Abstract
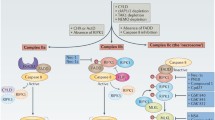
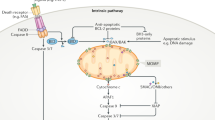
The cytoprotective effects of glycine against cell death have been recognized for over 28 years. They are expressed in multiple cell types and injury settings that lead to necrosis, but are still not widely appreciated or considered in the conceptualization of cell death pathways. In this paper, we review the available data on the expression of this phenomenon, its relationship to major pathophysiologic pathways that lead to cell death and immunomodulatory effects, the hypothesis that it involves suppression by glycine of the development of a hydrophilic death channel of molecular dimensions in the plasma membrane, and evidence for its impact on disease processes in vivo.

Similar content being viewed by others
Abbreviations
- CCCP:
-
carbonylcyanide m-chlorophenylhydrazone
- COP:
-
Cytolytic oncotic pore
- ∆ ψ m :
-
Mitochondrial membrane potential
- EthBr:
-
Ethidium bromide
- LDH:
-
Lactate dehydrogenase
- MPT:
-
Mitochondrial permeability transition
- NHE:
-
Sodium-hydrogen exchanger
- P2X7R:
-
P2X7 purinergic receptor
References
Fournier N, Ducet G, Crevat A (1987) Action of cyclosporine on mitochondrial calcium fluxes. J Bioenerg Biomembr 19(3):297–303
Gunter TE, Pfeiffer DR (1990) Mechanisms by which mitochondria transport calcium. Am J Physiol 258(5):C755–C786
Halestrap AP, Davidson AM (1990) Inhibition of Ca2+-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J 268:153–160
Bernardi P, Rasola A, Forte M, Lippe G (2015) The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol Rev 95(4):1111–1155. doi:10.1152/physrev.00001.2015
Weinberg JM, Davis JA, Abarzua M, Rajan T (1987) Cytoprotective effects of glycine and glutathione against hypoxic injury to renal tubules. J Clin Invest 80(5):1446–1454. doi:10.1172/JCI113224
Weinberg JM (1990) The effect of amino acids on ischemic and toxic injury to the kidney. Semin Nephrol 10(5):491–500
Weinberg JM (1991) The cell biology of ischemic renal injury. Kidney Int 39(3):476–500
Bonventre JV, Weinberg JM (1992) Kidney preservation ex vivo for transplantation. Annu Rev Med 43:523–553. doi:10.1146/annurev.me.43.020192.002515
Weinberg JM (1992) Glutathione and glycine in acute renal failure. Ren Fail 14(3):311–319
Hall JC (1998) Glycine. JPEN J Parenter Enteral Nutr 22(6):393–398
Wheeler MD, Ikejema K, Enomoto N, Stacklewitz RF, Seabra V, Zhong Z, Yin M, Schemmer P, Rose ML, Rusyn I, Bradford B, Thurman RG (1999) Glycine: a new anti-inflammatory immunonutrient. Cell Mol Life Sci 56(9–10):843–856
Habib MM, Hodgson HJ, Davidson BR (2006) The role of glycine in hepatic ischemia-reperfusion injury. Curr Pharm Des 12(23):2953–2967
Gundersen RY, Vaagenes P, Breivik T, Fonnum F, Opstad PK (2005) Glycine–an important neurotransmitter and cytoprotective agent. Acta Anaesthesiol Scand 49(8):1108–1116. doi:10.1111/j.1399-6576.2005.00786.x
Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, Bunzendaul H, Bradford B, Lemasters JJ (2003) l-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care 6(2):229–240. doi:10.1097/01.mco.0000058609.19236.a4
Petrat F, Boengler K, Schulz R, de Groot H (2012) Glycine, a simple physiological compound protecting by yet puzzling mechanism(s) against ischaemia-reperfusion injury: current knowledge. Br J Pharmacol 165(7):2059–2072. doi:10.1111/j.1476-5381.2011.01711.x
Weinberg JM, Molitoris BA (2009) Illuminating mitochondrial function and dysfunction using multiphoton technology. J Am Soc Nephrol 20(6):1164–1166. doi:10.1681/Asn.2009040419
Mandel LJ, Schnellmann RG, Jacobs WR (1990) Intracellular glutathione in the protection from anoxic injury in renal proximal tubules. J Clin Invest 85(2):316–324. doi:10.1172/JCI114440
Weinberg JM, Venkatachalam MA, Garzo-Quintero R, Roeser NF, Davis JA (1990) Structural requirements for protection by small amino acids against hypoxic injury in kidney proximal tubules. FASEB J 4(15):3347–3354
Weinberg JM, Roeser NF, Davis JA, Venkatachalam MA (1997) Glycine-protected, hypoxic, proximal tubules develop severely compromised energetic function. Kidney Int 52(1):140–151
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760
Weinberg JM, Davis JA, Abarzua M, Kiani T (1989) Relationship between cell ATP and glutathione content and protection by glycine against hypoxic proximal tubule cell injury. J Lab Clin Med 113:612–623
Weinberg JM, Davis JA, Abarzua M, Kiani T, Kunkel R (1990) Protection by glycine of proximal tubules from injury due to inhibitors of mitochondrial Atp production. Am J Physiol 258(6):C1127–C1140
Weinberg JM, Davis JA, Abarzua M, Smith RK, Kunkel R (1990) Ouabain-induced lethal proximal tubule cell injury is prevented by glycine. Am J Physiol 258(2):F346–F355
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072. doi:10.1016/j.cell.2012.03.042
Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A, Stockwell BR (2014) Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc 136(12):4551–4556. doi:10.1021/ja411006a
Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz PS, Weinlich R, Vanden Berghe T, Vandenabeele P, Pasparakis M, Bleich M, Weinberg JM, Reichel CA, Brasen JH, Kunzendorf U, Anders HJ, Stockwell BR, Green DR, Krautwald S (2014) Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci USA 111(47):16836–16841. doi:10.1073/pnas.1415518111
Wu GY, Wu ZL, Dai ZL, Yang Y, Wang WW, Liu C, Wang B, Wang JJ, Yin YL (2013) Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids 44(4):1107–1113. doi:10.1007/s00726-012-1444-2
Wang W, Wu Z, Dai Z, Yang Y, Wang J, Wu G (2013) Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids 45(3):463–477. doi:10.1007/s00726-013-1493-1
Weinberg JM, Buchanan DN, Davis JA, Abarzua M (1991) Metabolic aspects of protection by glycine against hypoxic injury to isolated proximal tubules. J Am Soc Nephrol 1(7):949–958
Weinberg JM, Nissim I, Roeser NF, Davis JA, Schultz S, Nissim I (1991) Relationships between intracellular amino-acid levels and protection against injury to isolated proximal tubules. Am J Physiol 260(3):F410–F419
Locasale JW (2013) Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer 13(8):572–583. doi:10.1038/nrc3557
Saunder A, Ametani MS, Belzer FO, Southard JH (1993) Cytoprotective effect of glycine in cold stored canine renal tubules. Cryobiology 30(3):243–249. doi:10.1006/cryo.1993.1022
Garza-Quintero R, Ortega-Lopez J, Stein JH, Venkatachalam MA (1990) Alanine protects rabbit proximal tubules against anoxic injury in vitro. Am J Physiol 258(4 Pt 2):F1075–F1083
Aleo MD, Schnellmann RG (1992) The neurotoxicants strychnine and bicuculline protect renal proximal tubules from mitochondrial inhibitor-induced cell death. Life Sci 51(23):1783–1787
Wetzels JFM, Wang X, Gengaro PE, Nemenoff RA, Burke TJ, Schrier RW (1993) Glycine protection against hypoxic but not phospholipase A2-induced injury in rat proximal tubules. Am J Physiol Renal Fluid Electrolyte Physiol 264:F94–F99
