Abstract
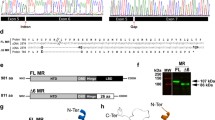
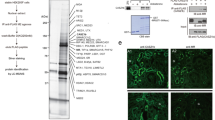
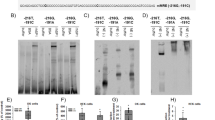
Mineralocorticoid receptor (MR) mediates the sodium-retaining action of aldosterone in the distal nephron. Herein, we decipher mechanisms by which hypotonicity increases MR expression in renal principal cells. We identify HuR (human antigen R), an mRNA-stabilizing protein, as an important posttranscriptional regulator of MR expression. Hypotonicity triggers a rapid and reversible nuclear export of HuR in renal KC3AC1 cells, as quantified by high-throughput microscopy. We also identify a key hairpin motif in the 3′-untranslated region of MR transcript, pivotal for the interaction with HuR and its stabilizing function. Next, we show that hypotonicity increases MR recruitment onto Sgk1 promoter, a well-known MR target gene, thereby enhancing aldosterone responsiveness. Our data shed new light on the crucial role of HuR as a stabilizing factor for the MR transcript and provide evidence for a short autoregulatory loop in which expression of a nuclear receptor transcriptionally regulating water and sodium balance is controlled by osmotic tone.




Similar content being viewed by others
Abbreviations
- MR:
-
Mineralocorticoid receptor
- HuR:
-
Human antigen R
- Sgk1:
-
Serum and glucocorticoid-regulated kinase 1
- Tis11b:
-
Tetradecanoyl phorbol acetate inducible sequence 11b
- RBP:
-
RNA-binding protein
- 3′-UTR:
-
3′-Untranslated region
- ELAVL1:
-
Embryonic lethal, abnormal vision, Drosophila homolog-like 1
- EGF:
-
Epidermal growth factor
- T3:
-
Triiodothyronine
- DCC:
-
Dextran charcoal-coated
- RNP-IP:
-
Ribonucleoprotein immunoprecipitation
- Luc:
-
Luciferase
- DNA-ChiP:
-
DNA-chromatin immunoprecipitation
- Iso:
-
Isotonicity
- Hypo:
-
Hypotonicity
- HTM:
-
High-throughput microscopy
- LMB:
-
Leptomycin B
- PKC:
-
Protein kinase C
- miRNA:
-
MicroRNA
- PIS:
-
Preimmune serum
- Ucp1:
-
Uncoupling protein 1
- MTA:
-
Metastasis-associate 1
- PTMA:
-
Prothymosin
References
Viengchareun S, Le Menuet D, Martinerie L et al (2007) The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl Recept Signal 5:e012. doi:10.1621/nrs.05012
Quinkler M, Zehnder D, Eardley KS et al (2005) Increased expression of mineralocorticoid effector mechanisms in kidney biopsies of patients with heavy proteinuria. Circulation 112:1435–1443. doi:10.1161/CIRCULATIONAHA.105.539122
Shibata S, Nagase M, Yoshida S et al (2008) Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med 14:1370–1376. doi:10.1038/nm.1879
Martinerie L, Viengchareun S, Delezoide A-L et al (2009) Low renal mineralocorticoid receptor expression at birth contributes to partial aldosterone resistance in neonates. Endocrinology 150:4414–4424. doi:10.1210/en.2008-1498
Geller DS, Zhang J, Zennaro M-C et al (2006) Autosomal dominant pseudohypoaldosteronism type 1: mechanisms, evidence for neonatal lethality, and phenotypic expression in adults. J Am Soc Nephrol JASN 17:1429–1436. doi:10.1681/ASN.2005111188
Doucet A, Katz AI (1981) Mineralcorticoid receptors along the nephron: [3H]aldosterone binding in rabbit tubules. Am J Physiol 241:F605–F611
Fenton RA, Knepper MA (2007) Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev 87:1083–1112. doi:10.1152/physrev.00053.2006
Viengchareun S, Kamenicky P, Teixeira M et al (2009) Osmotic stress regulates mineralocorticoid receptor expression in a novel aldosterone-sensitive cortical collecting duct cell line. Mol Endocrinol Baltim Md 23:1948–1962. doi:10.1210/me.2009-0095
