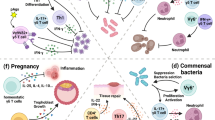
Abstract
Objective
γδ T cells are a distinct subset of unconventional T cells, which link innate and adaptive immunity by secreting cytokines and interacting with other immune cells, thereby modulating immune responses. As the first line of host defense, γδ T cells are essential for mucosal homeostasis and immune surveillance. When abnormally activated or impaired, γδ T cells can contribute to pathogenic processes. Accumulating evidence has revealed substantial impacts of γδ T cells on the pathogenesis of cancers, infections, and immune-inflammatory diseases. γδ T cells exhibit dual roles in cancers, promoting or inhibiting tumor growth, depending on their phenotypes and the clinical stage of cancers. During infections, γδ T cells exert high cytotoxic activity in infectious diseases, which is essential for combating bacterial and viral infections by recognizing foreign antigens and activating other immune cells. γδ T cells are also implicated in the onset and progression of immune-inflammatory diseases. However, the specific involvement and underlying mechanisms of γδ T cells in oral diseases have not been systematically discussed.
Methods
We conducted a systematic literature review using the PubMed/MEDLINE databases to identify and analyze relevant literature on the roles of γδ T cells in oral diseases.
Results
The literature review revealed that γδ T cells play a pivotal role in maintaining oral mucosal homeostasis and are involved in the pathogenesis of oral cancers, periodontal diseases, graft-versus-host disease (GVHD), oral lichen planus (OLP), and oral candidiasis. γδ T cells mainly influence various pathophysiological processes, such as anti-tumor activity, eradication of infection, and immune response regulation.
Conclusion
This review focuses on the involvement of γδ T cells in oral diseases, with a particular emphasis on the main functions and underlying mechanisms by which γδ T cells influence the pathogenesis and progression of these conditions. This review underscores the potential of γδ T cells as therapeutic targets in managing oral health issues.


Similar content being viewed by others
Data availability
In this manuscript, we conducted a systematic literature review using the PubMed/MEDLINE databases to identify and analyze relevant literature on the roles of γδ T cells in oral diseases.
References
Ribot JC, Lopes N, Silva-Santos B. γδ T cells in tissue physiology and surveillance. Nat Rev Immunol. 2021;21(4):221–32. https://doi.org/10.1038/s41577-020-00452-4.
Muñoz-Ruiz M, Sumaria N, Pennington DJ, et al. Thymic determinants of γδ T cell differentiation. Trends Immunol. 2017;38(5):336–44. https://doi.org/10.1016/j.it.2017.01.007.
Jensen KD, Su X, Shin S, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29(1):90–100. https://doi.org/10.1016/j.immuni.2008.04.022.
Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10(7):467–78. https://doi.org/10.1038/nri2781.
Papotto PH, Reinhardt A, Prinz I, et al. Innately versatile: γδ17T cells in inflammatory and autoimmune diseases. J Autoimmun. 2018. https://doi.org/10.1016/j.jaut.2017.11.006.
Zhao Y, Niu C, Cui J. Gamma-delta (γδ) T cells: friend or foe in cancer development? J Transl Med. 2018;16(1):3. https://doi.org/10.1186/s12967-017-1378-2.
Paul S, Lal G. Regulatory and effector functions of gamma-delta (γδ) T cells and their therapeutic potential in adoptive cellular therapy for cancer. Int J Cancer. 2016;139(5):976–85. https://doi.org/10.1002/ijc.30109.
Vavassori S, Kumar A, Wan GS, et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat Immunol. 2013;14(9):908–16. https://doi.org/10.1038/ni.2665.
Salim M, Knowles TJ, Baker AT, et al. BTN3A1 discriminates γδ T cell phosphoantigens from nonantigenic small molecules via a conformational sensor in Its B30.2 domain. ACS Chem Biol. 2017;12(10):2631–43. https://doi.org/10.1021/acschembio.7b00694.
Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309(5732):264–8. https://doi.org/10.1126/science.1110267.
Papotto PH, Ribot JC, Silva-Santos B. IL-17(+) γδ T cells as kick-starters of inflammation. Nat Immunol. 2017;18(6):604–11. https://doi.org/10.1038/ni.3726.
Isailovic N, Daigo K, Mantovani A, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015. https://doi.org/10.1016/j.jaut.2015.04.006.
Sutton CE, Lalor SJ, Sweeney CM, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31(2):331–41. https://doi.org/10.1016/j.immuni.2009.08.001.
Hosokawa H, Rothenberg EV. How transcription factors drive choice of the T cell fate. Nat Rev Immunol. 2021;21(3):162–76. https://doi.org/10.1038/s41577-020-00426-6.