Almeida AR, Wetzels JF, Bunnachak D, Burke TJ, Chaimovitz C, Hammond WS, Schrier RW (1992) Acute phosphate depletion and in vitro rat proximal tubule injury: protection by glycine and acidosis. Kidney Int 41(6):1494–1500
Baines AD, Shaikh N, Ho P (1990) Mechanisms of perfused kidney cytoprotection by alanine and glycine. Am J Physiol 259(1 Pt 2):F80–F87
Heyman S, Spokes K, Rosen S, Epstein FH (1992) Mechanism of glycine protection in hypoxic injury: analogies with glycine receptor. Kidney Int 42(1):41–45
Silva P, Rosen S, Spokes K, Epstein FH (1991) Effect of glycine on medullary thick ascending limb injury in perfused kidneys. Kidney Int 39(4):653–658
Weinberg JM, Venkatachalam MA, Roeser NF, Davis JA, Varani J, Johnson KJ (1991) Amino acid protection of cultured kidney tubule cells against calcium ionophore-induced lethal cell injury. Lab Invest 65(6):671–678
Weinberg JM, Varani J, Johnson KJ, Roeser NF, Dame MK, Davis JA, Venkatachalam MA (1992) Protection of human umbilical vein endothelial cells by glycine and structurally similar amino acids against calcium and hydrogen peroxide-induced lethal cell injury. Am J Pathol 140(2):457–471
Currin RT, Caldwell-Kenkel JC, Lichtman SN, Bachmann S, Takei Y, Kawano S, Thurman RG, Lemasters JJ (1996) Protection by Carolina rinse solution, acidotic pH, and glycine against lethal reperfusion injury to sinusoidal endothelial cells of rat livers stored for transplantation. Transplantation 62(11):1549–1558
Nishimura Y, Lemasters JJ (2001) Glycine blocks opening of a death channel in cultured hepatic sinusoidal endothelial cells during chemical hypoxia. Cell Death Differ 8(8):850–858. doi:10.1038/sj.cdd.4400877
Estacion M, Weinberg JS, Sinkins WG, Schilling WP (2003) Blockade of maitotoxin-induced endothelial cell lysis by glycine and l-alanine. Am J Physiol Cell Physiol 284(4):C1006–C1020. doi:10.1152/ajpcell.00258.2002
Dickson RC, Bronk SF, Gores GJ (1992) Glycine cytoprotection during lethal hepatocellular injury from adenosine triphosphate depletion. Gastroenterology 102(6):2098–2107
Frank A, Rauen U, de Groot H (2000) Protection by glycine against hypoxic injury of rat hepatocytes: inhibition of ion fluxes through nonspecific leaks. J Hepatol 32(1):58–66. doi:10.1016/S0168-8278(00)80190-7
Marsh DC, Vreugdenhil PK, Mack VE, Belzer FO, Southard JH (1993) Glycine protects hepatocytes from injury caused by anoxia, cold ischemia and mitochondrial inhibitors, but not injury caused by calcium ionophores or oxidative stress. Hepatology 17(1):91–98. doi:10.1016/0270-9139(93)90197-U
Nyberg SL, Hardin JA, Matos LE, Rivera DJ, Misra SP, Gores GJ (2000) Cytoprotective influence of ZVAD-fmk and glycine on gel-entrapped rat hepatocytes in a bioartificial liver. Surgery 127(4):447–455
Qian T, Nieminen AL, Herman B, Lemasters JJ (1997) Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am J Physiol 273(6 Pt 1):C1783–C1792
Brennan MA, Cookson BT (2000) Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol 38(1):31–40
Stockbauer KE, Foreman-Wykert AK, Miller JF (2003) Bordetella type III secretion induces caspase 1-independent necrosis. Cell Microbiol 5(2):123–132
Fink SL, Cookson BT (2006) Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol 8(11):1812–1825. doi:10.1111/j.1462-5822.2006.00751.x
Verhoef PA, Kertesy SB, Lundberg K, Kahlenberg JM, Dubyak GR (2005) Inhibitory effects of chloride on the activation of caspase-1, IL-1 beta secretion, and cytolysis by the P2X7 receptor. J Immunol 175(11):7623–7634
Goldmann O, Sastalla I, Wos-Oxley M, Rohde M, Medina E (2009) Streptococcus pyogenes induces oncosis in macrophages through the activation of an inflammatory programmed cell death pathway. Cell Microbiol 11(1):138–155. doi:10.1111/j.1462-5822.2008.01245.x
Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A (2010) Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11(12):U1136–U1194. doi:10.1038/ni.1960
Wellington M, Koselny K, Sutterwala FS, Krysan DJ (2014) Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot Cell 13(2):329–340. doi:10.1128/EC.00336-13
Ikejima K, Qu W, Stachlewitz RF, Thurman RG (1997) Kupffer cells contain a glycine-gated chloride channel. Am J Physiol 272(6 Pt 1):G1581–G1586
Schilling T, Eder C (2004) A novel physiological mechanism of glycine-induced immunomodulation: Na+-coupled amino acid transporter currents in cultured brain macrophages. J Physiol 559(Pt 1):35–40. doi:10.1113/jphysiol.2004.070763
Carmans S, Hendriks JJ, Thewissen K, Van den Eynden J, Stinissen P, Rigo JM, Hellings N (2010) The inhibitory neurotransmitter glycine modulates macrophage activity by activation of neutral amino acid transporters. J Neurosci Res 88(11):2420–2430. doi:10.1002/jnr.22395
Van den Eynden J, Notelaers K, Brone B, Janssen D, Nelissen K, Sahebali S, Smolders I, Hellings N, Steels P, Rigo JM (2011) Glycine enhances microglial intracellular calcium signaling. A role for sodium-coupled neutral amino acid transporters. Pflugers Arch 461(4):481–491. doi:10.1007/s00424-011-0939-0
Komm B, Beyreis M, Kittl M, Jakab M, Ritter M, Kerschbaum HH (2014) Glycine modulates membrane potential, cell volume, and phagocytosis in murine microglia. Amino Acids 46(8):1907–1917. doi:10.1007/s00726-014-1745-8
Mangino JE, Kotadia B, Mangino MJ (1996) Characterization of hypothermic intestinal ischemia-reperfusion injury in dogs. Effects of glycine. Transplantation 62(2):173–178
Iijima S, Shou J, Naama H, Calvano SE, Daly JM (1997) Beneficial effect of enteral glycine in intestinal ischemia/reperfusion injury. J Gastrointest Surg 1(1):61–67 (discussion 67–68)
Kallakuri S, Ascher E, Pagala M, Gade P, Hingorani A, Scheinman M, Mehraein K, Jacob T (2003) Protective effect of glycine in mesenteric ischemia and reperfusion injury in a rat model. J Vasc Surg 38(5):1113–1120. doi:10.1016/S0741
Lee MA, McCauley RD, Kong SE, Hall JC (2002) Influence of glycine on intestinal ischemia-reperfusion injury. JPEN J Parenter Enteral Nutr 26(2):130–135
Diestel CF, Marques RG, Lopes-Paulo F, Paiva D, Horst NL, Caetano CE, Portela MC (2007) Role of l-glutamine and glycine supplementation on irradiated colonic wall. Int J Colorectal Dis 22(12):1523–1529. doi:10.1007/s00384-007-0341-8
Tsune I, Ikejima K, Hirose M, Yoshikawa M, Enomoto N, Takei Y, Sato N (2003) Dietary glycine prevents chemical-induced experimental colitis in the rat. Gastroenterology 125(3):775–785
Stoffels B, Turler A, Schmidt J, Nazir A, Tsukamoto T, Moore BA, Schnurr C, Kalff JC, Bauer AJ (2011) Anti-inflammatory role of glycine in reducing rodent postoperative inflammatory ileus. Neurogastroenterol Motil 23(1):76–87. doi:10.1111/j.1365-2982.2010.01603.x (e78)
Petrat F, Drowatzky J, Boengler K, Finckh B, Schmitz KJ, Schulz R, de Groot H (2011) Protection from glycine at low doses in ischemia-reperfusion injury of the rat small intestine. Eur Surg Res 46(4):180–187. doi:10.1159/000324393
