Abstract
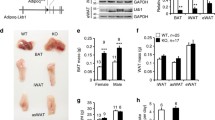
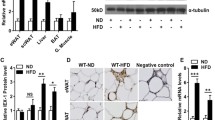
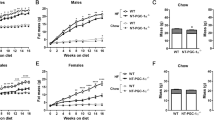
RDH1 is one of the several enzymes that catalyze the first of the two reactions to convert retinol into all-trans-retinoic acid (atRA). Here, we show that Rdh1-null mice fed a low-fat diet gain more weight as adiposity (17% males, 13% females) than wild-type mice by 20 weeks old, despite neither consuming more calories nor decreasing activity. Glucose intolerance and insulin resistance develop following increased adiposity. Despite the increase in white fat pads, epididymal white adipose does not express Rdh1, nor does muscle. Brown adipose tissue (BAT) and liver express Rdh1 at relatively high levels compared to other tissues. Rdh1 ablation lowered body temperatures during ambient conditions. Given the decreased body temperature, we focused on BAT. A lack of differences in BAT adipogenic gene expression between Rdh1-null mice and wild-type mice, including Pparg, Prdm16, Zfp516 and Zfp521, indicated that the phenotype was not driven by brown adipose hyperplasia. Rather, Rdh1 ablation eliminated the increase in BAT atRA that occurs after re-feeding. This disruption of atRA homeostasis increased fatty acid uptake, but attenuated lipolysis in primary brown adipocytes, resulting in increased lipid content and larger lipid droplets. Rdh1 ablation also decreased mitochondrial proteins, including CYCS and UCP1, the mitochondria oxygen consumption rate, and disrupted the mitochondria membrane potential, further reflecting impaired BAT function, resulting in both BAT and white adipose hypertrophy. RNAseq revealed dysregulation of 424 BAT genes in null mice, which segregated predominantly into differences after fasting vs after re-feeding. Exceptions were Rbp4 and Gbp2b, which increased during both dietary conditions. Rbp4 encodes the serum retinol-binding protein—an insulin desensitizer. Gbp2b encodes a GTPase. Because Gbp2b increased several hundred-fold, we overexpressed it in brown adipocytes. This caused a shift to larger lipid droplets, suggesting that GBP2b affects signaling downstream of the β-adrenergic receptor during basal thermogenesis. Thus, Rdh1-generated atRA in BAT regulates multiple genes that promote BAT adaptation to whole-body energy status, such as fasting and re-feeding. These gene expression changes promote optimum mitochondria function and thermogenesis, limiting adiposity. Attenuation of adiposity and insulin resistance suggests that RDH1 mitigates metabolic syndrome.








Similar content being viewed by others
References
McLaren DS, Kraemer K (2012) Vitamin A in health. World Rev Nutr Diet 103:33–51. https://doi.org/10.1159/000170954
Li Y, Wong K, Walsh K et al (2013) Retinoic acid receptor β stimulates hepatic induction of fibroblast growth factor 21 to promote fatty acid oxidation and control whole-body energy homeostasis in mice. J Biol Chem 288:10490–10504. https://doi.org/10.1074/jbc.M112.429852
Yang D, Vuckovic MG, Smullin CP et al (2018) Modest decreases in endogenous all-trans-retinoic acid produced by a mouse Rdh10 heterozygote provoke major abnormalities in adipogenesis and lipid metabolism. Diabetes 67:662–673. https://doi.org/10.2337/db17-0946
Alvarez R, de Andrés J, Yubero P et al (1995) A novel regulatory pathway of brown fat thermogenesis. Retinoic acid is a transcriptional activator of the mitochondrial uncoupling protein gene. J Biol Chem 270:5666–5673
Mercader J, Palou A, Bonet ML (2010) Induction of uncoupling protein-1 in mouse embryonic fibroblast-derived adipocytes by retinoic acid. Obesity 18:655–662. https://doi.org/10.1038/oby.2009.330
Berry DC, DeSantis D, Soltanian H et al (2012) Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes 61:1112–1121. https://doi.org/10.2337/db11-1620
