Abstract
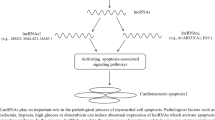
Loss of functional cardiomyocytes is a major underlying mechanism for myocardial remodeling and heart diseases, due to the limited regenerative capacity of adult myocardium. Apoptosis, programmed necrosis, and autophagy contribute to loss of cardiac myocytes that control the balance of cardiac cell death and cell survival through multiple intricate signaling pathways. In recent years, non-coding RNAs (ncRNAs) have received much attention to uncover their roles in cell death of cardiovascular diseases, such as myocardial infarction, cardiac hypertrophy, and heart failure. In addition, based on the view that mitochondrial morphology is linked to three types of cell death, ncRNAs are able to regulate mitochondrial fission/fusion of cardiomyocytes by targeting genes involved in cell death pathways. This review focuses on recent progress regarding the complex relationship between apoptosis/necrosis/autophagy and ncRNAs in the context of myocardial cell death in response to stress. This review also provides insight into the treatment for heart diseases that will guide novel therapies in the future.

Similar content being viewed by others
References
Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL (1994) Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest 94:1621–1628
Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA et al (1997) Apoptosis in the failing human heart. New Engl J Med 336:1131–1141
Konstantinidis K, Whelan RS, Kitsis RN (2012) Mechanisms of cell death in heart disease. Arterioscler Throm Vasc 32:1552–1562
Wang K, Zhou LY, Wang JX, Wang Y, Sun T, Zhao B et al (2015) E2F1-dependent miR-421 regulates mitochondrial fragmentation and myocardial infarction by targeting Pink1. Nat Commun 6:7619
Small EM, Frost RJA, Olson EN (2010) MicroRNAs add a new dimension to cardiovascular disease. Circulation 121:U1022–U1066
Leite-Moreira AM, Lourenco AP, Falcao-Pires I, Leite-Moreira AF (2013) Pivotal role of microRNAs in cardiac physiology and heart failure. Drug Discov Today 18:1243–1249
Kumarswamy R, Thum T (2013) Non-coding RNAs in cardiac remodeling and heart failure. Circ Res 113:676–689
Boon RA, Dimmeler S (2015) MicroRNAs in myocardial infarction. Nat Rev Cardiol 12:135–142
Filipowicz W, Bhattacharyya SN, Sonenberg N (2008) Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 9:102–114
Chekulaeva M, Filipowicz W (2009) Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr Opin Cell Biol 21:452–460
Berezikov E (2011) Evolution of microRNA diversity and regulation in animals. Nat Rev Genet 12:846–860
Graves P, Zeng Y (2012) Biogenesis of mammalian microRNAs: a global view. Genom Proteom Bioinform 10:239–245
Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH et al (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16:3–11
Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7:99–109
Orogo AM, Gustafsson AB (2013) Cell death in the myocardium: my heart won’t go on. IUBMB Life 65:651–656
Skommer J, Rana I, Marques FZ, Zhu W, Du Z, Charchar FJ (2014) Small molecules, big effects: the role of microRNAs in regulation of cardiomyocyte death. Cell Death Dis 5:e1325
Gao CF, Ren S, Zhang LL, Nakajima T, Ichinose S, Hara T et al (2001) Caspase-dependent cytosolic release of cytochrome c and membrane translocation of Bax in p53-induced apoptosis. Exp Cell Res 265:145–151
Jiang XJ, Wang XD (2000) Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to Apaf-1. J Biol Chem 275:31199–31203
Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ (2010) Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121(18):2012–2022
Clerk A, Cullingford TE, Fuller SJ, Giraldo A, Markou T, Pikkarainen S et al (2007) Signaling pathways mediating cardiac myocyte gene expression in physiological and stress responses. J Cell Physiol 212:311–322
Bostjancic E, Zidar N, Glavac D (2009) MicroRNA microarray expression profiling in human myocardial infarction. Dis Mark 27:255–268
