Abstract
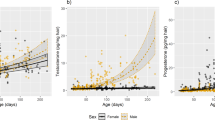
Hormones have not been found in concentrations of orders of magnitude higher than nanograms per milliliter. Here, we report urine concentrations of a catecholamine (norepinephrine) ranging from 0.05 to 0.5 g/l, and concentrations of its metabolite dl-3,4-dihydroxyphenyl glycol (DOPEG) ranging from 1.0 to 44.5 g/l, in wild male red deer Cervus elaphus hispanicus after LC–MS analyses. The dark ventral patch of male red deer, a recently described sexually selected signal, contains high amounts of DOPEG (0.9–266.9 mg/l) stuck in the hairs, while DOPEG is not present in non-darkened hair. The formation of this dark patch is explained by the chemical structure of DOPEG, which is a catecholamine-derived o-diphenol susceptible to be oxidized by air and form allomelanins, nitrogen-free pigments similar to cutaneous melanins; by its high concentration in urine; and by the urine spraying behavior of red deer by which urine is spread through the ventral body area. Accordingly, the size of the dark ventral patch was positively correlated with the concentration of DOPEG in urine, which was in turn correlated with DOPEG absorbed in ventral hair. These findings represent catecholamine concentrations about one million higher than those previously reported for any hormone in an organism. This may have favored the evolution of the dark ventral patch of red deer by transferring information on the fighting capacity to rivals and mates. Physiological limits for hormone production in animals are thus considerably higher than previously thought. These results also unveil a novel mechanism of pigmentation based on the self-application of urine over the fur.


Photographs by Rafael Palomo Santana (a) and Eva de la Peña (b)