Howard A, Tahir I, Javed S, Waring SM, Ford D, Hirst BH (2010) Glycine transporter GLYT1 is essential for glycine-mediated protection of human intestinal epithelial cells against oxidative damage. J Physiol Lond 588(6):995–1009. doi:10.1113/jphysiol.2009.186262
Ruiz-Meana M, Pina P, Garcia-Dorado D, Rodriguez-Sinovas A, Barba I, Miro-Casas E, Mirabet M, Soler-Soler J (2004) Glycine protects cardiomyocytes against lethal reoxygenation injury by inhibiting mitochondrial permeability transition. J Physiol 558(Pt 3):873–882. doi:10.1113/jphysiol.2004.068320
Rodriguez-Sinovas A, Garcia-Dorado D, Pina P, Ruiz-Meana M, Soler-Soler J (2005) Effect of sarcolemmal rupture on myocardial electrical impedance during oxygen deprivation. Am J Physiol Heart Circ Physiol 288(3):H1396–H1403. doi:10.1152/ajpheart.00768.2004
Zhang K, Weinberg JM, Venkatachalam MA, Dong Z (2003) Glycine protection of PC-12 cells against injury by ATP-depletion. Neurochem Res 28(6):893–901
Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP, Oliet SH (2012) Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 150(3):633–646. doi:10.1016/j.cell.2012.06.029
Newell DW, Barth A, Ricciardi TN, Malouf AT (1997) Glycine causes increased excitability and neurotoxicity by activation of NMDA receptors in the hippocampus. Exp Neurol 145(1):235–244. doi:10.1006/exnr.1997.6463
Patel J, Zinkand WC, Thompson C, Keith R, Salama A (1990) Role of glycine in the N-methyl-d-aspartate-mediated neuronal cytotoxicity. J Neurochem 54(3):849–854
Yao W, Ji F, Chen Z, Zhang N, Ren SQ, Zhang XY, Liu SY, Lu W (2012) Glycine exerts dual roles in ischemic injury through distinct mechanisms. Stroke 43(8):2212–2220. doi:10.1161/STROKEAHA.111.645994
Tonshin AA, Lobysheva NV, Yaguzhinsky LS, Bezgina EN, Moshkov DA, Nartsissov YR (2007) Effect of the inhibitory neurotransmitter glycine on slow destructive processes in brain cortex slices under anoxic conditions. Biochemistry (Mosc) 72(5):509–517
Zhao P, Qian H, Xia Y (2005) GABA and glycine are protective to mature but toxic to immature rat cortical neurons under hypoxia. Eur J Neurosci 22(2):289–300. doi:10.1111/j.1460-9568.2005.04222.x
Aprison MH, Werman R (1965) The distribution of glycine in cat spinal cord and roots. Life Sci 4(21):2075–2083
Eulenburg V, Armsen W, Betz H, Gomeza J (2005) Glycine transporters: essential regulators of neurotransmission. Trends Biochem Sci 30(6):325–333. doi:10.1016/j.tibs.2005.04.004
Weinberg JM, Davis JA, Abarzua M, Kiani T (1990) Glycine-dependent protection of proximal tubules against lethal cell injury due to inhibitors of mitochondrial ATP production. Am J Physiol 258:C1127–C1140
Garza-Quintero R, Weinberg JM, Ortega-Lopez J, Davis JA, Venkatachalam MA (1993) Conservation of structure in ATP-depleted proximal tubules: role of calcium, polyphosphoinositides, and glycine. Am J Physiol 265(5 Pt 2):F605–F623
Venkatachalam MA, Weinberg JM, Patel Y, Hussong U, Davis JA (1995) Effects of Ca++ and glycine on lipid breakdown and death of ATP-depleted MDCK cells. Kidney Int 48(1):118–128
Meng X, Reeves WB (2000) Effects of chloride channel inhibitors on H(2)O(2)-induced renal epithelial cell injury. Am J Physiol Renal Physiol 278(1):F83–F90
Sogabe K, Roeser NF, Venkatachalam MA, Weinberg JM (1996) Differential cytoprotection by glycine against oxidant damage to proximal tubule cells. Kidney Int 50(3):845–854
Miller GW, Lock EA, Schnellmann RG (1994) Strychnine and glycine protect renal proximal tubules from various nephrotoxicants and act in the late-phase of necrotic cell injury. Toxicol Appl Pharmacol 125(2):192–197. doi:10.1006/taap.1994.1064
Zager RA, Johnson AC, Hanson SY, Wasse H (2002) Parenteral iron formulations: a comparative toxicologic analysis and mechanisms of cell injury. Am J Kidney Dis 40(1):90–103. doi:10.1053/ajkd.2002.33917
Bhattacharyya S, Ghosh J, Sil PC (2012) Iron induces hepatocytes death via MAPK activation and mitochondria-dependent apoptotic pathway: beneficial role of glycine. Free Radic Res 46(10):1296–1307. doi:10.3109/10715762.2012.712690
Zager RA, Burkhart KM, Conrad DS, Gmur DJ (1995) Iron, heme oxygenase, and glutathione: effects on myohemoglobinuric proximal tubular injury. Kidney Int 48(5):1624–1634
Fink SL, Bergsbaken T, Cookson BT (2008) Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci USA 105(11):4312–4317. doi:10.1073/pnas.0707370105
Verhoef PA, Kertesy SB, Estacion M, Schilling WP, Dubyak GR (2004) Maitotoxin induces biphasic interleukin-1 beta secretion and membrane blebbing in murine macrophages. Mol Pharmacol 66(4):909–920
Venkatachalam MA, Patel YJ, Kreisberg JI, Weinberg JM (1988) Energy thresholds that determine membrane integrity and injury in a renal epithelial-cell line (Llc-Pk1)—relationships to phospholipid degradation and unesterified fatty-acid accumulation. J Clin Investig 81(3):745–758. doi:10.1172/Jci113380
Anundi I, de Groot H (1989) Hypoxic liver cell death: critical Po2 and dependence of viability on glycolysis. Am J Physiol 257(1 Pt 1):G58–G64
Weinberg JM, Davis JA, Roeser NF, Venkatachalam MA (1991) Role of increased cytosolic free calcium in the pathogenesis of rabbit proximal tubule cell injury and protection by glycine or acidosis. J Clin Invest 87(2):581–590. doi:10.1172/JCI115033
Nurko S, Sogabe K, Davis JA, Roeser NF, Defrain M, Chien A, Hinshaw D, Athey B, Meixner W, Venkatachalam MA, Weinberg JM (1996) Contribution of actin cytoskeletal alterations to ATP depletion and calcium-induced proximal tubule cell injury. Am J Physiol 270(1 Pt 2):F39–F52
Sogabe K, Roeser NF, Davis JA, Nurko S, Venkatachalam MA, Weinberg JM (1996) Calcium dependence of integrity of the actin cytoskeleton of proximal tubule cell microvilli. Am J Physiol 271(2 Pt 2):F292–F303
Venkatachalam MA, Weinberg JM, Patel Y, Saikumar P, Dong Z (1996) Cytoprotection of kidney epithelial cells by compounds that target amino acid gated chloride channels. Kidney Int 49(2):449–460. doi:10.1038/ki.1996.64
Dong Z, Patel Y, Saikumar P, Weinberg JM, Venkatachalam MA (1998) Development of porous defects in plasma membranes of ATP-depleted Madin-Darby canine kidney cells and its inhibition by glycine. Lab Invest 78:657–668
Weinberg JM, Davis JA, Venkatachalam MA (1997) Cytosolic-free calcium increases to greater than 100 micromolar in ATP-depleted proximal tubules. J Clin Invest 100(3):713–722. doi:10.1172/JCI119584
Chen J, Dai JW, Grant RL, Doctor RB, Sheetz MP, Mandel LJ (1997) Loss of cytoskeletal support is not sufficient for anoxic plasma membrane disruption in renal cells. Am J Physiol Cell Physiol 272:C1319–C1328
Soltoff SP, Mandel LJ (1984) Active ion transport in the renal proximal tubule. III. The ATP dependence of the Na pump. J Gen Physiol 84(4):643–662
Gao GF, Wang WW, Tadagavadi RK, Briley NE, Love MI, Miller BA, Reeves WB (2014) TRPM2 mediates ischemic kidney injury and oxidant stress through RAC1. J Clin Investig 124(11):4989–5001. doi:10.1172/Jci76042
