Abstract
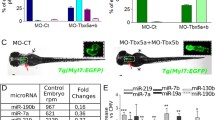
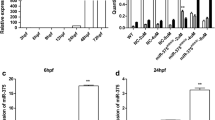
To dissect the TBX5 regulatory circuit, we focused on microRNAs (miRNAs) that collectively contribute to make TBX5 a pivotal cardiac regulator. We profiled miRNAs in hearts isolated from wild-type, CRE, Tbx5lox/+and Tbx5del/+ mice using a Next Generation Sequencing (NGS) approach. TBX5 deficiency in cardiomyocytes increased the expression of the miR-183 cluster family that is controlled by Kruppel-like factor 4, a transcription factor repressed by TBX5. MiR-182-5p, the most highly expressed miRNA of this family, was functionally analyzed in zebrafish. Transient overexpression of miR-182-5p affected heart morphology, calcium handling and the onset of arrhythmias as detected by ECG tracings. Accordingly, several calcium channel proteins identified as putative miR-182-5p targets were downregulated in miR-182-5p overexpressing hearts. In stable zebrafish transgenic lines, we demonstrated that selective miRNA-182-5p upregulation contributes to arrhythmias. Moreover, cardiac-specific down-regulation of miR-182-5p rescued cardiac defects in a zebrafish model of Holt–Oram syndrome. In conclusion, miR-182-5p exerts an evolutionarily conserved role as a TBX5 effector in the onset of cardiac propensity for arrhythmia, and constitutes a relevant target for mediating the relationship between TBX5, arrhythmia and heart development.





Similar content being viewed by others
References
Bruneau BG, Logan M, Davis N, Levi T, Tabin CJ, Seidman JG, Seidman CE (1999) Chamber-specific cardiac expression of Tbx5 and heart defects in Holt-Oram syndrome. Dev Biol 211(1):100–108
Basson CT, Bachinsky DR, Lin RC, Levi T, Elkins JA, Soults J, Grayzel D, Kroumpouzou E, Traill TA, Leblanc-Straceski J, Renault B, Kucherlapati R, Seidman JG, Seidman CE (1997) Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet 15(1):30–35. https://doi.org/10.1038/ng0197-30
Christophersen IE, Rienstra M, Roselli C, Yin X, Geelhoed B, Barnard J, Lin H, Arking DE, Smith AV, Albert CM, Chaffin M, Tucker NR, Li M, Klarin D, Bihlmeyer NA, Low SK, Weeke PE, Muller-Nurasyid M, Smith JG, Brody JA, Niemeijer MN, Dorr M, Trompet S, Huffman J, Gustafsson S, Schurmann C, Kleber ME, Lyytikainen LP, Seppala I, Malik R, Horimoto A, Perez M, Sinisalo J, Aeschbacher S, Theriault S, Yao J, Radmanesh F, Weiss S, Teumer A, Choi SH, Weng LC, Clauss S, Deo R, Rader DJ, Shah SH, Sun A, Hopewell JC, Debette S, Chauhan G, Yang Q, Worrall BB, Pare G, Kamatani Y, Hagemeijer YP, Verweij N, Siland JE, Kubo M, Smith JD, Van Wagoner DR, Bis JC, Perz S, Psaty BM, Ridker PM, Magnani JW, Harris TB, Launer LJ, Shoemaker MB, Padmanabhan S, Haessler J, Bartz TM, Waldenberger M, Lichtner P, Arendt M, Krieger JE, Kahonen M, Risch L, Mansur AJ, Peters A, Smith BH, Lind L, Scott SA, Lu Y, Bottinger EB, Hernesniemi J, Lindgren CM, Wong JA, Huang J, Eskola M, Morris AP, Ford I, Reiner AP, Delgado G, Chen LY, Chen YI, Sandhu RK, Boerwinkle E, Eisele L, Lannfelt L, Rost N, Anderson CD, Taylor KD, Campbell A, Magnusson PK, Porteous D, Hocking LJ, Vlachopoulou E, Pedersen NL, Nikus K, Orho-Melander M, Hamsten A, Heeringa J, Denny JC, Kriebel J, Darbar D, Newton-Cheh C, Shaffer C, Macfarlane PW, Heilmann-Heimbach S, Almgren P, Huang PL, Sotoodehnia N, Soliman EZ, Uitterlinden AG, Hofman