Viengchareun S, Lema I, Lamribet K et al (2014) Hypertonicity compromises renal mineralocorticoid receptor signaling through Tis11b-mediated post-transcriptional control. J Am Soc Nephrol JASN 25:2213–2221. doi:10.1681/ASN.2013091023
Hinman MN, Lou H (2008) Diverse molecular functions of Hu proteins. Cell Mol Life Sci CMLS 65:3168–3181. doi:10.1007/s00018-008-8252-6
Keene JD (1999) Why is Hu where? Shuttling of early-response-gene messenger RNA subsets. Proc Natl Acad Sci USA 96:5–7
Doller A, Pfeilschifter J, Eberhardt W (2008) Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell Signal 20:2165–2173. doi:10.1016/j.cellsig.2008.05.007
Abdelmohsen K, Lal A, Kim HH, Gorospe M (2007) Posttranscriptional orchestration of an anti-apoptotic program by HuR. Cell Cycle Georget Tex 6:1288–1292
Katsanou V, Milatos S, Yiakouvaki A et al (2009) The RNA-binding protein Elavl1/HuR is essential for placental branching morphogenesis and embryonic development. Mol Cell Biol 29:2762–2776. doi:10.1128/MCB.01393-08
Chamboredon S, Ciais D, Desroches-Castan A et al (2011) Hypoxia-inducible factor-1α mRNA: a new target for destabilization by tristetraprolin in endothelial cells. Mol Biol Cell 22:3366–3378. doi:10.1091/mbc.E10-07-0617
Le Billan F, Khan JA, Lamribet K et al (2015) Cistrome of the aldosterone-activated mineralocorticoid receptor in human renal cells. FASEB J Off Publ Fed Am Soc Exp Biol 29:3977–3989. doi:10.1096/fj.15-274266
Amazit L, Roseau A, Khan JA et al (2011) Ligand-dependent degradation of SRC-1 is pivotal for progesterone receptor transcriptional activity. Mol Endocrinol Baltim Md 25:394–408. doi:10.1210/me.2010-0458
Amazit L, Le Billan F, Kolkhof P et al (2015) Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J Biol Chem 290:21876–21889. doi:10.1074/jbc.M115.657957
Ma WJ, Cheng S, Campbell C et al (1996) Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J Biol Chem 271:8144–8151
Zhang J, Bowden GT (2008) UVB irradiation regulates Cox-2 mRNA stability through AMPK and HuR in human keratinocytes. Mol Carcinog 47:974–983. doi:10.1002/mc.20450
Cherradi N, Lejczak C, Desroches-Castan A, Feige J-J (2006) Antagonistic functions of tetradecanoyl phorbol acetate-inducible-sequence 11b and HuR in the hormonal regulation of vascular endothelial growth factor messenger ribonucleic acid stability by adrenocorticotropin. Mol Endocrinol Baltim Md 20:916–930. doi:10.1210/me.2005-0121
López de Silanes I, Zhan M, Lal A et al (2004) Identification of a target RNA motif for RNA-binding protein HuR. Proc Natl Acad Sci USA 101:2987–2992. doi:10.1073/pnas.0306453101
Lebedeva S, Jens M, Theil K et al (2011) Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol Cell 43:340–352. doi:10.1016/j.molcel.2011.06.008
Mukherjee N, Corcoran DL, Nusbaum JD et al (2011) Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol Cell 43:327–339. doi:10.1016/j.molcel.2011.06.007
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415
Soler DM, Ghosh A, Chen F, Shneider BL (2014) A single element in the 3′UTR of the apical sodium-dependent bile acid transporter controls both stabilization and destabilization of mRNA. Biochem J 462:547–553. doi:10.1042/BJ20140070
Komnenov D, Scipione CA, Bazzi ZA et al (2015) Pro-inflammatory cytokines reduce human TAFI expression via tristetraprolin-mediated mRNA destabilisation and decreased binding of HuR. Thromb Haemost 114:337–349. doi:10.1160/TH14-08-0653