Tang Y, Zheng J, Sun Y, Wu Z, Liu Z, Huang G (2009) MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int Heart J 50:377–387
Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B et al (2007) The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med 13:486–491
Xu C, Lu Y, Pan Z, Chu W, Luo X, Lin H et al (2007) The muscle-specific microRNAs miR-1 and miR-133 produce opposing effects on apoptosis by targeting HSP60, HSP70 and caspase-9 in cardiomyocytes. J Cell Sci 120:3045–3052
Matkovich SJ, Wang W, Tu Y, Eschenbacher WH, Dorn LE, Condorelli G et al (2010) MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res 106:166–175
Wang HJ, Li J, Chi HJ, Zhang F, Zhu XM, Cai J et al (2015) MicroRNA-181c targets Bcl-2 and regulates mitochondrial morphology in myocardial cells. J Cell Mol Med 19:2084–2097
Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C (2009) MicroRNA-21 protects against the H(2)O(2)-induced injury on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol 47:5–14
Li J, Donath S, Li Y, Qin D, Prabhakar BS, Li P (2010) miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS Genet 6:e1000795
Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J et al (2009) Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res 104:879–886
Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K et al (2009) MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation 119:2357–2366
Wang JX, Gao J, Ding SL, Wang K, Jiao JQ, Wang Y et al (2015) Oxidative modification of miR-184 enables It to target Bcl-xL and Bcl-w. Mol Cell 59:50–61
Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T et al (2008) Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell 14:193–204
Li P (2010) MicroRNAs in cardiac apoptosis. J Cardiovasc Transl Res 3:219–224
Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F et al (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1:515–525
Li JC, Donath S, Li YR, Qin D, Prabhakar BS, Li PF (2016) miR-30 Regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. Plos Genet 6(1):e1000795
Wang JX, Jiao JQ, Li QA, Long B, Wang K, Liu JP et al (2011) miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med 17(1):71–78
Li JC, Zhou J, Li YR, Qin DN, Li PF (2010) Mitochondrial fission controls DNA fragmentation by regulating endonuclease G. Free Radic Biol Med 49:622–631
Li JC, Li YZ, Jiao JQ, Wang JX, Li YR, Qin DN et al (2014) Mitofusin 1 is negatively regulated by microRNA 140 in cardiomyocyte apoptosis. Mol Cell Biol 34:1788–1799
Dagda RK, Cherra SJ, Kulich SM, Tandon A, Park D, Chu CT (2009) Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem 284:13843–13855
Wang K, Zhou LY, Wang JX, Wang Y, Sun T, Zhao B et al (2015) E2F1-dependent miR-421 regulates mitochondrial fragmentation and myocardial infarction by targeting Pink1. Nat Commun 6:7619
Wang K, Liu CY, Zhang XJ, Feng C, Zhou LY, Zhao Y et al (2015) miR-361-regulated prohibitin inhibits mitochondrial fission and apoptosis and protects heart from ischemia injury. Cell Death Differ 22(6):1058–1068
Wang K, Zhang DL, Long B, An T, Zhang J, Zhou LY et al (2015) NFAT4-dependent miR-324-5p regulates mitochondrial morphology and cardiomyocyte cell death by targeting Mtfr1. Cell Death Dis 6:e2007
Singal PK, Iliskovic N (1998) Doxorubicin-induced cardiomyopathy. New Engl J Med 339(13):900–905
Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL (2012) Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 52:1213–1225
Tony H, Yu K, Qiutang Z (2015) MicroRNA-208a silencing attenuates doxorubicin induced myocyte apoptosis and cardiac dysfunction. Oxidative Med Cell Longev 2015:597032
Wang JX, Zhang XJ, Feng C, Sun T, Wang K, Wang Y et al (2015) MicroRNA-532-3p regulates mitochondrial fission through targeting apoptosis repressor with caspase recruitment domain in doxorubicin cardiotoxicity. Cell Death Dis 6:e1677
Tong Z, Jiang B, Wu Y, Liu Y, Li Y, Gao M et al (2015) MiR-21 protected cardiomyocytes against doxorubicin-induced apoptosis by targeting BTG2. Int J Mol Sci 16(7):14511–14525