Jeyakumar SM, Vajreswari A, Giridharan NV (2006) Chronic dietary vitamin A supplementation regulates obesity in an obese mutant WNIN/Ob rat model. Obesity 14:52–59. https://doi.org/10.1038/oby.2006.7
Mercader J, Ribot J, Murano I et al (2006) Remodeling of white adipose tissue after retinoic acid administration in mice. Endocrinology 147:5325–5332. https://doi.org/10.1210/en.2006-0760
Hayes DP (2007) Nutritional hormesis. Eur J Clin Nutr 61:147–159. https://doi.org/10.1038/sj.ejcn.1602507
Obrochta KM, Kane MA, Napoli JL (2014) Effects of diet and strain on mouse serum and tissue retinoid concentrations. PLoS One 9:e99435. https://doi.org/10.1371/journal.pone.0099435
Stone RL, Bernlohr DA (1990) The molecular basis for inhibition of adipose conversion of murine 3T3-L1 cells by retinoic acid. Differ Res Biol Divers 45:119–127
Muenzner M, Tuvia N, Deutschmann C et al (2013) Retinol-binding protein 4 and its membrane receptor STRA6 control adipogenesis by regulating cellular retinoid homeostasis and retinoic acid receptor α activity. Mol Cell Biol 33:4068–4082. https://doi.org/10.1128/MCB.00221-13
Yang D, Krois CR, Huang P et al (2017) Raldh1 promotes adiposity during adolescence independently of retinal signaling. PLoS One 12:e0187669. https://doi.org/10.1371/journal.pone.0187669
Berry DC, Noy N (2009) All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol Cell Biol 29:3286–3296. https://doi.org/10.1128/MCB.01742-08
Mercader J, Madsen L, Felipe F et al (2007) All-trans retinoic acid increases oxidative metabolism in mature adipocytes. Cell Physiol Biochem 20:1061–1072. https://doi.org/10.1159/0000110717
Hernandez A, de Mena RM, Martin E, Obregon M-J (2011) Differences in the response of UCP1 mRNA to hormonal stimulation between rat and mouse primary cultures of brown adipocytes. Cell Physiol Biochem 28:969–980. https://doi.org/10.1159/000335810
Murholm M, Isidor MS, Basse AL et al (2013) Retinoic acid has different effects on UCP1 expression in mouse and human adipocytes. BMC Cell Biol 14:41. https://doi.org/10.1186/1471-2121-14-41
Kedishvili NY (2016) Retinoic acid synthesis and degradation. Subcell Biochem 81:127–161. https://doi.org/10.1007/978-94-024-0945-1_5
Napoli JL (2017) Cellular retinoid binding-proteins, CRBP, CRABP, FABP5: effects on retinoid metabolism, function and related diseases. Pharmacol Ther 173:19–33. https://doi.org/10.1016/j.pharmthera.2017.01.004
Harrison EH (2012) Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim Biophys Acta 1821:70–77. https://doi.org/10.1016/j.bbalip.2011.06.002
Wang S, Yu J, Jones JW et al (2018) Retinoic acid signaling promotes the cytoskeletal rearrangement of embryonic epicardial cells. FASEB J https://doi.org/10.1096/fj.201701038R
Ross AC, Zolfaghari R (2011) Cytochrome P450 s in the regulation of cellular retinoic acid metabolism. Annu Rev Nutr 31:65–87. https://doi.org/10.1146/annurev-nutr-072610-145127
Nelson CH, Buttrick BR, Isoherranen N (2013) Therapeutic potential of the inhibition of the retinoic acid hydroxylases CYP26A1 and CYP26B1 by xenobiotics. Curr Top Med Chem 13:1402–1428
Zhai Y, Sperkova Z, Napoli JL (2001) Cellular expression of retinal dehydrogenase types 1 and 2: effects of vitamin A status on testis mRNA. J Cell Physiol 186:220–232. https://doi.org/10.1002/1097-4652(200102)186:2%3c220:AID-JCP1018%3e3.0.CO;2-N
Jiang W, Napoli JL (2013) The retinol dehydrogenase Rdh10 localizes to lipid droplets during acyl ester biosynthesis. J Biol Chem 288:589–597. https://doi.org/10.1074/jbc.M112.402883
Yang D, Vuckovic MG, Smullin CP et al (2018) Modest decreases in endogenous all-trans-retinoic acid produced by a MouseRdh10Heterozygote provoke major abnormalities in adipogenesis and lipid metabolism. Diabetes 67:662–673. https://doi.org/10.2337/db17-0946