Carini R, Bellomo G, Grazia De Cesaris M, Albano E (1997) Glycine protects against hepatocyte killing by KCN or hypoxia by preventing intracellular Na+ overload in the rat. Hepatology 26(1):107–112. doi:10.1002/hep.510260114
Chen J, Mandel LJ (1997) Role of water and electrolyte influxes in anoxic plasma membrane disruption. Am J Physiol 273(4 Pt 1):C1341–C1348
Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA (1997) Internucleosomal DNA cleavage triggered by plasma membrane damage during necrotic cell death—involvement of serine but not cysteine proteases. Am J Pathol 151(5):1205–1213
Carini R, Alchera E, Baldanzi G, Piranda D, Splendore R, Grazia De Cesaris M, Caraceni P, Graziani A, Albano E (2007) Role of p38 map kinase in glycine-induced hepatocyte resistance to hypoxic injury. J Hepatol 46(4):692–699. doi:10.1016/j.jhep.2006.10.014
Stefureac R, Long YT, Kraatz HB, Howard P, Lee JS (2006) Transport of alpha-helical peptides through alpha-hemolysin and aerolysin pores. Biochemistry 45(30):9172–9179. doi:10.1021/bi0604835
Cascio M (2004) Structure and function of the glycine receptor and related nicotinicoid receptors. J Biol Chem 279(19):19383–19386. doi:10.1074/jbc.R300035200
Miller GW, Schnellmann RG (1993) Cytoprotection by inhibition of chloride channels: the mechanism of action of glycine and strychnine. Life Sci 53(15):1211–1215
Waters SL, Miller GW, Aleo MD, Schnellmann RG (1997) Neurosteroid inhibition of cell death. Am J Physiol 273(6 Pt 2):F869–F876
Miller GW, Schnellmann RG (1995) Inhibitors of renal chloride transport do not block toxicant- induced chloride influx in the proximal tubule. Toxicol Lett 76:179–184
Peters SM, de Jong MD, Bindels RJ, van Os CH, Wetzels JF (1998) Effects of renal cytoprotective agents on erythrocyte membrane stability. Life Sci 63(11):975–983
Kribben A, Wieder ED, Wetzels JF, Yu L, Gengaro PE, Burke TJ, Schrier RW (1994) Evidence for role of cytosolic free calcium in hypoxia-induced proximal tubule injury. J Clin Invest 93(5):1922–1929. doi:10.1172/JCI117183
Gronow G, Klause N, Malyusz M (1994) Restriction of hypoxic membrane defect by glycine improves mitochondrial and cellular function in reoxygenation renal tubules. In: Hogan MC, Mathieu-Costello O, Poole DC, Wagner PD (eds) Oxygen transport to tissues. Advances in experimental medicine and biology, vol XVI. Plenum Press Div Plenum Publishing Corp, New York, pp 585–589
Moran JH, Schnellmann RG (1997) Diverse cytoprotectants prevent cell lysis and promote recovery of respiration and ion transport. Biochem Biophys Res Commun 234(1):275–277. doi:10.1006/bbrc.1997.6625
Dong Z, Saikumar P, Griess GA, Weinberg JM, Venkatachalam MA (1998) Intracellular Ca2+ thresholds that determine survival or death of energy-deprived cells. Am J Pathol 152(1):231–240
Feldkamp T, Kribben A, Roeser NF, Senter RA, Weinberg JM (2006) Accumulation of nonesterified fatty acids causes the sustained energetic deficit in kidney proximal tubules after hypoxia–reoxygenation. Am J Physiol Renal Physiol 290(2):F465–F477. doi:10.1152/ajprenal.00305.2005
Weinberg JM, Venkatachalam MA, Goldberg H, Roeser NF, Davis JA (1995) Modulation by Gly, Ca, and acidosis of injury-associated unesterified fatty acid accumulation in proximal tubule cells. Am J Physiol 268(1 Pt 2):F110–F121
Weinberg JM (1985) Oxygen deprivation-induced injury to isolated rabbit kidney tubules. J Clin Invest 76:1193–1208
Zager RA, Schimpf BA, Gmur DJ (1993) Physiological pH: effects on posthypoxic proximal tubular injury. Circ Res 72:837–846
Weinberg JM, Davis JA, Roeser NF, Venkatachalam MA (1994) Role of intracellular pH during cytoprotection of proximal tubule cells by glycine or acidosis. J Am Soc Nephrol 5(6):1314–1323
Jansova H, Machacek M, Wang Q, Haskova P, Jirkovska A, Potuckova E, Kielar F, Franz KJ, Simunek T (2014) Comparison of various iron chelators and prochelators as protective agents against cardiomyocyte oxidative injury. Free Radic Biol Med 74:210–221. doi:10.1016/j.freeradbiomed.2014.06.019
Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7(2):99–109. doi:10.1038/nrmicro2070
Lamkanfi M, Dixit VM (2014) Mechanisms and functions of inflammasomes. Cell 157(5):1013–1022. doi:10.1016/j.cell.2014.04.007
Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM (2012) Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490(7419):288–291. doi:10.1038/nature11419
Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440(7081):228–232. doi:10.1038/nature04515
Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526(7575):666–671. doi:10.1038/nature15541
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526(7575):660–665. doi:10.1038/nature15514
Man SM, Kanneganti TD (2015) Gasdermin D: the long-awaited executioner of pyroptosis. Cell Res 25(11):1183–1184. doi:10.1038/cr.2015.124
Saikumar P, Dong Z, Patel Y, Hall K, Hopfer U, Weinberg JM, Venkatachalam MA (1998) Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene 17(26):3401–3415. doi:10.1038/sj.onc.1202590
Saikumar P, Dong Z, Weinberg JM, Venkatachalam MA (1998) Mechanisms of cell death in hypoxia/reoxygenation injury. Oncogene 17(25):3341–3349. doi:10.1038/sj.onc.1202579
Fink SL, Cookson BT (2005) Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73(4):1907–1916. doi:10.1128/IAI.73.4.1907-1916.2005
Yaqoob M, Edelstein CL, Wieder ED, Alkhunaizi AM, Gengaro PE, Nemenoff RA, Schrier RW (1996) Nitric oxide kinetics during hypoxia in proximal tubules: effects of acidosis and glycine. Kidney Int 49(5):1314–1319
Edelstein CL, Ling H, Gengaro PE, Nemenoff RA, Bahr BA, Schrier RW (1997) Effect of glycine on prelethal and postlethal increases in calpain activity in rat renal proximal tubules. Kidney Int 52(5):1271–1278
Tijsen MJ, Peters SM, Bindels RJ, van Os CH, Wetzels JF (1997) Glycine protection against hypoxic injury in isolated rat proximal tubules: the role of proteases. Nephrol Dial Transplant 12(12):2549–2556
Nissim I, Hardy M, Pleasure J, Nissim I, States B (1992) A mechanism of glycine and alanine cytoprotective action: stimulation of stress-induced HSP70 mRNA. Kidney Int 42(3):775–782
Grosser N, Oberle S, Berndt G, Erdmann K, Hemmerle A, Schroder H (2004) Antioxidant action of l-alanine: heme oxygenase-1 and ferritin as possible mediators. Biochem Biophys Res Commun 314(2):351–355. doi:10.1016/j.bbrc.2003.12.089
Jiang LL, Qin X, Zhong XZ, Liu L, Jiang L, Lu Y, Fan LM, He ZG, Chen Q (2011) Glycine-induced cytoprotection is mediated by ERK1/2 and AKT in renal cells with ATP depletion. Eur J Cell Biol 90(4):333–341. doi:10.1016/j.ejcb.2010.10.003
Weinberg JM, Venkatachalam MA, Roeser NF, Saikumar P, Dong Z, Senter RA, Nissim I (2000) Anaerobic and aerobic pathways for salvage of proximal tubules from hypoxia-induced mitochondrial injury. Am J Physiol Ren Physiol 279(5):F927–F943
Webb TI, Lynch JW (2007) Molecular pharmacology of the glycine receptor chloride channel. Curr Pharm Des 13(23):2350–2367