Roca-Alonso L, Castellano L, Mills A, Dabrowska AF, Sikkel MB, Pellegrino L et al (2015) Myocardial MiR-30 downregulation triggered by doxorubicin drives alterations in beta-adrenergic signaling and enhances apoptosis. Cell Death Dis 6:e1754
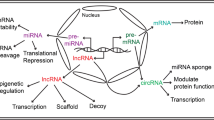
Papait R, Kunderfranco P, Stirparo GG, Latronico MVG, Condorelli G (2013) Long noncoding RNA: a new player of heart failure? J Cardiovasc Transl 6:876–883
Wang KC, Chang HY (2011) Molecular mechanisms of long noncoding RNAs. Mol Cell 43:904–914
Piccoli MT, Gupta SK, Thum T (2015) Noncoding RNAs as regulators of cardiomyocyte proliferation and death. J Mol Cell Cardiol 89:59–67
Ginger MR, Shore AN, Contreras A, Rijnkels M, Miller J, Gonzalez-Rimbau MF et al (2006) A noncoding RNA is a potential marker of cell fate during mammary gland development. Proc Natl Acad Sci USA 103:5781–5786
Kanduri C (2011) Kcnq1ot1: a chromatin regulatory RNA. Semin Cell Dev Biol 22:343–350
Grote P, Wittler L, Hendrix D, Koch F, Wahrisch S, Beisaw A et al (2013) The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell 24:206–214
Ishii N, Ozaki K, Sato H, Mizuno H, Saito S, Takahashi A et al (2006) Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J Hum Genet 51:1087–1099
Wang K, Long B, Zhou LY, Liu F, Zhou QY, Liu CY et al (2014) CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat Commun 5:3596
Hsu MT, Coca-Prados M (1979) Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature 280(5720):339–340
Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A et al (2013) Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495:333–338
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK et al (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495:384–388
Wang K, Long B, Liu F, Wang JX, Liu CY, Zhao B et al (2016) A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J 37(33):2602–2611
Koseki T, Inohara N, Chen S, Nunez G (1998) ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases. Proc Natl Acad Sci USA 95(9):5156–5160
Li YZ, Lu DY, Tan WQ, Wang JX, Li PF (2008) p53 initiates apoptosis by transcriptionally targeting the antiapoptotic protein ARC. Mol Cell Biol 28:564–574
Du WJ, Pan ZW, Chen X, Wang LM, Zhang Y, Li S et al (2014) By targeting Stat3 microRNA-17-5p promotes cardiomyocyte apoptosis in response to ischemia followed by reperfusion. Cell Physiol Biochem 34:955–965
Danielson LS, Park DS, Rotllan N, Chamorro-Jorganes A, Guijarro MV, Fernandez-Hernando C et al (2013) Cardiovascular dysregulation of miR-17-92 causes a lethal hypertrophic cardiomyopathy and arrhythmogenesis. Faseb J 27:1460–1467
Qin YJ, Yu YQ, Dong H, Bian XH, Guo X, Dong SM (2012) MicroRNA 21 inhibits left ventricular remodeling in the early phase of rat model with ischemia–reperfusion injury by suppressing cell apoptosis. Int J Med Sci 9:413–423
Ding SL, Wang JX, Jiao JQ, Tu X, Wang Q, Liu F et al (2013) A pre-microRNA-149 (miR-149) genetic variation affects miR-149 maturation and its ability to regulate the Puma protein in apoptosis. J Biol Chem 288:26865–26877
Long B, Wang K, Li N, Murtaza I, Xiao JY, Fan YY et al (2013) miR-761 regulates the mitochondrial network by targeting mitochondrial fission factor. Free Radic Biol Med 65:371–379
Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP et al (2011) miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med 17:71–78
Lv G, Shao S, Dong H, Bian X, Yang X, Dong S (2014) MicroRNA-214 protects cardiac myocytes against H2O2-induced injury. J Cell Biochem 115:93–101
Li R, Yan G, Li Q, Sun H, Hu Y, Sun J et al (2012) MicroRNA-145 protects cardiomyocytes against hydrogen peroxide (H(2)O(2))-induced apoptosis through targeting the mitochondria apoptotic pathway. PLoS One 7:e44907
Fang J, Song XW, Tian J, Chen HY, Li DF, Wang JF et al (2012) Overexpression of microRNA-378 attenuates ischemia-induced apoptosis by inhibiting caspase-3 expression in cardiac myocytes. Apoptosis 17:410–423
Zhu H, Yang Y, Wang Y, Li J, Schiller PW, Peng T (2011) MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc Res 92:75–84