Zhang M, Hu P, Krois CR et al (2007) Altered vitamin A homeostasis and increased size and adiposity in the rdh1-null mouse. FASEB J 21:2886–2896. https://doi.org/10.1096/fj.06-7964com
Pradhan RN, Zachara M, Deplancke B (2017) A systems perspective on brown adipogenesis and metabolic activation. Obes Rev 18(Suppl 1):65–81. https://doi.org/10.1111/obr.12512
Trayhurn P (2017) Origins and early development of the concept that brown adipose tissue thermogenesis is linked to energy balance and obesity. Biochimie 134:62–70. https://doi.org/10.1016/j.biochi.2016.09.007
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Heikkinen S, Argmann CA, Champy M-F, Auwerx J (2001) Evaluation of glucose homeostasis. In: Current protocols in molecular biology. Wiley
Kane MA, Napoli JL (2010) Quantification of endogenous retinoids. Methods Mol Biol 652:1–54. https://doi.org/10.1007/978-1-60327-325-1_1
van Dam AD, Nahon KJ, Kooijman S et al (2015) Salsalate activates brown adipose tissue in mice. Diabetes 64:1544–1554. https://doi.org/10.2337/db14-1125
Parlee SD, Lentz SI, Mori H, MacDougald OA (2014) Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol 537:93–122. https://doi.org/10.1016/B978-0-12-411619-1.00006-9
van Dam AD, Nahon KJ, Kooijman S et al (2015) Salsalate activates brown adipose tissue in mice. Diabetes 64:1544–1554. https://doi.org/10.2337/db14-1125
Rowan S, Jiang S, Korem T et al (2017) Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc Natl Acad Sci USA 114:E4472–E4481. https://doi.org/10.1073/pnas.1702302114
Khalil A, Cevik SE, Hung S et al (2018) Developmental exposure to 2,2′,4,4′-tetrabromodiphenyl ether permanently alters blood-liver balance of lipids in male mice. Front Endocrinol 9:548. https://doi.org/10.3389/fendo.2018.00548
Mackay H, Patterson ZR, Khazall R et al (2013) Organizational effects of perinatal exposure to bisphenol-A and diethylstilbestrol on arcuate nucleus circuitry controlling food intake and energy expenditure in male and female CD-1 mice. Endocrinology 154:1465–1475. https://doi.org/10.1210/en.2012-2044
Wahlang B, Falkner KC, Gregory B et al (2013) Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J Nutr Biochem 24:1587–1595. https://doi.org/10.1016/j.jnutbio.2013.01.009
Davidson SM, Papagiannakopoulos T, Olenchock BA et al (2016) environment impacts the metabolic dependencies of ras-driven non-small cell lung cancer. Cell Metab 23:517–528. https://doi.org/10.1016/j.cmet.2016.01.007
Turner SM, Murphy EJ, Neese RA et al (2003) Measurement of TG synthesis and turnover in vivo by 2H2O incorporation into the glycerol moiety and application of MIDA. Am J Physiol Endocrinol Metab 285:E790–E803. https://doi.org/10.1152/ajpendo.00402.2002
Lee WN, Bassilian S, Guo Z et al (1994) Measurement of fractional lipid synthesis using deuterated water (2H2O) and mass isotopomer analysis. Am J Physiol 266:E372–E383
Meyer CW, Ootsuka Y, Romanovsky AA (2017) Body temperature measurements for metabolic phenotyping in mice. Front Physiol 8:520. https://doi.org/10.3389/fphys.2017.00520
Christoffolete MA, Linardi CCG, de Jesus L et al (2004) Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes 53:577–584
Schweiger M, Eichmann TO, Taschler U et al (2014) Measurement of lipolysis. Methods Enzymol 538:171–193. https://doi.org/10.1016/B978-0-12-800280-3.00010-4
Rotondo F, Ho-Palma AC, Remesar X et al (2017) Glycerol is synthesized and secreted by adipocytes to dispose of excess glucose, via glycerogenesis and increased acyl-glycerol turnover. Sci Rep 7:8983. https://doi.org/10.1038/s41598-017-09450-4
Smith PK, Krohn RI, Hermanson GT et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Aune UL, Ruiz L, Kajimura S (2013) Isolation and differentiation of stromal vascular cells to beige/brite cells. J Vis Exp. https://doi.org/10.3791/50191