Eberhardt W, Badawi A, Biyanee A, Pfeilschifter J (2016) Cytoskeleton-dependent transport as a potential target for interfering with post-transcriptional HuR mRNA regulons. Front Pharmacol 7:251. doi:10.3389/fphar.2016.00251
Ge J, Chang N, Zhao Z et al (2016) Essential roles of RNA-binding protein HuR in activation of hepatic stellate cells induced by transforming growth factor-β1. Sci Rep 6:22141. doi:10.1038/srep22141
Fan XC, Steitz JA (1998) HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc Natl Acad Sci USA 95:15293–15298
Doller A, Gauer S, Sobkowiak E et al (2009) Angiotensin II induces renal plasminogen activator inhibitor-1 and cyclooxygenase-2 expression post-transcriptionally via activation of the mRNA-stabilizing factor human-antigen R. Am J Pathol 174:1252–1263. doi:10.2353/ajpath.2009.080652
Levy NS, Chung S, Furneaux H, Levy AP (1998) Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J Biol Chem 273:6417–6423
Mochizuki H, Murphy CJ, Brandt JD et al (2012) Altered stability of mRNAs associated with glaucoma progression in human trabecular meshwork cells following oxidative stress. Invest Ophthalmol Vis Sci 53:1734–1741. doi:10.1167/iovs.12-7938
St Laurent G, Shtokalo D, Heydarian M et al (2012) Insights from the HuR-interacting transcriptome: ncRNAs, ubiquitin pathways, and patterns of secondary structure dependent RNA interactions. Mol Genet Genomics MGG 287:867–879. doi:10.1007/s00438-012-0722-8
Barker A, Epis MR, Porter CJ et al (2012) Sequence requirements for RNA binding by HuR and AUF1. J Biochem (Tokyo) 151:423–437. doi:10.1093/jb/mvs010
Srikantan S, Tominaga K, Gorospe M (2012) Functional interplay between RNA-binding protein HuR and microRNAs. Curr Protein Pept Sci 13:372–379
He F, Peng F, Xia X et al (2014) MiR-135a promotes renal fibrosis in diabetic nephropathy by regulating TRPC1. Diabetologia 57:1726–1736. doi:10.1007/s00125-014-3282-0
Taruno A, Niisato N, Marunaka Y (2008) Intracellular calcium plays a role as the second messenger of hypotonic stress in gene regulation of SGK1 and ENaC in renal epithelial A6 cells. Am J Physiol Renal Physiol 294:F177–F186. doi:10.1152/ajprenal.00250.2007
Bockenhauer D, Bichet DG (2015) Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus. Nat Rev Nephrol 11:576–588. doi:10.1038/nrneph.2015.89
Shang J, Wan Q, Wang X et al (2015) Identification of NOD2 as a novel target of RNA-binding protein HuR: evidence form NAPDH oxidase-mediated HuR signaling in diabetic nephropathy. Free Radic Biol Med 79:217–227. doi:10.1016/freeradbiomed.2014.12.013
Acknowledgements
The authors are indebted to Julie Sappa for English editing.
Author information
Authors and Affiliations
Contributions
IL, AB, JF, NC, ML, and SV designed the study. IL, LA, KL, SV, NC, and ML performed the study. LA, IL, KL, AB, NC, ML, and SV analyzed the data, and IL, LA, NC, ML, and SV wrote the paper.
Corresponding authors
Ethics declarations
Funding
This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale, the Université Paris-Sud and Agence Nationale de la Recherche (Grant 11-BSV1-028-01). Ingrid Lema held a fellowship from the Cardiovasculaire-Obésité-Rein-Diabète-Domaine d’Intérêt Majeur (Région Ile-de-France) and HAC Pharma & SFE (Société Française d’Endocrinologie).
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lema, I., Amazit, L., Lamribet, K. et al. RNA-binding protein HuR enhances mineralocorticoid signaling in renal KC3AC1 cells under hypotonicity. Cell. Mol. Life Sci. 74, 4587–4597 (2017). https://doi.org/10.1007/s00018-017-2594-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2594-x