A, Franco OH, Volker U, Jockel KH, Sinner MF, Lin HJ, Guo X, Dichgans M, Ingelsson E, Kooperberg C, Melander O, Loos RJF, Laurikka J, Conen D, Rosand J, van der Harst P, Lokki ML, Kathiresan S, Pereira A, Jukema JW, Hayward C, Rotter JI, Marz W, Lehtimaki T, Stricker BH, Chung MK, Felix SB, Gudnason V, Alonso A, Roden DM, Kaab S, Chasman DI, Heckbert SR, Benjamin EJ, Tanaka T, Lunetta KL, Lubitz SA, Ellinor PT (2017) Large-scale analyses of common and rare variants identify 12 new loci associated with atrial fibrillation. Nat Genet 49(6):946–952. https://doi.org/10.1038/ng.3843
Ma JF, Yang F, Mahida SN, Zhao L, Chen X, Zhang ML, Sun Z, Yao Y, Zhang YX, Zheng GY, Dong J, Feng MJ, Zhang R, Sun J, Li S, Wang QS, Cao H, Benjamin EJ, Ellinor PT, Li YG, Tian XL (2016) TBX5 mutations contribute to early-onset atrial fibrillation in Chinese and caucasians. Cardiovasc Res 109(3):442–450. https://doi.org/10.1093/cvr/cvw003
Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, Conner DA, Gessler M, Nemer M, Seidman CE, Seidman JG (2001) A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell 106(6):709–721
Nadadur RD, Broman MT, Boukens B, Mazurek SR, Yang X, van den Boogaard M, Bekeny J, Gadek M, Ward T, Zhang M, Qiao Y, Martin JF, Seidman CE, Seidman J, Christoffels V, Efimov IR, McNally EM, Weber CR, Moskowitz IP (2016) Pitx2 modulates a Tbx5-dependent gene regulatory network to maintain atrial rhythm. Sci Transl Med 8(354):354
Garrity DM, Childs S, Fishman MC (2002) The heartstrings mutation in zebrafish causes heart/fin Tbx5 deficiency syndrome. Development 129(19):4635–4645
Mori AD, Zhu Y, Vahora I, Nieman B, Koshiba-Takeuchi K, Davidson L, Pizard A, Seidman JG, Seidman CE, Chen XJ, Henkelman RM, Bruneau BG (2006) Tbx5-dependent rheostatic control of cardiac gene expression and morphogenesis. Dev Biol 297(2):566–586
D’Aurizio R, Russo F, Chiavacci E, Baumgart M, Groth M, D’Onofrio M, Arisi I, Rainaldi G, Pitto L, Pellegrini M (2016) Discovering miRNA regulatory networks in Holt-Oram syndrome using a zebrafish model. Front Bioeng Biotechnol 4:60. https://doi.org/10.3389/fbioe.2016.00060
Chiavacci E, D’Aurizio R, Guzzolino E, Russo F, Baumgart M, Groth M, Mariani L, D’Onofrio M, Arisi I, Pellegrini M, Cellerino A, Cremisi F, Pitto L (2015) MicroRNA 19a replacement partially rescues fin and cardiac defects in zebrafish model of Holt Oram syndrome. Sci Rep 5:18240. https://doi.org/10.1038/srep18240
Chiavacci E, Dolfi L, Verduci L, Meghini F, Gestri G, Evangelista AMM, Wilson SW, Cremisi F, Pitto L (2012) MicroRNA 218 mediates the effects of tbx5a over-expression on zebrafish heart development. PLoS One 7 (11). doi:ARTN e50536 https://doi.org/10.1371/journal.pone.0050536
Guzzolino E, Chiavacci E, Ahuja N, Mariani L, Evangelista M, Ippolito C, Rizzo M, Garrity D, Cremisi F, Pitto L (2018) Post-transcriptional Modulation of Sphingosine-1-Phosphate Receptor 1 by miR-19a Affects Cardiovascular Development in Zebrafish. Front Cell Dev Biol. 6:58. https://doi.org/10.3389/fcell.2018.00058
Sakai K, Miyazaki J (1997) A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun 237(2):318–324
Baumgart M, Groth M, Priebe S, Appelt J, Guthke R, Platzer M, Cellerino A (2012) Age-dependent regulation of tumor-related microRNAs in the brain of the annual fish Nothobranchius furzeri. Mech Ageing Dev 133(5):226–233. https://doi.org/10.1016/j.mad.2012.03.015
Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11(10):R106. https://doi.org/10.1186/gb-2010-11-10-r106
Chi NC, Shaw RM, Jungblut B, Huisken J, Ferrer T, Arnaout R, Scott I, Beis D, Xiao T, Baier H, Jan LY, Tristani-Firouzi M, Stainier DY (2008) Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol 6(5):e109
Umemoto N, Nishimura Y, Shimada Y, Yamanaka Y, Kishi S, Ito S, Okamori K, Nakamura Y, Kuroyanagi J, Zhang Z, Zang L, Wang Z, Nishimura N, Tanaka T (2013) Fluorescent-based methods for gene knockdown and functional cardiac imaging in zebrafish. Mol Biotechnol 55(2):131–142. https://doi.org/10.1007/s12033-013-9664-6
Waldron L, Steimle JD, Greco TM, Gomez NC, Dorr KM, Kweon J, Temple B, Yang XH, Wilczewski CM, Davis IJ, Cristea IM, Moskowitz IP, Conlon FL (2016) The cardiac TBX5 interactome reveals a chromatin remodeling network essential for cardiac septation. Dev Cell 36(3):262–275. https://doi.org/10.1016/j.devcel.2016.01.009
Segura MF, Jubierre L, Li S, Soriano A, Koetz L, Gaziel-Sovran A, Masanas M, Kleffman K, Dankert JF, Walsh MJ, Hernando E (2017) Kruppel-like factor 4 (KLF4) regulates the miR-183 ~ 96–182 cluster under physiologic and pathologic conditions. Oncotarget 8(16):26298–26311. https://doi.org/10.18632/oncotarget.15459
Yang KC, Yamada KA, Patel AY, Topkara VK, George I, Cheema FH, Ewald GA, Mann DL, Nerbonne JM (2014) Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodeling with mechanical circulatory support. Circulation 129(9):1009–1021. https://doi.org/10.1161/circulationaha.113.003863
Cakmak HA, Coskunpinar E, Ikitimur B, Barman HA, Karadag B, Tiryakioglu NO, Kahraman K, Vural VA (2015) The prognostic value of circulating microRNAs in heart failure: preliminary results from a genome-wide expression study. J Cardiovasc Med (Hagerstown) 16(6):431–437. https://doi.org/10.2459/jcm.0000000000000233
Li N, Hwangbo C, Jaba IM, Zhang J, Papangeli I, Han J, Mikush N, Larrivee B, Eichmann A, Chun HJ, Young LH, Tirziu D (2016) miR-182 modulates myocardial hypertrophic response induced by angiogenesis in heart. Sci Rep 6:21228. https://doi.org/10.1038/srep21228
Kuppusamy KT, Jones DC, Sperber H, Madan A, Fischer KA, Rodriguez ML, Pabon L, Zhu WZ, Tulloch NL, Yang X, Sniadecki NJ, Laflamme MA, Ruzzo WL, Murry CE, Ruohola-Baker H (2015) Let-7 family of microRNA is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes. Proc Natl Acad Sci USA 112(21):E2785–2794. https://doi.org/10.1073/pnas.1424042112
Bakkers J (2011) Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc Res 91(2):279–288. https://doi.org/10.1093/cvr/cvr098
Huang CJ, Tu CT, Hsiao CD, Hsieh FJ, Tsai HJ (2003) Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev Dyn 228(1):30–40. https://doi.org/10.1002/dvdy.10356
Auman HJ, Coleman H, Riley HE, Olale F, Tsai HJ, Yelon D (2007) Functional modulation of cardiac form through regionally confined cell shape changes. PLoS Biol 5(3):e53. https://doi.org/10.1371/journal.pbio.0050053
Yelon D (2001) Cardiac patterning and morphogenesis in zebrafish. Dev Dyn 222(4):552–563. https://doi.org/10.1002/dvdy.1243
Georges R, Nemer G, Morin M, Lefebvre C, Nemer M (2008) Distinct expression and function of alternatively spliced Tbx5 isoforms in cell growth and differentiation. Mol Cell Biol 28(12):4052–4067. https://doi.org/10.1128/mcb.02100-07