Vandenberg RJ, French CR, Barry PH, Shine J, Schofield PR (1992) Antagonism of ligand-gated ion channel receptors: two domains of the glycine receptor alpha subunit form the strychnine-binding site. Proc Natl Acad Sci USA 89(5):1765–1769
Lewis TM, Schofield PR, McClellan AML (2003) Kinetic determinants of agonist action at the recombinant human glycine receptor. J Physiol Lond 549(2):361–374. doi:10.1113/jphysiol.2002.037796
Tokutomi N, Kaneda M, Akaike N (1989) What confers specificity on glycine for its receptor-site. Br J Pharmacol 97(2):353–360
Dong Z, Venkatachalam MA, Weinberg JM, Saikumar P, Patel Y (2001) Protection of ATP-depleted cells by impermeant strychnine derivatives: implications for glycine cytoprotection. Am J Pathol 158(3):1021–1028. doi:10.1016/S0002-9440(10)64049-7
Kessler M, Terramani T, Lynch G, Baudry M (1989) A glycine site associated with N-methyl-d-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem 52(4):1319–1328
Snell LD, Johnson KM (1988) Cycloleucine competitively antagonizes the strychnine-insensitive glycine receptor. Eur J Pharmacol 151(1):165–166
Wang X-N, Wetzels JFM, Arnold PE, Burke TJ, Schrier RW (1991) Strychnine protects against hypoxic injury in freshly isolated rat renal proximal tubules. J Am Soc Nephrol 2:656
Miller GW, Schnellmann RG (1993) A novel low-affinity strychnine binding site on renal proximal tubules: role in toxic cell death. Life Sci 53(15):1203–1209
Young AB, Snyder SH (1973) Strychnine binding associated with glycine receptors of the central nervous system. Proc Natl Acad Sci USA 70(10):2832–2836
Froh M, Thurman RG, Wheeler MD (2002) Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am J Physiol Gastrointest Liver Physiol 283(4):G856–G863. doi:10.1152/ajpgi.00503.2001
Yamashina S, Konno A, Wheeler MD, Rusyn I, Rusyn EV, Cox AD, Thurman RG (2001) Endothelial cells contain a glycine-gated chloride channel. Nutr Cancer 40(2):197–204. doi:10.1207/S15327914NC402_17
Miller GW, Schnellmann RG (1994) A putative cytoprotective receptor in the kidney: relation to the neuronal strychnine-sensitive glycine receptor. Life Sci 55(1):27–34
Sarang SS, Miller GW, Grant DF, Schnellmann RG (1999) Expression and localization of the neuronal glycine receptor beta-subunit in human, rabbit and rat kidneys. Nephron 82(3):254–260 (45410)
Lee JW, Chou CL, Knepper MA (2015) Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26(11):2669–2677. doi:10.1681/ASN.2014111067
Pan C, Bai XM, Fan LM, Ji Y, Li XY, Chen Q (2005) Cytoprotection by glycine against ATP-depletion-induced injury is mediated by glycine receptor in renal cells. Biochem J 390:447–453. doi:10.1042/Bj20050141
Lu Y, Zhang J, Ma B, Li K, Li X, Bai H, Yang Q, Zhu X, Ben J, Chen Q (2012) Glycine attenuates cerebral ischemia/reperfusion injury by inhibiting neuronal apoptosis in mice. Neurochem Int 61(5):649–658. doi:10.1016/j.neuint.2012.07.005
Thurman RG, Schemmer P, Zhong Z, Bunzendahl H, Von Frankenberg M, Lemasters JJ (1998) Kupffer cell-dependent reperfusion injury in liver transplantation: new clinically relevant use of glycine. Langenbecks Arch Chir Suppl Kongressbd 115(185–90):185–190
Schemmer P, Bradford BU, Rose ML, Bunzendahl H, Raleigh JA, Lemasters JJ, Thurman RG (1999) Intravenous glycine improves survival in rat liver transplantation. Am J Physiol 276(4 Pt 1):G924–G932
Wheeler MD, Thurman RG (1999) Production of superoxide and TNF-alpha from alveolar macrophages is blunted by glycine. Am J Physiol Lung Cell Mol Physiol 277(5):L952–L959
Spittler A, Reissner CM, Oehler R, Gornikiewicz A, Gruenberger T, Manhart N, Brodowicz T, Mittlboeck M, Boltz-Nitulescu G, Roth E (1999) Immunomodulatory effects of glycine on LPS-treated monocytes: reduced TNF-alpha production and accelerated IL-10 expression. FASEB J 13(3):563–571
Stachlewitz RF, Li X, Smith S, Bunzendahl H, Graves LM, Thurman RG (2000) Glycine inhibits growth of T lymphocytes by an IL-2-independent mechanism. J Immunol 164(1):176–182
Wheeler M, Stachlewitz RF, Yamashina S, Ikejima K, Morrow AL, Thurman RG (2000) Glycine-gated chloride channels in neutrophils attenuate calcium influx and superoxide production. FASEB J 14(3):476–484
Wang HD, Lu XX, Lu DX, Qi RB, Wang YP, Fu YM, Wang LW (2009) Glycine inhibits the LPS-induced increase in cytosolic Ca2+ concentration and TNF alpha production in cardiomyocytes by activating a glycine receptor. Acta Pharmacol Sin 30(8):1107–1114. doi:10.1038/aps.2009.106
Giambelluca MS, Gende OA (2009) Effect of glycine on the release of reactive oxygen species in human neutrophils. Int Immunopharmacol 9(1):32–37. doi:10.1016/j.intimp.2008.09.006
Choi JJ, Wang SG, Tung YS, Morrison B, Konofagou EE (2010) Molecules of various pharmacologically-relevant sizes can cross the ultrasound-induced blood-brain barrier opening in vivo. Ultrasound Med Biol 36(1):58–67. doi:10.1016/j.ultrasmedbio.2009.08.006
Chen J, Liu X, Mandel LJ, Schnellmann RG (2001) Progressive disruption of the plasma membrane during renal proximal tubule cellular injury. Toxicol Appl Pharmacol 171(1):1–11. doi:10.1006/taap.2000.9105
Schilling WP, Snyder D, Sinkins WG, Estacion M (2006) Palytoxin-induced cell death cascade in bovine aortic endothelial cells. Am J Physiol Cell Physiol 291(4):C657–C667. doi:10.1152/ajpcell.00063.2006
Wisnoskey BJ, Estacion M, Schilling WP (2004) Maitotoxin-induced cell death cascade in bovine aortic endothelial cells: divalent cation specificity and selectivity. Am J Physiol Cell Physiol 287(2):C345–C356. doi:10.1152/ajpcell.00473.2003
Bergstrom J, Alvestrand A, Furst P (1990) Plasma and muscle free amino-acids in maintenance hemodialysis-patients without protein-malnutrition. Kidney Int 38(1):108–114. doi:10.1038/ki.1990.174
Gannon MC, Nuttall JA, Nuttall FQ (2002) The metabolic response to ingested glycine. Am J Clin Nutr 76(6):1302–1307
Duran MA, Spencer D, Weise M, Kronfol NO, Spencer RF, Oken DE (1990) Renal epithelial amino acid concentrations in mercury-induced and postischemic acute renal failure. Toxicol Appl Pharmacol 105(2):183–194
Adibi SA, Morse EL (1982) Enrichment of glycine pool in plasma and tissues by glycine, di-, tri-, and tetraglycine. Am J Physiol 243(5):E413–E417
Quan H, Athirakul K, Wetsel WC, Torres GE, Stevens R, Chen YT, Coffman TM, Caron MG (2004) Hypertension and impaired glycine handling in mice lacking the orphan transporter XT2. Mol Cell Biol 24(10):4166–4173
Shalhoub R, Pitts RF, Glabman S, Dehaas J, Klein J, Webber W, Canessaf M (1963) Extraction of amino acids from and their addition to renal blood plasma. Am J Physiol 204(2):181–186
Block WD, Hubbard RW (1962) Amino acid content of rabbit urine and plasma. Arch Biochem Biophys 96:557–561
Dennis VW, Brazy PC (1978) Sodium, phosphate, glucose, bicarbonate, and alanine interactions in the isolated proximal convoluted tubule of the rabbit kidney. J Clin Invest 62(2):387–397. doi:10.1172/JCI109140