Briand N, Prado C, Mabilleau G et al (2014) Caveolin-1 expression and cavin stability regulate caveolae dynamics in adipocyte lipid store fluctuation. Diabetes 63:4032–4044. https://doi.org/10.2337/db13-1961
Listenberger LL, Brown DA (2007) Fluorescent detection of lipid droplets and associated proteins. Curr Protoc Cell Biol Chapter 24:Unit 24.2. https://doi.org/10.1002/0471143030.cb2402s35
Harjes U, Bridges E, Gharpure KM et al (2017) Antiangiogenic and tumour inhibitory effects of downregulating tumour endothelial FABP4. Oncogene 36:912–921. https://doi.org/10.1038/onc.2016.256
Liao J, Sportsman R, Harris J, Stahl A (2005) Real-time quantification of fatty acid uptake using a novel fluorescence assay. J Lipid Res 46:597–602. https://doi.org/10.1194/jlr.D400023-JLR200
Tharp KM, Kang MS, Timblin GA et al (2018) Actomyosin-mediated tension orchestrates uncoupled respiration in adipose tissues. Cell Metab 27:602.e4–615.e4. https://doi.org/10.1016/j.cmet.2018.02.005
Nguyen TB, Louie SM, Daniele JR et al (2017) DGAT1-dependent lipid droplet biogenesis protects mitochondrial function during starvation-induced autophagy. Dev Cell 42:9.e5–21.e5. https://doi.org/10.1016/j.devcel.2017.06.003
Jayaraman S (2005) Flow cytometric determination of mitochondrial membrane potential changes during apoptosis of T lymphocytic and pancreatic beta cell lines: comparison of tetramethylrhodamineethylester (TMRE), chloromethyl-X-rosamine (H2-CMX-Ros) and MitoTracker Red 580 (MTR580). J Immunol Methods 306:68–79. https://doi.org/10.1016/j.jim.2005.07.024
Nolan T, Hands RE, Bustin SA (2006) Quantification of mRNA using real-time RT-PCR. Nat Protoc 1:1559–1582. https://doi.org/10.1038/nprot.2006.236
Fink T, Lund P, Pilgaard L et al (2008) Instability of standard PCR reference genes in adipose-derived stem cells during propagation, differentiation and hypoxic exposure. BMC Mol Biol 9:98. https://doi.org/10.1186/1471-2199-9-98
Shinoda K, Luijten IHN, Hasegawa Y et al (2015) Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med 21:389–394. https://doi.org/10.1038/nm.3819
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Jo J, Gavrilova O, Pack S et al (2009) Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol 5:e1000324. https://doi.org/10.1371/journal.pcbi.1000324
Tschop MH, Speakman JR, Arch JRS et al (2012) A guide to analysis of mouse energy metabolism. Nat Methods 9:57–63. https://doi.org/10.1038/nmeth.1806
Zhang M, Chen W, Smith SM, Napoli JL (2001) Molecular characterization of a mouse short chain dehydrogenase/reductase active with all-trans-retinol in intact cells, mRDH1. J Biol Chem 276:44083–44090. https://doi.org/10.1074/jbc.M105748200
Niederreither K, Vermot J, Messaddeq N et al (2001) Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Dev Camb Engl 128:1019–1031
Obrochta KM, Krois CR, Campos B, Napoli JL (2015) Insulin regulates retinol dehydrogenase expression and all-trans-retinoic acid biosynthesis through FoxO1. J Biol Chem 290:7259–7268. https://doi.org/10.1074/jbc.M114.609313
Moutier E, Ye T, Choukrallah M-A et al (2012) Retinoic acid receptors recognize the mouse genome through binding elements with diverse spacing and topology. J Biol Chem 287:26328–26341. https://doi.org/10.1074/jbc.M112.361790
Mazzoni EO, Mahony S, Peljto M et al (2013) Saltatory remodeling of Hox chromatin in response to rostrocaudal patterning signals. Nat Neurosci 16:1191–1198. https://doi.org/10.1038/nn.3490
He Y, Tsuei J, Wan Y-JY (2014) Biological functional annotation of retinoic acid alpha and beta in mouse liver based on genome-wide binding. Am J Physiol Gastrointest Liver Physiol 307:G205–G218. https://doi.org/10.1152/ajpgi.00105.2014
Chatagnon A, Veber P, Morin V et al (2015) RAR/RXR binding dynamics distinguish pluripotency from differentiation associated cis-regulatory elements. Nucleic Acids Res 43:4833–4854. https://doi.org/10.1093/nar/gkv370
