneurol.2009.03.020
Wang Y, Luo W, Wang Y (2019) PARP-1 and its associated nucleases in DNA damage response. DNA Repair (Amst) 81:102651. https://doi.org/10.1016/j.dnarep.2019.102651
Abeti R, Abramov AY, Duchen MR (2011) Beta-amyloid activates PARP causing astrocytic metabolic failure and neuronal death. Brain 134(Pt 6):1658–1672. https://doi.org/10.1093/brain/awr104
Kam TI, Mao X, Park H, Chou SC, Karuppagounder SS, Umanah GE, Yun SP, Brahmachari S, Panicker N, Chen R, Andrabi SA, Qi C, Poirier GG, Pletnikova O, Troncoso JC, Bekris LM, Leverenz JB, Pantelyat A, Ko HS, Rosenthal LS, Dawson TM, Dawson VL (2018) Poly(ADP-ribose) drives pathologic alpha-synuclein neurodegeneration in Parkinson’s disease. Science. https://doi.org/10.1126/science.aat8407
Love S, Barber R, Wilcock GK (1999) Increased poly(ADP-ribosyl)ation of nuclear proteins in Alzheimer’s disease. Brain 122(Pt 2):247–253. https://doi.org/10.1093/brain/122.2.247
Paldino E, D’Angelo V, Laurenti D, Angeloni C, Sancesario G, Fusco FR (2020) Modulation of inflammasome and pyroptosis by olaparib, a PARP-1 inhibitor, in the R6/2 mouse model of Huntington’s disease. Cells. https://doi.org/10.3390/cells9102286
Rulten SL, Rotheray A, Green RL, Grundy GJ, Moore DA, Gomez-Herreros F, Hafezparast M, Caldecott KW (2014) PARP-1 dependent recruitment of the amyotrophic lateral sclerosis-associated protein FUS/TLS to sites of oxidative DNA damage. Nucleic Acids Res 42(1):307–314. https://doi.org/10.1093/nar/gkt835
Wang Y, Kim NS, Haince JF, Kang HC, David KK, Andrabi SA, Poirier GG, Dawson VL, Dawson TM (2011) Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase-1-dependent cell death (parthanatos). Sci Signal 4(167):ra20. https://doi.org/10.1126/scisignal.2000902
Newell-Rogers MK, Rogers SK, Tobin RP, Mukherjee S, Shapiro LA (2020) Antagonism of macrophage migration inhibitory factory (MIF) after traumatic brain injury ameliorates astrocytosis and peripheral lymphocyte activation and expansion. Int J Mol Sci. https://doi.org/10.3390/ijms21207448
Zhang S, Zhao J, Zhang Y, Zhang Y, Cai F, Wang L, Song W (2019) Upregulation of MIF as a defense mechanism and a biomarker of Alzheimer’s disease. Alzheimers Res Ther 11(1):54. https://doi.org/10.1186/s13195-019-0508-x
Nasiri E, Sankowski R, Dietrich H, Oikonomidi A, Huerta PT, Popp J, Al-Abed Y, Bacher M (2020) Key role of MIF-related neuroinflammation in neurodegeneration and cognitive impairment in Alzheimer’s disease. Mol Med 26(1):34. https://doi.org/10.1186/s10020-020-00163-5
Liu YC, Tsai YH, Tang SC, Liou HC, Kang KH, Liou HH, Jeng JS, Fu WM (2018) Cytokine MIF enhances blood–brain barrier permeability: impact for therapy in ischemic stroke. Sci Rep 8(1):743. https://doi.org/10.1038/s41598-017-16927-9
Inacio AR, Ruscher K, Leng L, Bucala R, Deierborg T (2011) Macrophage migration inhibitory factor promotes cell death and aggravates neurologic deficits after experimental stroke. J Cereb Blood Flow Metab 31(4):1093–1106. https://doi.org/10.1038/jcbfm.2010.194
Inacio AR, Bucala R, Deierborg T (2011) Lack of macrophage migration inhibitory factor in mice does not affect hallmarks of the inflammatory/immune response during the first week after stroke. J Neuroinflammation 8:75. https://doi.org/10.1186/1742-2094-8-75
Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL (2002) Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297(5579):259–263. https://doi.org/10.1126/science.1072221