Barfuss DW, Schafer JA (1979) Active amino acid absorption by proximal convoluted and proximal straight tubules. Am J Physiol 236(2):F149–F162
Parks LD, Barfuss DW (2002) Transepithelial transport and metabolism of glycine in S1, S2, and S3 cell types of the rabbit proximal tubule. Am J Physiol Renal Physiol 283(6):F1208–F1215. doi:10.1152/ajprenal.00021.2002
Beck FX, Ohno A, Dorge A, Thurau K (1995) Ischemia-induced changes in cell element composition and osmolyte contents of outer medulla. Kidney Int 48(2):449–457
Roux MJ, Supplisson S (2000) Neuronal and glial glycine transporters have different stoichiometries. Neuron 25(2):373–383
Richards DA, Silva MA, Murphy N, Wigmore SJ, Mirza DF (2007) Extracellular amino acid levels in the human liver during transplantation: a microdialysis study from donor to recipient. Amino Acids 33(3):429–437. doi:10.1007/s00726-006-0480-1
Yin M, Rusyn I, Schoonhoven R, Graves LM, Rusyn EV, Li X, Li F, Cox AD, Harding TW, Bunzendahl H, Swenberg JA, Thurman RG (2000) Inhibition of chronic rejection of aortic allografts by dietary glycine. Transplantation 69(5):773–780
Vieira CP, De Oliveira LP, Da Re Guerra F, Dos Santos De Almeida M, Marcondes MC, Pimentel ER (2015) Glycine improves biochemical and biomechanical properties following inflammation of the achilles tendon. Anat Rec (Hoboken) 298(3):538–545. doi:10.1002/ar.23041
Li X, Bradford BU, Wheeler MD, Stimpson SA, Pink HM, Brodie TA, Schwab JH, Thurman RG (2001) Dietary glycine prevents peptidoglycan polysaccharide-induced reactive arthritis in the rat: role for glycine-gated chloride channel. Infect Immun 69(9):5883–5891
Alvarado-Vasquez N, Lascurain R, Ceron E, Vanda B, Carvajal-Sandoval G, Tapia A, Guevara J, Montano LF, Zenteno E (2006) Oral glycine administration attenuates diabetic complications in streptozotocin-induced diabetic rats. Life Sci 79(3):225–232. doi:10.1016/j.lfs.2005.12.055
Alarcon-Aguilar FJ, Almanza-Perez J, Blancas G, Angeles S, Garcia-Macedo R, Roman R, Cruz M (2008) Glycine regulates the production of pro-inflammatory cytokines in lean and monosodium glutamate-obese mice. Eur J Pharmacol 599(1–3):152–158. doi:10.1016/j.ejphar.2008.09.047
Blancas-Flores G, Alarcon-Aguilar FJ, Garcia-Macedo R, Almanza-Perez JC, Flores-Saenz JL, Roman-Ramos R, Ventura-Gallegos JL, Kumate J, Zentella-Dehesa A, Cruz M (2012) Glycine suppresses TNF-alpha-induced activation of NF-kappa B in differentiated 3T3-L1 adipocytes. Eur J Pharmacol 689(1–3):270–277. doi:10.1016/j.ejphar.2012.06.025
Garcia-Macedo R, Sanchez-Munoz F, Almanza-Perez JC, Duran-Reyes G, Alarcon-Aguilar F, Cruz M (2008) Glycine increases mRNA adiponectin and diminishes pro-inflammatory adipokines expression in 3T3-L1 cells. Eur J Pharmacol 587(1–3):317–321. doi:10.1016/j.ejphar.2008.03.051
Tastesen HS, Keenan AH, Madsen L, Kristiansen K, Liaset B (2014) Scallop protein with endogenous high taurine and glycine content prevents high-fat, high-sucrose-induced obesity and improves plasma lipid profile in male C57BL/6J mice. Amino Acids 46(7):1659–1671. doi:10.1007/s00726-014-1715-1
Ruiz-Ramirez A, Ortiz-Balderas E, Cardozo-Saldana G, Diaz-Diaz E, El-Hafidi M (2014) Glycine restores glutathione and protects against oxidative stress in vascular tissue from sucrose-fed rats. Clin Sci (Lond) 126(1):19–29. doi:10.1042/CS20130164
Sekhar RV, McKay SV, Patel SG, Guthikonda AP, Reddy VT, Balasubramanyam A, Jahoor F (2011) Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 34(1):162–167. doi:10.2337/dc10-1006
Nguyen D, Hsu JW, Jahoor F, Sekhar RV (2014) Effect of increasing glutathione with cysteine and glycine supplementation on mitochondrial fuel oxidation, insulin sensitivity, and body composition in older HIV-infected patients. J Clin Endocrinol Metab 99(1):169–177. doi:10.1210/jc.2013-2376
El Hafidi M, Perez I, Zamora J, Soto V, Carvajal-Sandoval G, Banos G (2004) Glycine intake decreases plasma free fatty acids, adipose cell size, and blood pressure in sucrose-fed rats. Am J Physiol Regul Integr Comp Physiol 287(6):R1387–R1393. doi:10.1152/ajpregu.00159.2004
El Hafidi M, Perez I, Banos G (2006) Is glycine effective against elevated blood pressure? Curr Opin Clin Nutr Metab Care 9(1):26–31
Cruz M, Maldonado-Bernal C, Mondragon-Gonzalez R, Sanchez-Barrera R, Wacher NH, Carvajal-Sandoval G, Kumate J (2008) Glycine treatment decreases proinflammatory cytokines and increases interferon-gamma in patients with type 2 diabetes. J Endocrinol Invest 31(8):694–699
Lustgarten MS, Price LL, Phillips EM, Fielding RA (2013) Serum glycine is associated with regional body fat and insulin resistance in functionally-limited older adults. PLoS ONE. 8(12):e84034. doi:10.1371/journal.pone.0084034
Rose ML, Madren J, Bunzendahl H, Thurman RG (1999) Dietary glycine inhibits the growth of B16 melanoma tumors in mice. Carcinogenesis 20(5):793–798
Amin K, Li J, Chao WR, Dewhirst MW, Haroon ZA (2003) Dietary glycine inhibits angiogenesis during wound healing and tumor growth. Cancer Biol Ther 2(2):173–178
Bruns H, Petrulionis M, Schultze D, Al Saeedi M, Lin S, Yamanaka K, Ambrazevicius M, Strupas K, Schemmer P (2014) Glycine inhibits angiogenic signaling in human hepatocellular carcinoma cells. Amino Acids 46(4):969–976. doi:10.1007/s00726-013-1662-2
Melendez-Hevia E, de Paz-Lugo P, Cornish-Bowden A, Cardenas ML (2009) A weak link in metabolism: the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. J Biosci 34(6):853–872
Nissim I, Weinberg JM (1996) Glycine attenuates Fanconi syndrome induced by maleate or ifosfamide in rats. Kidney Int 49(3):684–695
Bannai M, Kawai N (2012) New therapeutic strategy for amino acid medicine: glycine improves the quality of sleep. J Pharmacol Sci 118(2):145–148
Evins AE, Fitzgerald SM, Wine L, Rosselli R, Goff DC (2000) Placebo-controlled trial of glycine added to clozapine in schizophrenia. Am J Psychiatry 157(5):826–828. doi:10.1176/appi.ajp.157.5.826
Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M (1999) Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry 56(1):29–36
Yin M, Zhong Z, Connor HD, Bunzendahl H, Finn WF, Rusyn I, Li X, Raleigh JA, Mason RP, Thurman RG (2002) Protective effect of glycine on renal injury induced by ischemia-reperfusion in vivo. Am J Physiol Renal Physiol 282(3):F417–F423. doi:10.1152/ajprenal.00011.2001
Wetzels JFM, Yu L, Shanley PF, Burke TJ, Schrier RW (1993) Infusion of glycine does not attenuate invivo ischemic acute-renal-failure in the rat. J Lab Clin Med 121(2):263–267
Collentine GE Jr (1948) On the efficacy and safety of glycine administered by vein. J Lab Clin Med 33(12):1555–1562
Croner RS, Kulu Y, Hoerer E, Peters V, Schmidt-Mader B, Schemmer P, Herfarth C, Klar E (2005) Intravenous glycine after cecal ligation and puncture has no effect on impaired hepatic microperfusion, leukocyte adhesion, and mortality in septic rats. Microvasc Res 69(1–2):71–78. doi:10.1016/j.mvr.2005.01.001