Hiroi Y, Kudoh S, Monzen K, Ikeda Y, Yazaki Y, Nagai R, Komuro I (2001) Tbx5 associates with Nkx2-5 and synergistically promotes cardiomyocyte differentiation. Nat Genet 28(3):276–280. https://doi.org/10.1038/90123
Parrie LE, Renfrew EM, Wal AV, Mueller RL, Garrity DM (2013) Zebrafish tbx5 paralogs demonstrate independent essential requirements in cardiac and pectoral fin development. Dev Dyn 242(5):485–502. https://doi.org/10.1002/dvdy.23953
Chernyavskaya Y, Ebert AM, Milligan E, Garrity DM (2012) Voltage-gated calcium channel CACNB2 (beta2.1) protein is required in the heart for control of cell proliferation and heart tube integrity. Dev Dyn 241(4):648–662. https://doi.org/10.1002/dvdy.23746
Nielsen BS, Moller T, Holmstrom K (2012) Combined MicroRNA in situ hybridization and immunohistochemical detection of protein markers. Eur J Cancer 48:S216–S216. https://doi.org/10.1016/s0959-8049(12)71524-1
Lorenzon A, Calore M, Poloni G, De Windt LJ, Braghetta P, Rampazzo A (2017) Wnt/beta-catenin pathway in arrhythmogenic cardiomyopathy. Oncotarget 8(36):60640–60655. https://doi.org/10.18632/oncotarget.17457
Cohen ED, Tian Y, Morrisey EE (2008) Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development 135(5):789–798. https://doi.org/10.1242/dev.016865
Ghigo A, Laffargue M, Li M, Hirsch E (2017) PI3K and calcium signaling in cardiovascular disease. Circ Res 121(3):282–292. https://doi.org/10.1161/circresaha.117.310183
Sciarretta S, Forte M, Frati G, Sadoshima J (2018) New insights into the role of mTOR signaling in the cardiovascular system. Circ Res 122(3):489–505. https://doi.org/10.1161/circresaha.117.311147
Ehler E (2018) Actin-associated proteins and cardiomyopathy-the ‘unknown’ beyond troponin and tropomyosin. Biophys Rev 10(4):1121–1128. https://doi.org/10.1007/s12551-018-0428-1
Landstrom AP, Dobrev D, Wehrens XHT (2017) Calcium signaling and cardiac arrhythmias. Circ Res 120(12):1969–1993. https://doi.org/10.1161/circresaha.117.310083
Bennett JS, Stroud DM, Becker JR, Roden DM (2013) Proliferation of embryonic cardiomyocytes in zebrafish requires the sodium channel scn5Lab. Genesis 51(8):562–574. https://doi.org/10.1002/dvg.22400
Cassis P, Cerullo D, Zanchi C, Corna D, Lionetti V, Giordano F, Novelli R, Conti S, Casieri V, Matteucci M, Locatelli M, Taraboletti G, Villa S, Gastoldi S, Remuzzi G, Benigni A, Zoja C (2018) ADAMTS13 deficiency shortens the life span of mice with experimental diabetes. Diabetes 67(10):2069–2083. https://doi.org/10.2337/db17-1508
Sehnert AJ, Stainier DY (2002) A window to the heart: can zebrafish mutants help us understand heart disease in humans? Trends Genet 18(10):491–494
Goetz SC, Brown DD, Conlon FL (2006) TBX5 is required for embryonic cardiac cell cycle progression. Development 133(13):2575–2584
Sun H, Kerfant BG, Zhao D, Trivieri MG, Oudit GY, Penninger JM, Backx PH (2006) Insulin-like growth factor-1 and PTEN deletion enhance cardiac L-type Ca2 + currents via increased PI3Kalpha/PKB signaling. Circ Res 98(11):1390–1397
Subramanyam P, Obermair GJ, Baumgartner S, Gebhart M, Striessnig J, Kaufmann WA, Geley S, Flucher BE (2009) Activity and calcium regulate nuclear targeting of the calcium channel beta4b subunit in nerve and muscle cells. Channels (Austin) 3(5):343–355. https://doi.org/10.4161/chan.3.5.9696