Ciofani M, ZúñIGA-PFLüCKER JC. Determining γδ versus αß T cell development. Nat Rev Immunol. 2010;10(9):657–63. https://doi.org/10.1038/nri2820.
Hu Y, Hu Q, Li Y, et al. γδ T cells: origin and fate, subsets, diseases and immunotherapy. Signal Transduct Target Ther. 2023;8(1):434. https://doi.org/10.1038/s41392-023-01653-8.
Boehme L, Roels J, Taghon T,. Development of γδ T cells in the thymus—A human perspective. Semin Immunol. 2022. https://doi.org/10.1016/j.smim.2022.101662.
Fichtner AS, Ravens S, Prinz I,. Human γδ TCR repertoires in health and disease. Cells. 2020. https://doi.org/10.3390/cells9040800.
Qi C, Wang Y, Li P, et al. Gamma Delta T cells and their pathogenic role in psoriasis. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.627139.
Yang Y, Li L, Yuan L, et al. A structural change in butyrophilin upon phosphoantigen binding underlies phosphoantigen-mediated Vγ9Vδ2 T cell activation. Immunity. 2019;50(4):1043-53.e5. https://doi.org/10.1016/j.immuni.2019.02.016.
Koren N, Zubeidat K, Saba Y, et al. Maturation of the neonatal oral mucosa involves unique epithelium-microbiota interactions. Cell Host Microbe. 2021;29(2):197-209.e5. https://doi.org/10.1016/j.chom.2020.12.006.
Lin D, Yang L, Wen L, et al. Crosstalk between the oral microbiota, mucosal immunity, and the epithelial barrier regulates oral mucosal disease pathogenesis. Mucosal Immunol. 2021;14(6):1247–58. https://doi.org/10.1038/s41385-021-00413-7.
Tsukasaki M, Komatsu N, Nagashima K, et al. Host defense against oral microbiota by bone-damaging T cells. Nat Commun. 2018;9(1):701. https://doi.org/10.1038/s41467-018-03147-6.
Fleming C, Cai Y, Sun X, et al. Microbiota-activated CD103(+) DCs stemming from microbiota adaptation specifically drive γδT17 proliferation and activation. Microbiome. 2017;5(1):46. https://doi.org/10.1186/s40168-017-0263-9.
Papotto PH, Yilmaz B, Silva-Santos B. Crosstalk between γδ T cells and the microbiota. Nat Microbiol. 2021;6(9):1110–7. https://doi.org/10.1038/s41564-021-00948-2.
Lin D, Hu Q, Yang L, et al. The niche-specialist and age-related oral microbial ecosystem: crosstalk with host immune cells in homeostasis. Microb Genom. 2022. https://doi.org/10.1099/mgen.0.000811.
Wilharm A, Tabib Y, Nassar M, et al. Mutual interplay between IL-17-producing γδT cells and microbiota orchestrates oral mucosal homeostasis. Proc Natl Acad Sci U S A. 2019;116(7):2652–61. https://doi.org/10.1073/pnas.1818812116.
Gober HJ, Kistowska M, Angman L, et al. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197(2):163–8. https://doi.org/10.1084/jem.20021500.
Wang X, Lin X, Zheng Z, et al. Host-derived lipids orchestrate pulmonary γδ T cell response to provide early protection against influenza virus infection. Nat Commun. 2021;12(1):1914. https://doi.org/10.1038/s41467-021-22242-9.
Gao Y, Yang W, Pan M, et al. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198(3):433–42. https://doi.org/10.1084/jem.20030584.
Park JH, Lee HK. Function of gammadelta T cells in tumor immunology and their application to cancer therapy. Exp Mol Med. 2021;53(3):318–27. https://doi.org/10.1038/s12276-021-00576-0.
Couzi L, Pitard V, Sicard X, et al. Antibody-dependent anti-cytomegalovirus activity of human γδ T cells expressing CD16 (FcγRIIIa). Blood. 2012;119(6):1418–27. https://doi.org/10.1182/blood-2011-06-363655.
Yazdanifar M, Barbarito G, Bertaina A, et al. γδ T cells: the Ideal tool for cancer immunotherapy. Cells. 2020. https://doi.org/10.3390/cells9051305.
McGinley AM, Edwards SC, Raverdeau M, et al. Th17 cells, γδ T cells and their interplay in EAE and multiple sclerosis. J Autoimmun. 2018. https://doi.org/10.1016/j.jaut.2018.01.001.