Collins JW, Macdermott S, Bradbrook RA, Keeley FX Jr, Timoney AG (2005) A comparison of the effect of 1.5 % glycine and 5 % glucose irrigants on plasma serum physiology and the incidence of transurethral resection syndrome during prostate resection. BJU Int 96(3):368–372. doi:10.1111/j.1464-410X.2005.05633.x
Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM (2009) Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 457(7231):910–914. doi:10.1038/nature07762
Chaneton B, Hillmann P, Zheng L, Martin ACL, Maddocks ODK, Chokkathukalam A, Coyle JE, Jankevics A, Holding FP, Vousden KH, Frezza C, O’Reilly M, Gottlieb E (2012) Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature 491(7424):458–462. doi:10.1038/nature11540
Kim D, Fiske BP, Birsoy K, Freinkman E, Kami K, Possemato RL, Chudnovsky Y, Pacold ME, Chen WW, Cantor JR, Shelton LM, Gui DY, Kwon M, Ramkissoon SH, Ligon KL, Kang SW, Snuderl M, Vander Heiden MG, Sabatini DM (2015) SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature 520(7547):363–367. doi:10.1038/nature14363
Labuschagne CF, van den Broek NJ, Mackay GM, Vousden KH, Maddocks OD (2014) Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep 7(4):1248–1258
Johannesen J, Lie M, Kiil F (1977) Effects of glycine and glucagon on glomerular filtration and renal metabolic rates. Am J Physiol 233:F61–F66
Heyman SN, Brezis M, Epstein FH, Spokes K, Rosen S (1992) Effect of glycine and hypertrophy on renal outer medulla hypoxic injury in ischemia reflow and contrast nephropathy. Am J Kidney Dis 19:578–586
Deng A, Thomson SC (2009) Renal NMDA receptors independently stimulate proximal reabsorption and glomerular filtration. Am J Physiol Renal Physiol 296(5):F976–F982. doi:10.1152/ajprenal.90391.2008
Arora S, Kaur T, Kaur A, Singh AP (2014) Glycine aggravates ischemia reperfusion-induced acute kidney injury through N-Methyl-d-Aspartate receptor activation in rats. Mol Cell Biochem 393(1–2):123–131. doi:10.1007/s11010-014-2052-0
Zager RA, Venkatachalam MA (1983) Potentiation of ischemic renal injury by amino acid infusion. Kidney Int 24(5):620–625
Mishra RC, Tripathy S, Quest D, Desai KM, Akhtar J, Dattani ID, Gopalakrishnan V (2008) l-Serine lowers while glycine increases blood pressure in chronic l-NAME-treated and spontaneously hypertensive rats. J Hypertens 26(12):2339–2348. doi:10.1097/HJH.0b013e328312c8a3
Bienholz A, Petrat F, Wenzel P, Ickerott P, Weinberg JM, Witzke O, Kribben A, de Groot H, Feldkamp T (2012) Adverse effects of alpha-ketoglutarate/malate in a rat model of acute kidney injury. Am J Physiol Renal Physiol 303(1):F56–F63
Zager RA, Johnson AC, Naito M, Bomsztyk K (2008) Maleate nephrotoxicity: mechanisms of injury and correlates with ischemic/hypoxic tubular cell death. Am J Physiol Renal Physiol 294(1):F187–F197. doi:10.1152/ajprenal.00434.2007
Heyman SN, Rosen S, Silva P, Spokes K, Egorin MJ, Epstein FH (1991) Protective action of glycine in cisplatin nephropathy. Kidney Int 40:273–279
Thurman RG, Zhong Z, Mv Frankenberg, Stachlewitz RF, Bunzendahl H (1997) Prevention of cyclosporin-induced nephrotoxicity with dietary glycine. Transplantation 63:1661–1667
Zhong Z, Connor HD, Yin M, Moss N, Mason RP, Bunzendahl H, Forman DT, Thurman RG (1999) Dietary glycine and renal denervation prevents cyclosporin A-induced hydroxyl radical production in rat kidney. Mol Pharmacol 56(3):455–463
Zhong Z, Jones S, Thurman RG (1996) Glycine minimizes reperfusion injury in a low-flow, reflow liver perfusion model in the rat. Am J Physiol 270(2 Pt 1):G332–G338
Yin M, Ikejima K, Arteel GE, Seabra V, Bradford BU, Kono H, Rusyn I, Thurman RG (1998) Glycine accelerates recovery from alcohol-induced liver injury. J Pharmacol Exp Ther 286(2):1014–1019
Rivera CA, Bradford BU, Hunt KJ, Adachi Y, Schrum LW, Koop DR, Burchardt ER, Rippe RA, Thurman RG (2001) Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol 281(1):G200–G207
Froh M, Zhong Z, Walbrun P, Lehnert M, Netter S, Wiest R, Conzelmann L, Gabele E, Hellerbrand C, Scholmerich J, Thurman RG (2008) Dietary glycine blunts liver injury after bile duct ligation in rats. World J Gastroenterol 14(39):5996–6003
Yamanouchi K, Eguchi S, Kamohara Y, Yanaga K, Okudaira S, Tajima Y, Kanematsu T (2007) Glycine reduces hepatic warm ischaemia-reperfusion injury by suppressing inflammatory reactions in rats. Liver Int 27(9):1249–1254. doi:10.1111/j.1478-3231.2007.01564.x
Ito K, Ozasa H, Noda Y, Koike Y, Arii S, Horikawa S (2008) Effect of non-essential amino acid glycine administration on the liver regeneration of partially hepatectomized rats with hepatic ischemia/reperfusion injury. Clin Nutr 27(5):773–780. doi:10.1016/j.clnu.2008.06.012
Hafez T, Sheth H, Glantzounis G, Parkes H, Seifalian A, Fuller B, Davidson B (2008) Glycine protects bile physiology and biliary-specific liver cell metabolism from ischemia-reperfusion injury: a H-1 NMR study. Cell preservation technology 6(3):173–180
Duenschede F, Westermann S, Riegler N, Miesner I, Erbes K, Ewald P, Kircher A, Schaefer H, Schneider J, Schad A, Dutkowski P, Kiemer AK, Junginger T (2006) Different protection mechanisms after pretreatment with glycine or alpha-lipoic acid in a rat model of warm hepatic ischemia. Eur Surg Res 38(6):503–512. doi:10.1159/000096061
Hoffmann K, Buchler MW, Schemmer P (2011) Supplementation of amino acids to prevent reperfusion injury after liver surgery and transplantation - Where do we stand today? Clinical Nutrition 30(2):143–147
Wang YS, Yan YH, Zou XF (2010) Protective effect of glycine on liver injury during liver transplantation. Chin Med J (Engl) 123(14):1931–1938
Stachlewitz RF, Seabra V, Bradford B, Bradham CA, Rusyn I, Germolec D, Thurman RG (1999) Glycine and uridine prevent d-galactosamine hepatotoxicity in the rat: role of Kupffer cells. Hepatology 29(3):737–745. doi:10.1002/hep.510290335
Schneider L, Hackert T, Longerich T, Hartwig W, Fritz S, Krych R, Fortunato F, Gebhard MM, Werner J (2010) Effects of gadolinium chloride and glycine on hepatic and pancreatic tissue damage in alcoholic pancreatitis. Pancreas 39(4):502–509. doi:10.1097/MPA.0b013e3181bd6470
Rentsch M, Puellmann K, Sirek S, Iesalnieks I, Kienle K, Mueller T, Bolder U, Geissler E, Jauch KW, Beham A (2005) Benefit of Kupffer cell modulation with glycine versus Kupffer cell depletion after liver transplantation in the rat: effects on postischemic reperfusion injury, apoptotic cell death graft regeneration and survival. Transpl Int 18(9):1079–1089. doi:10.1111/j.1432-2277.2005.00185.x
Bruns H, Watanpour I, Gebhard MM, Flechtenmacher C, Galli U, Schulze-Bergkamen H, Zorn M, Buchler MW, Schemmer P (2011) Glycine and taurine equally prevent fatty livers from Kupffer cell-dependent injury: an in vivo microscopy study. Microcirculation 18(3):205–213. doi:10.1111/j.1549-8719.2010.00078.x