Tabuchi T, Satoh M, Itoh T, Nakamura M (2012) MicroRNA-34a regulates the longevity-associated protein SIRT1 in coronary artery disease: effect of statins on SIRT1 and microRNA-34a expression. Clin Sci (Lond) 123:161–171
Wu W, Liu P, Li J (2012) Necroptosis: an emerging form of programmed cell death. Crit Rev Oncol Hematol 82:249–258
Galluzzi L, Kepp O, Kroemer G (2009) RIP kinases initiate programmed necrosis. J Mol Cell Biol 1:8–10
Fiers W, Beyaert R, Boone E, Cornelis S, Declercq W, Decoster E et al (1995) TNF-induced intracellular signaling leading to gene induction or to cytotoxicity by necrosis or by apoptosis. J Inflamm 47:67–75
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S et al (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1:489–495
Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X et al (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4:313–321
Whelan RS, Kaplinskiy V, Kitsis RN (2010) Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol 72:19–44
Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA et al (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434:658–662
Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H et al (2005) Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434:652–658
Wang K, An T, Zhou LY, Liu CY, Zhang XJ, Feng C et al (2015) E2F1-regulated miR-30b suppresses cyclophilin D and protects heart from ischemia/reperfusion injury and necrotic cell death. Cell Death Differ 22:743–754
Liu J, van Mil A, Vrijsen K, Zhao J, Gao L, Metz CH et al (2011) MicroRNA-155 prevents necrotic cell death in human cardiomyocyte progenitor cells via targeting RIP1. J Cell Mol Med 15:1474–1482
Wang K, Liu F, Zhou LY, Ding SL, Long B, Liu CY et al (2013) miR-874 regulates myocardial necrosis by targeting caspase-8. Cell Death Dis 4:e709
Lee EW, Kim JH, Ahn YH, Seo J, Ko A, Jeong M et al (2012) Ubiquitination and degradation of the FADD adaptor protein regulate death receptor-mediated apoptosis and necroptosis. Nat Commun 3:978
Wang JX, Zhang XJ, Li Q, Wang K, Wang Y, Jiao JQ et al (2015) MicroRNA-103/107 regulate programmed necrosis and myocardial ischemia/reperfusion injury through targeting FADD. Circ Res 117:352–363
Wang K, Long B, Li N, Li L, Liu CY, Dong YH et al (2016) MicroRNA-2861 regulates programmed necrosis in cardiomyocyte by impairing adenine nucleotide translocase 1 expression. Free Radic Biol Med 91:58–67
Walther T, Tschope C, Sterner-Kock A, Westermann D, Heringer-Walther S, Riad A et al (2007) Accelerated mitochondrial adenosine diphosphate/adenosine triphosphate transport improves hypertension-induced heart disease. Circulation 115:333–344
Wang K, Liu F, Liu CY, An T, Zhang J, Zhou LY et al (2016) The long noncoding RNA NRF regulates programmed necrosis and myocardial injury during ischemia and reperfusion by targeting miR-873. Cell Death Differ 23(8):1394–1405
He CC, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132(1):27–42
Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I et al (2010) Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 6(5):600–606
Sciarretta S, Volpe M, Sadoshima J (2014) Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res 114(3):549–564
Li Q, Xie J, Li R, Shi J, Sun J, Gu R et al (2014) Overexpression of microRNA-99a attenuates heart remodelling and improves cardiac performance after myocardial infarction. J Cell Mol Med 18:919–928
Su M, Wang J, Wang C, Wang X, Dong W, Qiu W et al (2015) MicroRNA-221 inhibits autophagy and promotes heart failure by modulating the p27/CDK2/mTOR axis. Cell Death Differ 22:986–999
Bo L, Su-Ling D, Fang L, Lu-Yu Z, Tao A, Stefan D et al (2014) Autophagic program is regulated by miR-325. Cell Death Differ 21:967–977
Pan W, Zhong Y, Cheng C, Liu B, Wang L, Li A et al (2013) MiR-30-regulated autophagy mediates angiotensin II-induced myocardial hypertrophy. PLoS One 8:e53950
Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW et al (2007) MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation 116:258–267
Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S et al (2012) The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun 3:1078
Song L, Su M, Wang S, Zou Y, Wang X, Wang Y et al (2014) MiR-451 is decreased in hypertrophic cardiomyopathy and regulates autophagy by targeting TSC1. J Cell Mol Med 18:2266–2274
Gupta SK, Foinquinos A, Thum S, Remke J, Zimmer K, Bauters C et al (2016) Preclinical development of a MicroRNA-based therapy for elderly patients with myocardial infarction. J Am Coll Cardiol 68:1557–1571
Wang K, Liu CY, Zhou LY, Wang JX, Wang M, Zhao B et al (2015) APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat Commun 6:6779
Huang J, Sun W, Huang H, Ye J, Pan W, Zhong Y et al (2014) miR-34a modulates angiotensin II-induced myocardial hypertrophy by direct inhibition of ATG9A expression and autophagic activity. PLoS One 9:e94382
Li XX, Zeng Z, Li Q, Xu QL, Xie JH, Hao HX et al (2015) Inhibition of microRNA-497 ameliorates anoxia/reoxygenation injury in cardiomyocytes by suppressing cell apoptosis and enhancing autophagy. Oncotarget 6:18829–18844
Nishida K, Yamaguchi O, Otsu K (2008) Crosstalk between autophagy and apoptosis in heart disease. Circ Res 103:343–351
Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z (2007) Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 26:1749–1760
Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S et al (2003) Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci USA 100:15883–15888
Marquez RT, Xu L (2012) Bcl-2: beclin 1 complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am J Cancer Res 2:214–221
Crighton D, Wilkinson S, O’Prey J, Syed N, Smith P, Harrison PR et al (2006) DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell 126:121–134
Pyo JO, Jang MH, Kwon YK, Lee HJ, Jun JIL, Woo HN et al (2005) Essential roles of Atg5 and FADD in autophagic cell death—dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem 280:20722–20729
Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S et al (2012) Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci USA 109:6566–6571
Diwan A, Matkovich SJ, Yuan QY, Zhao W, Yatani A, Brown JH et al (2009) Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death. J Clin Investig 119:203–212
Ng F, Tang BL (2013) Sirtuins’ modulation of autophagy. J Cell Physiol 228:2262–2270
Ham O, Lee SY, Lee CY, Park JH, Lee J, Seo HH, et al (2015) Let-7b suppresses apoptosis and autophagy of human mesenchymal stem cells transplanted into ischemia/reperfusion injured heart 7 by targeting caspase-3. Stem Cell Res Ther 6
Zou Y, Liu W, Zhang J, Xiang D (2016) miR-153 regulates apoptosis and autophagy of cardiomyocytes by targeting Mcl-1. Mol Med Rep 14:1033–1039
Widera C, Gupta SK, Lorenzen JM, Bang C, Bauersachs J, Bethmann K et al (2011) Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J Mol Cell Cardiol 51(5):872–875
Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C et al (2010) Circulating microRNAs in patients with coronary artery disease. Circ Res 107(5):677–684
Acknowledgements
We thank Chao Chen of the Institute for Translational Medicine, Qingdao University, China for his generous assistance with literature searches.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding information
This work was supported by the Natural Science Foundation of China (Grant nos: 31430041, 81470522, 81522005), Applied Basic Research Programs of Qingdao, China (Grant no: 17-1-1-46-jch), and Shandong Provincial Natural Science Foundation, China (Grant no: ZR2016CQ31).
Conflict of interest
The authors have declared there are no potential conflicts of interest, funding, acknowledgements.
Rights and permissions
About this article
Cite this article
Dong, Y., Liu, C., Zhao, Y. et al. Role of noncoding RNAs in regulation of cardiac cell death and cardiovascular diseases. Cell. Mol. Life Sci. 75, 291–300 (2018). https://doi.org/10.1007/s00018-017-2640-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2640-8