Lowell BB, S-Susulic V, Hamann A et al (1993) Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366:740–742. https://doi.org/10.1038/366740a0
Enerbäck S (2010) Human brown adipose tissue. Cell Metab 11:248–252. https://doi.org/10.1016/j.cmet.2010.03.008
Kajimura S, Saito M (2014) A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol 76:225–249. https://doi.org/10.1146/annurev-physiol-021113-170252
Rothwell NJ, Stock MJ (1981) Regulation of energy balance. Annu Rev Nutr 1:235–256. https://doi.org/10.1146/annurev.nu.01.070181.001315
Virtanen KA, Lidell ME, Orava J et al (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525. https://doi.org/10.1056/NEJMoa0808949
Cypess AM, White AP, Vernochet C et al (2013) Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 19:635–639. https://doi.org/10.1038/nm.3112
Long JZ, Svensson KJ, Bateman LA et al (2016) The secreted enzyme PM20D1 regulates lipidated amino acid uncouplers of mitochondria. Cell 166:424–435. https://doi.org/10.1016/j.cell.2016.05.071
Wang XL, Herzog B, Waltner-Law M et al (2004) The synergistic effect of dexamethasone and all-trans-retinoic acid on hepatic phosphoenolpyruvate carboxykinase gene expression involves the coactivator p300. J Biol Chem 279:34191–34200. https://doi.org/10.1074/jbc.M403455200
He Y, Gong L, Fang Y et al (2013) The role of retinoic acid in hepatic lipid homeostasis defined by genomic binding and transcriptome profiling. BMC Genomics 14:575. https://doi.org/10.1186/1471-2164-14-575
Palmer AC, West KP, Dalmiya N, Schultink W (2012) The use and interpretation of serum retinol distributions in evaluating the public health impact of vitamin A programmes. Public Health Nutr 15:1201–1215. https://doi.org/10.1017/S1368980012000560
Tanumihardjo SA, Russell RM, Stephensen CB et al (2016) Biomarkers of nutrition for development (BOND)-vitamin A review. J Nutr 146:1816S–1848S. https://doi.org/10.3945/jn.115.229708
Ribot J, Felipe F, Bonet ML, Palou A (2001) Changes of adiposity in response to vitamin A status correlate with changes of PPAR gamma 2 expression. Obes Res 9:500–509. https://doi.org/10.1038/oby.2001.65
Kamm JJ (1982) Toxicology, carcinogenicity, and teratogenicity of some orally administered retinoids. J Am Acad Dermatol 6:652–659
Park SH, Gray WC, Hernandez I et al (2000) Phase I trial of all-trans retinoic acid in patients with treated head and neck squamous carcinoma. Clin Cancer 6:847–854
Cheruvattath R, Orrego M, Gautam M et al (2006) Vitamin A toxicity: when one a day doesn’t keep the doctor away. Liver Transpl 12:1888–1891. https://doi.org/10.1002/lt.21007
Benn CS, Aaby P, Arts RJW et al (2015) An enigma: why vitamin A supplementation does not always reduce mortality even though vitamin A deficiency is associated with increased mortality. Int J Epidemiol 44:906–918. https://doi.org/10.1093/ije/dyv117
Zong H, Armoni M, Harel C et al (2012) Cytochrome P-450 CYP2E1 knockout mice are protected against high-fat diet-induced obesity and insulin resistance. Am J Physiol Endocrinol Metab 302:E532–E539. https://doi.org/10.1152/ajpendo.00258.2011
Quiroga AD, Lehner R (2018) Pharmacological intervention of liver triacylglycerol lipolysis: the good, the bad and the ugly. Biochem Pharmacol 155:233–241. https://doi.org/10.1016/j.bcp.2018.07.005
Delacroix L, Moutier E, Altobelli G et al (2010) Cell-specific interaction of retinoic acid receptors with target genes in mouse embryonic fibroblasts and embryonic stem cells. Mol Cell Biol 30:231–244. https://doi.org/10.1128/MCB.00756-09
Zhang Y, Li Y, Niepel MW et al (2012) Targeted deletion of thioesterase superfamily member 1 promotes energy expenditure and protects against obesity and insulin resistance. Proc Natl Acad Sci USA 109:5417–5422. https://doi.org/10.1073/pnas.1116011109
