Abstract
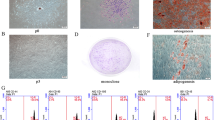
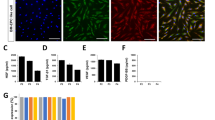
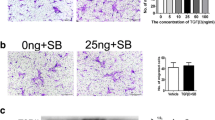
Mesenchymal stem cells (MSCs) are heterogeneous likely consisting of subpopulations with various therapeutic potentials. Here we attempted to acquire a subset of MSCs with enhanced effect in wound healing. We found that human placental MSCs expressing platelet-derived growth factor (PDGF) receptor (PDGFR)-β exhibited greater proliferation rates and generated more colony-forming unit-fibroblast (CFU-F), compared to PDGFR-β− MSCs. Notably, PDGFR-β+ MSCs expressed higher levels of pro-angiogenic factors such as Ang1, Ang2, VEGF, bFGF and PDGF. When 106 GFP-expressing MSCs were topically applied into excisional wounds in mice, PDGFR-β+ MSCs actively incorporated into the wound tissue, resulting in enhanced engraftment (3.92 ± 0.31 × 105 remained in wound by 7 days) and accelerated wound closure; meanwhile, PDGFR-β− MSCs tended to remain on the top of the wound bed with significantly fewer cells (2.46 ± 0.26 × 105) engrafted into the wound, suggesting enhanced chemotactic migration and engraftment of PDGFR-β+ MSCs into the wound. Real-Time PCR and immunostain analyses revealed that the expression of PDGF-B was upregulated after wounding; transwell migration assay showed that PDGFR-β+ MSCs migrated eightfold more than PDGFR-β− MSCs toward PDGF-BB. Intriguingly, PDGFR-β+ MSC-treated wounds showed significantly enhanced angiogenesis compared to PDGFR-β− MSC- or vehicle-treated wounds. Thus, our results indicate that PDGFR-β identifies a subset of MSCs with enhanced chemotactic migration to wound injury and effect in promoting angiogenesis and wound healing, implying a greater therapeutic potential for certain diseases.









Similar content being viewed by others
References
Trounson A, McDonald C (2015) Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 17(1):11–22. doi:10.1016/j.stem.2015.06.007
Parekkadan B, Milwid JM (2010) Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng 12:87–117. doi:10.1146/annurev-bioeng-070909-105309
Mo M, Wang S, Zhou Y et al (2016) Mesenchymal stem cell subpopulations: phenotype, property and therapeutic potential. Cell Mol Life Sci 73(17):3311–3321. doi:10.1007/s00018-016-2229-7
Phinney DG (2012) Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem 113(9):2806–2812. doi:10.1002/jcb.24166
Hellstrom M, Kalen M, Lindahl P et al (1999) Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126(14):3047–3055
Rajkumar VS, Shiwen X, Bostrom M et al (2006) Platelet-derived growth factor-beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am J Pathol 169(6):2254–2265
Tokunaga A, Oya T, Ishii Y et al (2008) PDGF receptor beta is a potent regulator of mesenchymal stromal cell function. J Bone Miner Res 23(9):1519–1528. doi:10.1359/Jbmr.080409
Lin RZ, Moreno-Luna R, Li D et al (2014) Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc Natl Acad Sci USA 111(28):10137–10142. doi:10.1073/pnas.1405388111
Falanga V (2005) Wound healing and its impairment in the diabetic foot. Lancet 366(9498):1736–1743. doi:10.1016/S0140-6736(05)67700-8
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341(10):738–746. doi:10.1056/NEJM199909023411006
Cha J, Falanga V (2007) Stem cells in cutaneous wound healing. Clin Dermatol 25(1):73–78. doi:10.1016/j.clindermatol.2006.10.002
Otero-Vinas M, Falanga V (2016) Mesenchymal stem cells in chronic wounds: the spectrum from basic to advanced therapy. Adv Wound Care 5(4):149–163. doi:10.1089/wound.2015.0627
DiPietro LA (2016) Angiogenesis and wound repair: when enough is enough. J Leukoc Biol 100(5):979–984. doi:10.1189/jlb.4MR0316-102R
Wu Y, Chen L, Scott PG et al (2007) Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25(10):2648–2659. doi:10.1634/stemcells.2007-0226
Wu Y, Zhao RC, Tredget EE (2010) Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells 28(5):905–915. doi:10.1002/stem.420
Li M, Zhao Y, Hao H et al (2015) Mesenchymal stem cell-based therapy for nonhealing wounds: today and tomorrow. Wound Repair Regeneration 23(4):465–482. doi:10.1111/wrr.12304
Chen L, Tredget EE, Wu PY et al (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3(4):e1886. doi:10.1371/journal.pone.0001886
Li Z, Liu C, Xie Z et al (2011) Epigenetic dysregulation in mesenchymal stem cell aging and spontaneous differentiation. PLoS One 6(6):e20526. doi:10.1371/journal.pone.0020526
Wang S, Guo L, Ge J et al (2015) Excess integrins cause lung entrapment of mesenchymal stem cells. Stem Cells 33(11):3315–3326. doi:10.1002/stem.2087