Zhong X, Li X, Qian L, Xu Y, Lu Y, Zhang J, Li N, Zhu X, Ben J, Yang Q, Chen Q (2012) Glycine attenuates myocardial ischemia-reperfusion injury by inhibiting myocardial apoptosis in rats. J Biomed Res 26(5):346–354. doi:10.7555/JBR.26.20110124
Zhang Y, Lv SJ, Yan H, Wang L, Liang GP, Wan QX, Peng X (2013) Effects of glycine supplementation on myocardial damage and cardiac function after severe burn. Burns 39(4):729–735. doi:10.1016/j.burns.2012.09.006
Ascher E, Hanson JN, Cheng W, Hingorani A, Scheinman M (2001) Glycine preserves function and decreases necrosis in skeletal muscle undergoing ischemia and reperfusion injury. Surgery 129(2):231–235. doi:10.1067/msy.2001.112594
Crinnion JN, Homer-Vanniasinkam S, Hatton R, Parkin SM, Gough MJ (1994) Role of neutrophil depletion and elastase inhibition in modifying skeletal muscle reperfusion injury. Cardiovasc Surg 2(6):749–753
Gohrbandt B, Fischer S, Warnecke G, Avsar M, Sommer SP, Haverich A, Strueber M (2006) Glycine intravenous donor preconditioning is superior to glycine supplementation to low-potassium dextran flush preservation and improves graft function in a large animal lung transplantation model after 24 h of cold ischemia. J Thorac Cardiovasc Surg 131(3):724–729. doi:10.1016/j.jtcvs.2005.09.049
Sommer SP, Sommer S, Sinha B, Leyh RG (2012) Glycine preconditioning to ameliorate pulmonary ischemia reperfusion injury in rats. Interact CardioVasc Thorac Surg 14(5):521–525. doi:10.1093/icvts/ivs008
Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I (2000) Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci USA 97(6):2826–2831
Gusev EI, Skvortsova VI, Dambinova SA, Raevskiy KS, Alekseev AA, Bashkatova VG, Kovalenko AV, Kudrin VS, Yakovleva EV (2000) Neuroprotective effects of glycine for therapy of acute ischaemic stroke. Cerebrovasc Dis 10(1):49–60 (16025)
Oda M, Kure S, Sugawara T, Yamaguchi S, Kojima K, Shinka T, Sato K, Narisawa A, Aoki Y, Matsubara Y, Omae T, Mizoi K, Kinouchi H (2007) Direct correlation between ischemic injury and extracellular glycine concentration in mice with genetically altered activities of the glycine cleavage multienzyme system. Stroke 38(7):2157–2164. doi:10.1161/Strokeaha.106.477026
Tanabe M, Nitta A, Ono H (2010) Neuroprotection via strychnine-sensitive glycine receptors during post-ischemic recovery of excitatory synaptic transmission in the hippocampus. J Pharmacol Sci 113(4):378–386
Selin AA, Lobysheva NV, Vorontsova ON, Tonshin AA, Yaguzhinsky LS, Nartsissov YR (2012) Mechanism underlying the protective effect of glycine in energetic disturbances in brain tissues under hypoxic conditions. Bull Exp Biol Med 153(1):44–47
Chen Z, Hu B, Wang F, Du L, Huang B, Li L, Qi J, Wang X (2015) Glycine bidirectionally regulates ischemic tolerance via different mechanisms including NR2A-dependent CREB phosphorylation. J Neurochem 133(3):397–408. doi:10.1111/jnc.12994
Wheeler MD, Rose ML, Yamashima S, Enomoto N, Seabra V, Madren J, Thurman RG (2000) Dietary glycine blunts lung inflammatory cell influx following acute endotoxin. Am J Physiol Lung Cell Mol Physiol 279(2):L390–L398
Ikejima K, Iimuro Y, Forman DT, Thurman RG (1996) A diet containing glycine improves survival in endotoxin shock in the rat. Am J Physiol 271(1 Pt 1):G97–G103
Yang S, Koo DJ, Chaudry IH, Wang P (2001) Glycine attenuates hepatocellular depression during early sepsis and reduces sepsis-induced mortality. Crit Care Med 29(6):1201–1206
Grotz MR, Pape HC, van Griensven M, Stalp M, Rohde F, Bock D, Krettek C (2001) Glycine reduces the inflammatory response and organ damage in a two-hit sepsis model in rats. Shock 16(2):116–121
Zhong Z, Enomoto N, Connor HD, Moss N, Mason RP, Thurman RG (1999) Glycine improves survival after hemorrhagic shock in the rat. Shock 12(1):54–62
Gundersen Y, Vaagenes P, Os O, Pillgram-Larsen J, Sundnes KO, Opstad PK (2007) Capacity of glycine to modulate early inflammatory disturbances after serious gunshot injuries in the pig. Scand J Clin Lab Invest 67(2):143–153. doi:10.1080/00365510600995226
Aziz M, Jacob A, Wang P (2014) Revisiting caspases in sepsis. Cell Death Dis 5:e1526. doi:10.1038/cddis.2014.488
Zager RA, Johnson AC, Lund S, Randolph-Habecker J (2007) Toll-like receptor (TLR4) shedding and depletion: acute proximal tubular cell responses to hypoxic and toxic injury. Am J Physiol Renal Physiol 292(1):F304–F312. doi:10.1152/ajprenal.00237.2006
Zager RA, Johnson AC, Hanson SY (2004) Proximal tubular cytochrome c efflux: determinant, and potential marker, of mitochondrial injury. Kidney Int 65(6):2123–2134. doi:10.1111/j.1523-1755.2004.00638.x
Zager RA, Johnson AC, Hanson SY (2003) Radiographic contrast media-induced tubular injury: evaluation of oxidant stress and plasma membrane integrity. Kidney Int 64(1):128–139. doi:10.1046/j.1523-1755.2003.00059.x
Zager RA (2000) Plasma membrane cholesterol: a critical determinant of cellular energetics and tubular resistance to attack. Kidney Int 58(1):193–205. doi:10.1046/j.1523-1755.2000.00154.x
Aki T, Egashira N, Yamauchi Y, Hama M, Yano T, Itoh Y, Yamada T, Oishi R (2008) Protective effects of amino acids against gabexate mesilate-induced cell injury in porcine aorta endothelial cells. J Pharmacol Sci 107(3):238–245
Pelegrin P, Barroso-Gutierrez C, Surprenant A (2008) P2X(7) receptor differentially couples to distinct release pathways for IL-1 beta in mouse macrophage. J Immunol 180(11):7147–7157
Feldkamp T, Park JS, Pasupulati R, Amora D, Roeser NF, Venkatachalam MA, Weinberg JM (2009) Regulation of the mitochondrial permeability transition in kidney proximal tubules and its alteration during hypoxia-reoxygenation. Am J Physiol Renal Physiol 297(6):F1632–F1646. doi:10.1152/ajprenal.00422.2009
Park JS, Pasupulati R, Feldkamp T, Roeser NF, Weinberg JM (2011) Cyclophilin D and the mitochondrial permeability transition in kidney proximal tubules after hypoxic and ischemic injury. Am J Physiol Renal Physiol 301(1):F134–F150. doi:10.1152/ajprenal.00033.2011
Reeves WB (1997) Effects of chloride channel blockers on hypoxic injury in rat proximal tubules. Kidney Int 51(5):1529–1534
Schulz R, Gorge PM, Gorbe A, Ferdinandy P, Lampe PD, Leybaert L (2015) Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol Therapeut 153:90–106. doi:10.1016/j.pharmthera.2015.06.005
Acknowledgments
Preparation of this manuscript was supported by Merit Review # I01 BX002367 (JMW) from the United States (US) Department of Veterans Affairs. Original investigations by the authors cited here were supported by NIH Grants, DK-34275 (JMW), DK-37139, and DK-48417 (MAV), the Office of Naval Research (JMW), the Department of Veterans Affairs (JMW), and the Dr. Werner Jackstaedt-Foundation (AB). The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weinberg, J.M., Bienholz, A. & Venkatachalam, M.A. The role of glycine in regulated cell death. Cell. Mol. Life Sci. 73, 2285–2308 (2016). https://doi.org/10.1007/s00018-016-2201-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2201-6