He J, Yang D, Wang C, Liu W, Liao J, Xu T, Bai C, Chen J, Lin K, Huang C, Dong Q (2011) Chronic zebrafish low dose decabrominated diphenyl ether (BDE-209) exposure affected parental gonad development and locomotion in F1 offspring. Ecotoxicology 20(8):1813–1822. https://doi.org/10.1007/s10646-011-0720-3
Burashnikov E, Pfeiffer R, Barajas-Martinez H, Delpon E, Hu D, Desai M, Borggrefe M, Haissaguerre M, Kanter R, Pollevick GD, Guerchicoff A, Laino R, Marieb M, Nademanee K, Nam GB, Robles R, Schimpf R, Stapleton DD, Viskin S, Winters S, Wolpert C, Zimmern S, Veltmann C, Antzelevitch C (2010) Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm 7(12):1872–1882. https://doi.org/10.1016/j.hrthm.2010.08.026
Arnolds DE, Liu F, Fahrenbach JP, Kim GH, Schillinger KJ, Smemo S, McNally EM, Nobrega MA, Patel VV, Moskowitz IP (2012) TBX5 drives Scn5a expression to regulate cardiac conduction system function. J Clin Invest 122(7):2509–2518
Zhu Y, Gramolini AO, Walsh MA, Zhou YQ, Slorach C, Friedberg MK, Takeuchi JK, Sun H, Henkelman RM, Backx PH, Redington AN, Maclennan DH, Bruneau BG (2008) Tbx5-dependent pathway regulating diastolic function in congenital heart disease. Proc Natl Acad Sci USA 105(14):5519–5524. https://doi.org/10.1073/pnas.0801779105
Torrado M, Franco D, Lozano-Velasco E, Hernandez-Torres F, Calvino R, Aldama G, Centeno A, Castro-Beiras A, Mikhailov A (2015) A MicroRNA-transcription factor blueprint for early atrial arrhythmogenic remodeling. Biomed Res Int 2015:263151. https://doi.org/10.1155/2015/263151
Hegyi B, Bossuyt J, Griffiths LG, Shimkunas R, Coulibaly Z, Jian Z, Grimsrud KN, Sondergaard CS, Ginsburg KS, Chiamvimonvat N, Belardinelli L, Varro A, Papp JG, Pollesello P, Levijoki J, Izu LT, Boyd WD, Banyasz T, Bers DM, Chen-Izu Y (2018) Complex electrophysiological remodeling in postinfarction ischemic heart failure. Proc Natl Acad Sci USA 115(13):E3036–E3044. https://doi.org/10.1073/pnas.1718211115
Acknowledgements
We are grateful to Dr.Elena Chiavacci (International Centre for Genetic Engineering and Biotechnology,Trieste, Italy) for experimental assistance, Dr. Marnie Halpern (Carnegie Institution Baltimore, Maryland) for kindly providing the pME-Galff-2a-mCherry and 4XNR UAS GFP plasmids, Dr. Héctor Sanchez Iranzo (Centro Nacional de Investigaciones Cardiovasculares Carlos III, 28029 Madrid,Spain) for kindly providing pDestCrysGFP plasmid and Prof.Sheng-Ping L. Hwang (Institute of Cellular and Organismic Biology Academia Sinica Taiwan) for the kind gift of the T7TS-klf4a plasmid for dre klf4 overexpression in zebrafish embryos. RF thanks Didier Stainier (Department of Developmental Genetics, Max Planck Institute for Heart and Lung Research, Bad Nauheim, Germany) for support.
Funding
The project was supported by grants from the American Heart Association (17AIREA33660773) and PCOM Center for Chronic Disorders of Aging to C.J. Hatcher and by the American Heart Association 17GRNT33460256 to D.Garrity.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guzzolino, E., Pellegrino, M., Ahuja, N. et al. miR-182-5p is an evolutionarily conserved Tbx5 effector that impacts cardiac development and electrical activity in zebrafish. Cell. Mol. Life Sci. 77, 3215–3229 (2020). https://doi.org/10.1007/s00018-019-03343-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03343-7