Guo L, Zhou Y, Wang S et al (2014) Epigenetic changes of mesenchymal stem cells in three-dimensional (3D) spheroids. J Cell Mol Med 18(10):2009–2019. doi:10.1111/jcmm.12336
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Wang X, Ge J, Tredget EE et al (2013) The mouse excisional wound splinting model, including applications for stem cell transplantation. Nat Protoc 8(2):302–309. doi:10.1038/nprot.2013.002
Honczarenko M, Le Y, Swierkowski M et al (2006) Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 24(4):1030–1041. doi:10.1634/stemcells.2005-0319
Lv FJ, Tuan RS, Cheung KM et al (2014) Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells 32(6):1408–1419. doi:10.1002/stem.1681
Chandrakanthan V, Yeola A, Kwan JC et al (2016) PDGF-AB and 5-Azacytidine induce conversion of somatic cells into tissue-regenerative multipotent stem cells. Proc Natl Acad Sci USA 113(16):E2306–E2315. doi:10.1073/pnas.1518244113
Zhang Y, Cao N, Huang Y et al (2016) Expandable cardiovascular progenitor cells reprogrammed from fibroblasts. Cell Stem Cell 18(3):368–381. doi:10.1016/j.stem.2016.02.001
Ball SG, Shuttleworth A, Kielty CM (2012) Inhibition of platelet-derived growth factor receptor signaling regulates Oct4 and Nanog expression, cell shape, and mesenchymal stem cell potency. Stem Cells 30(3):548–560. doi:10.1002/stem.1015
Hung SC, Pochampally RR, Chen SC et al (2007) Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells 25(9):2363–2370. doi:10.1634/stemcells.2006-0686
Tao H, Han Z, Han ZC et al (2016) Proangiogenic features of mesenchymal stem cells and their therapeutic applications. Stem Cells Int 2016:1314709. doi:10.1155/2016/1314709
Laschober GT, Brunauer R, Jamnig A et al (2011) Age-specific changes of mesenchymal stem cells are paralleled by upregulation of CD106 expression as a response to an inflammatory environment. Rejuvenation Res 14(2):119–131. doi:10.1089/rej.2010.1077
Winkler EA, Bell RD, Zlokovic BV (2010) Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegeneration 5:32. doi:10.1186/1750-1326-5-32
Wu Y, Zhao RC (2012) The role of chemokines in mesenchymal stem cell homing to myocardium. Stem Cell Rev 8(1):243–250. doi:10.1007/s12015-011-9293-z
Guo L, Ge J, Zhou Y et al (2014) Three-dimensional spheroid-cultured mesenchymal stem cells devoid of embolism attenuate brain stroke injury after intra-arterial injection. Stem Cells Dev 23(9):978–989. doi:10.1089/scd.2013.0338
Baxter MA, Wynn RF, Jowitt SN et al (2004) Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 22(5):675–682. doi:10.1634/stemcells.22-5-675
Krampera M, Pasini A, Rigo A et al (2005) HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood 106(1):59–66. doi:10.1182/blood-2004-09-3645
Fiedler J, Etzel N, Brenner RE (2004) To go or not to go: migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. J Cell Biochem 93(5):990–998. doi:10.1002/jcb.20219
Ball SG, Shuttleworth CA, Kielty CM (2007) Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J Cell Biol 177(3):489–500. doi:10.1083/jcb.200608093
Boomsma RA, Geenen DL (2012) Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One 7(4):e35685. doi:10.1371/journal.pone.0035685
Nuschke A (2014) Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis 10(1):29–37. doi:10.4161/org.27405
Gong Z, Calkins G, Cheng EC et al (2009) Influence of culture medium on smooth muscle cell differentiation from human bone marrow-derived mesenchymal stem cells. Tissue Eng Part A 15(2):319–330. doi:10.1089/ten.tea.2008.0161
Tamama K, Sen CK, Wells A (2008) Differentiation of bone marrow mesenchymal stem cells into the smooth muscle lineage by blocking ERK/MAPK signaling pathway. Stem Cells Dev 17(5):897–908. doi:10.1089/scd.2007.0155
Acknowledgements
We gratefully thank Bing Yu for assistance in confocal analysis. This work was supported by grants from Natural Science Foundation of China (Nos. 31371404, 31571429), Natural Science Foundation of Guangdong (2015A030311041), and Shenzhen Science and Technology Innovation Committee (JCY20160301150838144).
Author information
Authors and Affiliations
Contributions
SW: performed experiments and data analysis; SS, MM, JW, BS: performed experiments; LY: provided materials and designed experiments; XF, ET: designed experiments; YW: designed experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, S., Mo, M., Wang, J. et al. Platelet-derived growth factor receptor beta identifies mesenchymal stem cells with enhanced engraftment to tissue injury and pro-angiogenic property. Cell. Mol. Life Sci. 75, 547–561 (2018). https://doi.org/10.1007/s00018-017-2641-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2641-7