Guo D, Zeng L, Brody DL, Wong M (2013) Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS One 8(5):e64078. https://doi.org/10.1371/journal.pone.0064078
Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, Huganir RL, Ginty DD, Kolodkin AL (2009) Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature 462(7276):1065–1069. https://doi.org/10.1038/nature08628
Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H (2007) Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130(6):1146–1158. https://doi.org/10.1016/j.cell.2007.07.010
Woolley CS, Gould E, Frankfurt M, McEwen BS (1990) Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci 10(12):4035–4039
Sanchez-Bezanilla S, TeBay C, Nilsson M, Walker FR, Ong LK (2019) Visual discrimination impairment after experimental stroke is associated with disturbances in the polarization of the astrocytic aquaporin-4 and increased accumulation of neurotoxic proteins. Exp Neurol 318:232–243. https://doi.org/10.1016/j.expneurol.2019.05.001
Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD (1996) Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke 27(9):1616–1622. https://doi.org/10.1161/01.str.27.9.1616
Savitt JM, Jang SS, Mu W, Dawson VL, Dawson TM (2005) Bcl-x is required for proper development of the mouse substantia nigra. J Neurosci 25(29):6721–6728. https://doi.org/10.1523/JNEUROSCI.0760-05.2005
Schmued LC, Hopkins KJ (2000) Fluoro-Jade: novel fluorochromes for detecting toxicant-induced neuronal degeneration. Toxicol Pathol 28(1):91–99. https://doi.org/10.1177/019262330002800111
Simoes AP, Silva CG, Marques JM, Pochmann D, Porciuncula LO, Ferreira S, Oses JP, Beleza RO, Real JI, Kofalvi A, Bahr BA, Lerma J, Cunha RA, Rodrigues RJ (2018) Glutamate-induced and NMDA receptor-mediated neurodegeneration entails P2Y1 receptor activation. Cell Death Dis 9(3):297. https://doi.org/10.1038/s41419-018-0351-1
Chen Y, Zhang B, Bao L, Jin L, Yang M, Peng Y, Kumar A, Wang JE, Wang C, Zou X, Xing C, Wang Y, Luo W (2018) ZMYND8 acetylation mediates HIF-dependent breast cancer progression and metastasis. J Clin Investig 128(5):1937–1955. https://doi.org/10.1172/JCI95089
Sakata K, Jin L, Jha S (2010) Lack of promoter IV-driven BDNF transcription results in depression-like behavior. Genes Brain Behav 9(7):712–721. https://doi.org/10.1111/j.1601-183X.2010.00605.x
Luong TN, Carlisle HJ, Southwell A, Patterson PH (2011) Assessment of motor balance and coordination in mice using the balance beam. J Vis Exp. https://doi.org/10.3791/2376
Harper JM, Wilkinson JE, Miller RA (2010) Macrophage migration inhibitory factor-knockout mice are long lived and respond to caloric restriction. FASEB J 24(7):2436–2442. https://doi.org/10.1096/fj.09-152223
Das R, Chinnathambi S (2019) Microglial priming of antigen presentation and adaptive stimulation in Alzheimer’s disease. Cell Mol Life Sci 76(19):3681–3694. https://doi.org/10.1007/s00018-019-03132-2
Bolos M, Perea JR, Avila J (2017) Alzheimer’s disease as an inflammatory disease. Biomol Concepts 8(1):37–43. https://doi.org/10.1515/bmc-2016-0029
Stoica BA, Loane DJ, Zhao Z, Kabadi SV, Hanscom M, Byrnes KR, Faden AI (2014) PARP-1 inhibition attenuates neuronal loss, microglia activation and neurological deficits after traumatic brain injury. J Neurotrauma 31(8):758–772. https://doi.org/10.1089/neu.2013.3194
LaPlaca MC, Zhang J, Raghupathi R, Li JH, Smith F, Bareyre FM, Snyder SH, Graham DI, McIntosh TK (2001) Pharmacologic inhibition of poly(ADP-ribose) polymerase is neuroprotective following traumatic brain injury in rats. J Neurotrauma 18(4):369–376. https://doi.org/10.1089/089771501750170912