Photograph by Rafael Palomo Santana


Similar content being viewed by others
References
Petrie M, Schwabl H, Brande-Lavridsen N, Burke T (2001) Maternal investment: sex differences in avian yolk hormone levels. Nature 412:498
Ganguly S, Weller JL, Ho A, Chemineau P, Malpaux B, Klein DC (2005) Melatonin synthesis: 14-3-3-dependent activation and inhibition of arylalkylamine N-acetyltransferase mediated by phosphoserine-205. Proc Natl Acad Sci USA 102:1222–1227
Stafflinger E, Hansen KK, Hauser F, Schneider M, Cazzamali G, Williamson M, Grimmelikhuijzen CJ (2008) Cloning and identification of an oxytocin/vasopressin-like receptor and its ligand from insects. Proc Natl Acad Sci USA 105:3262–3267
Vitousek MN, Johnson MA, Donald JW, Francis CD, Fuxjager MJ, Goymann W, Hau M, Husak JF, Kircher BK, Knapp R, Martin LB, Miller ET, Schoenle LA, Uehling JJ, Williams TD (2018) HormoneBase, a population-level database of steroid hormone levels across vertebrates. Sci Data 5:180097
Axelrod J, Reisine TD (1984) Stress hormones: their interaction and regulation. Science 224:452–459
Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA (2010) Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal 3:ra70
Künzl C, Kaiser S, Meier E, Sachser N (2003) Is a wild mammal kept and reared in captivity still a wild animal? Horm Behav 43:187–196
Silverberg AB, Shah SD, Haymond MW, Cryer PE (1978) Norepinephrine: hormone and neurotransmitter in man. Am J Physiol Endocrinol Metab 234:E252–E256
Ito S, Yamanaka Y, Ojika M, Wakamatsu K (2016) The metabolic fate of ortho-quinones derived from catecholamine metabolites. Int J Mol Sci 17:164
Rang HP, Ritter JM, Flower R, Henderson G (eds) (2016) Noradrenergic transmission. In: Rang Dale’s pharmacology, 8th edn. Elsevier Churchill Livingstone, New York, pp 177–196
Nohta H, Yamaguchi E, Ohkura Y, Watanabe H (1989) Measurement of catecholamines, their precursor and metabolites in human urine and plasma by solid-phase extraction followed by high-performance liquid chromatography with fluorescence derivatization. J Chromatogr 493:15–26
Kanamori T, Funatsu T, Tsunoda M (2016) Determination of catecholamines and related compounds in mouse urine using column-switching HPLC. Analyst 141:2568–2573
Martín J, Carranza J, López P, Alarcos S, Pérez-González J (2014) A new sexual signal in rutting male red deer: age related chemical scent constituents in the belly black spot. Mamm Biol 79:362–368
Eisenhofer G, Kopin IJ, Goldstein DS (2004) Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 56:331–349
Carranza J, Alvarez F, Redondo T (1990) Territoriality as a mating strategy in red deer. Anim Behav 40:79–88
Carranza J, Alarcos S, Sánchez-Prieto CB, Valencia J, Mateos C (2004) Disposable-soma senescence mediated by sexual selection in an ungulate. Nature 432:215–218
Mitchell B (1967) Growth layers in dental cement for determining the age of red deer (Cervus elaphus L.). J Anim Ecol 36:279–293
Lv C, Li Q, Liu X, He B, Sui Z, Xu H, Yin Y, Liu R, Bi K (2015) Determination of catecholamines and their metabolites in rat urine by ultra-performance liquid chromatography-tandem mass spectrometry for the study of identifying potential markers for Alzheimer’s disease. J Mass Spectrom 50:354–363
Baetge EE, Behringer RR, Messing A, Brinster RL, Palmiter RD (1988) Transgenic mice express the human phenylethanolamine N-methyltransferase gene in adrenal medulla and retina. Proc Natl Acad Sci USA 85:3648–3652
Mårdh G, Dingley AL, Auld DS, Vallee BL (1986) Human class II (π) alcohol dehydrogenase has a redox-specific function in norepinephrine metabolism. Proc Natl Acad Sci USA 83:8908–8912
Lincoln GA, Guinness F, Short RV (1972) The way in which testosterone controls the social and sexual behavior of the red deer stag (Cervus elaphus). Horm Behav 3:375–396
Altendorf KB, Laundré JW, López González CA, Brown JS (2001) Assessing effects of predation risk on foraging behavior of mule deer. J Mammal 82:430–439
Barbas I, Fatouros IG, Douroudos II, Chatzinikolaou A, Michailidis Y, Draganidis D, Jamurtas AZ, Nikolaidis MG, Parotsidis C, Theodorou AA, Katrabasas I, Margonis K, Papassotiriou I, Taxildaris K (2011) Physiological and performance adaptations of elite Greco-Roman wrestlers during a one-day tournament. Eur J Appl Physiol 111:1421–1436
Varga M, Berkesi O, Darula Z, May NV, Palágyi A (2016) Structural characterization of allomelanin from black oat. Phytochemistry 130:313–320
Kruuk LE, Slate J, Pemberton JM, Brotherstone S, Guinness F, Clutton-Brock T (2002) Antler size in red deer: heritability and selection but no evolution. Evolution 56:1683–1695
Galván I, Garrido-Fernández J, Ríos J, Pérez-Gálvez A, Rodríguez-Herrera B, Negro JJ (2016) Tropical bat as mammalian model for skin carotenoid metabolism. Proc Natl Acad Sci USA 113:10932–10937
Acknowledgements
Two anonymous reviewers made comments that improved the manuscript. Rafael Palomo Santana kindly allowed us to reproduce his red deer photographs, shown in Figs. 2 and 3. IG benefits from a Ramón y Cajal Fellowship (RYC-2012-10237) from Spain’s Ministry of Science, Innovation and Universities, and MZ benefits from an “INCRECYT” research contract. Financial support was obtained from projects CGL2013-48122-P, CGL2015-67796-P and CTQ2016-78793-P from Spain’s Ministry of Science, Innovation and Universities, and from project SBPLY/17/180501/000262 from Junta de Comunidades de Castilla-La Mancha.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
All experiments were performed in compliance with the relevant laws and institutional guidelines in Spain.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Galván, I., Solano, F., Zougagh, M. et al. Unprecedented high catecholamine production causing hair pigmentation after urinary excretion in red deer. Cell. Mol. Life Sci. 76, 397–404 (2019). https://doi.org/10.1007/s00018-018-2962-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2962-1