Marsili A, Aguayo-Mazzucato C, Chen T et al (2011) Mice with a targeted deletion of the type 2 deiodinase are insulin resistant and susceptible to diet induced obesity. PLoS One 6:e20832. https://doi.org/10.1371/journal.pone.0020832
Berry DC, Jin H, Majumdar A, Noy N (2011) Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc Natl Acad Sci USA 108:4340–4345. https://doi.org/10.1073/pnas.1011115108
Mercader J, Granados N, Bonet ML, Palou A (2008) All-trans retinoic acid decreases murine adipose retinol binding protein 4 production. Cell Physiol Biochem 22:363–372. https://doi.org/10.1159/000149815
Kim B-H, Shenoy AR, Kumar P et al (2011) A family of IFN-γ-inducible 65-kD GTPases protects against bacterial infection. Science 332:717–721. https://doi.org/10.1126/science.1201711
Laudanna C, Campbell JJ, Butcher EC (1996) Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science 271:981–983
McBeath R, Pirone DM, Nelson CM et al (2004) Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6:483–495
Pope BD, Warren CR, Parker KK, Cowan CA (2016) Microenvironmental control of adipocyte fate and function. Trends Cell Biol 26:745–755. https://doi.org/10.1016/j.tcb.2016.05.005
Dicker KT, Gurski LA, Pradhan-Bhatt S et al (2014) Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater 10:1558–1570. https://doi.org/10.1016/j.actbio.2013.12.019
Acknowledgements
This work was supported by grants from the National Institutes of Health DK102014 and DK112754 (JLN), UC Berkeley College of Natural Resources Sponsored Projects for Undergraduate Research (MW), and a UC Berkeley NIH Bridges to Baccalaureate Grant R25GM095401 (CEG). CRK and KMO were supported by NIH Predoctoral Training Grant DK061918. The authors would like to thank Milena Tintcheva for KO mouse colony management and technical help with cell culture studies. Di Yang preformed immunolabeling for F4/80 in BAT. We thank Valerie Hynh, Jared Williams, and AgroSUP Dijon interns Julie Contini, Jeremy Rolland, Fabrice Orellana and Paul Snocks for assisting with microscopy, data collection and LD morphology analysis, and Julie Brayer for assistance with western blot experiments. Special thanks to Steve Ruzin and Denise Schichnes from the UC Berkeley CNR Biological Imaging Facility (BIF) for support of this project. Research reported in this publication was supported in part by the National Institutes of Health S10 program under award number 1S10RR026866-01 to UC Berkeley BIF. Maureen Kane and Jinshan Wang contributed to preliminary retinoid measurements. Matthew Bruss and Simply FlorCruz performed preliminary MIDA experiments. Mariarita Perri assisted with serum lipid measurements. Lilyana Chandra, Audrey Kim, Jane Kim, William Whang, Nicholas Bonniot, and Delphine Foucault assisted with animal studies.
Author information
Authors and Affiliations
Contributions
CRK, MGV, and JLN contributed to designing the study, analyzing the data and writing the manuscript. CRK, MGV, PH, CG, CZ, CSL, KMO, JHM, CBH, MRW and ACT performed experimental work and reviewed the manuscript. SPG analyzed RNAseq data. IDS analyzed RARE data. MKH reviewed mass isotopic dilution analysis studies.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Availability of data and materials
Data generated during this project are reported in this article and the supplementary information. Reagents specific to this project are available upon request from the corresponding author.
Declaration
These experiments comply with the laws of the USA.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Krois, C.R., Vuckovic, M.G., Huang, P. et al. RDH1 suppresses adiposity by promoting brown adipose adaptation to fasting and re-feeding. Cell. Mol. Life Sci. 76, 2425–2447 (2019). https://doi.org/10.1007/s00018-019-03046-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03046-z