Whalen MJ, Clark RS, Dixon CE, Robichaud P, Marion DW, Vagni V, Graham SH, Virag L, Hasko G, Stachlewitz R, Szabo C, Kochanek PM (1999) Reduction of cognitive and motor deficits after traumatic brain injury in mice deficient in poly(ADP-ribose) polymerase. J Cereb Blood Flow Metab 19(8):835–842. https://doi.org/10.1097/00004647-199908000-00002
Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL (1997) Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med 3(10):1089–1095. https://doi.org/10.1038/nm1097-1089
Zhang J, Dawson VL, Dawson TM, Snyder SH (1994) Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science 263(5147):687–689. https://doi.org/10.1126/science.8080500
Merk M, Mitchell RA, Endres S, Bucala R (2012) D-dopachrome tautomerase (D-DT or MIF-2): doubling the MIF cytokine family. Cytokine 59(1):10–17. https://doi.org/10.1016/j.cyto.2012.03.014
Calandra T, Roger T (2003) Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol 3(10):791–800. https://doi.org/10.1038/nri1200
Grieb G, Merk M, Bernhagen J, Bucala R (2010) Macrophage migration inhibitory factor (MIF): a promising biomarker. Drug News Perspect 23(4):257–264. https://doi.org/10.1358/dnp.2010.23.4.1453629
Liu S, Luo W, Wang Y (2021) Emerging role of PARP-1 and PARthanatos in ischemic stroke. J Neurochem. https://doi.org/10.1111/jnc.15464
Wang Y, Chen Y, Wang C, Yang M, Wang Y, Bao L, Wang JE, Kim B, Chan KY, Xu W, Capota E, Ortega J, Nijhawan D, Li GM, Luo W, Wang Y (2021) MIF is a 3′ flap nuclease that facilitates DNA replication and promotes tumor growth. Nat Commun 12(1):2954. https://doi.org/10.1038/s41467-021-23264-z
Acknowledgements
We thank Dr. W. Lee Kraus for providing PARP-1 KO mice and the UT Southwestern Medical Center Next Generation Sequencing Core for assistance with RNA-Seq.
Funding
This work was supported by grants from the National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke R00NS078049, National Institute of General Medical Sciences R35GM124693, and National Institute on Aging R01AG066166, Darrell K Royal Research Fund (DKR), Welch Foundation (I-1939-20170325), CPRIT-HIHR RP170671, TIBIR pilot Grant, the University of Texas (UT) Southwestern Medical Center Startup funds and UT Rising Stars to Y.W., and the Welch Foundation I-1903-20190330 to W.L.
Author information
Authors and Affiliations
Contributions
YW and WL conceived the idea, designed research, analyzed data and wrote the paper. ZR initiated the project and performed the majority of the experiments including TBI surgery, Nissl staining, Golgi staining, and neuron morphology tracing and analysis. QL continued the project and performed TBI surgery, Nissl staining, Golgi staining, and neuron morphology tracing and analysis. JEW maintained the mouse lines and performed mouse genotyping, TBI surgery on both MIF and PARP mice, and animal behavioral tests. MZ performed mRNA sequencing studies. SL performed FJC and microglial activation experiments. HZ performed PARP inhibitor injection study. AD contributed to the blind animal behavior data analysis. YJW performed MIF western blots. YNW performed PARP-1 KO western blots. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ruan, Z., Lu, Q., Wang, J.E. et al. MIF promotes neurodegeneration and cell death via its nuclease activity following traumatic brain injury. Cell. Mol. Life Sci. 79, 39 (2022). https://doi.org/10.1007/s00018-021-04037-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-021-04037-9