Petermann F, Rothhammer V, Claussen MC, et al. γδ T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33(3):351–63. https://doi.org/10.1016/j.immuni.2010.08.013.
Maniar A, Zhang X, Lin W, et al. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116(10):1726–33. https://doi.org/10.1182/blood-2009-07-234211.
Sabbione F, Gabelloni ML, Ernst G, et al. Neutrophils suppress γδ T-cell function. Eur J Immunol. 2014;44(3):819–30. https://doi.org/10.1002/eji.201343664.
Münz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202(2):203–7. https://doi.org/10.1084/jem.20050810.
Leslie DS, Vincent MS, Spada FM, et al. CD1-mediated gamma/delta T cell maturation of dendritic cells. J Exp Med. 2002;196(12):1575–84. https://doi.org/10.1084/jem.20021515.
Conti L, Casetti R, Cardone M, et al. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated gammadelta T cells: role of CD86 and inflammatory cytokines. J Immunol. 2005;174(1):252–60. https://doi.org/10.4049/jimmunol.174.1.252.
Devilder MC, Maillet S, Bouyge-Moreau I, et al. Potentiation of antigen-stimulated V gamma 9V delta 2 T cell cytokine production by immature dendritic cells (DC) and reciprocal effect on DC maturation. J Immunol. 2006;176(3):1386–93. https://doi.org/10.4049/jimmunol.176.3.1386.
Peters C, Kabelitz D, Wesch D. Regulatory functions of γδ T cells. Cell Mol Life Sci. 2018;75(12):2125–35. https://doi.org/10.1007/s00018-018-2788-x.
Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol. 2015;8(9):11884–94.
Ghantous Y, Abu EI. Global incidence and risk factors of oral cancer. Harefuah. 2017;156(10):645–9.
Silva-Santos B, Serre K, Norell H. γδ T cells in cancer. Nat Rev Immunol. 2015;15(11):683–91. https://doi.org/10.1038/nri3904.
Lafont V, Sanchez F, Laprevotte E, et al. Plasticity of γδ t cells: impact on the anti-tumor response. Front Immunol. 2014. https://doi.org/10.3389/fimmu.2014.00622.
Lo Presti E, Dieli F, Meraviglia S. Tumor-infiltrating γδ T lymphocytes: pathogenic role, clinical significance, and differential programing in the tumor microenvironment. Front Immunol. 2014. https://doi.org/10.3389/fimmu.2014.00607.
Lopes N, Silva-Santos B. Functional and metabolic dichotomy of murine γδ T cell subsets in cancer immunity. Eur J Immunol. 2021;51(1):17–26. https://doi.org/10.1002/eji.201948402.
Lo Presti E, Toia F, Oieni S, et al. Squamous cell tumors recruit γδ T cells producing either IL17 or IFNγ depending on the tumor stage. Cancer Immunol Res. 2017;5(5):397–407. https://doi.org/10.1158/2326-6066.Cir-16-0348.
Wu P, Wu D, Ni C, et al. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40(5):785–800. https://doi.org/10.1016/j.immuni.2014.03.013.
Girardi M, Oppenheim DE, Steele CR, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294(5542):605–9. https://doi.org/10.1126/science.1063916.
Sureshbabu SK, Chaukar D, Chiplunkar SV. Hypoxia regulates the differentiation and anti-tumor effector functions of γδT cells in oral cancer. Clin Exp Immunol. 2020;201(1):40–57. https://doi.org/10.1111/cei.13436.
Laad AD, Thomas ML, Fakih AR, et al. Human gamma delta T cells recognize heat shock protein-60 on oral tumor cells. Int J Cancer. 1999;80(5):709–14. https://doi.org/10.1002/(sici)1097-0215(19990301)80:5%3c709::aid-ijc14%3e3.0.co;2-r.
Domae E, Hirai Y, Ikeo T, et al. Human Vγ9Vδ2 T cells show potent antitumor activity against zoledronate-sensitized OSCC cell lines. J buon. 2018;23(7):132–8.
O’neill K, Pastar I, Tomic-Canic M, et al. Perforins expression by cutaneous gamma delta T Cells. Front Immunol. 2020. https://doi.org/10.3389/fimmu.2020.01839.
Wakita D, Sumida K, Iwakura Y, et al. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol. 2010;40(7):1927–37. https://doi.org/10.1002/eji.200940157.
Wei W, Li J, Shen X, et al. (2022) Oral microbiota from periodontitis promote oral squamous cell carcinoma development via γδ T cell activation. Systems. 2022;7(5):e0046922. https://doi.org/10.1128/msystems.00469-22.
Li L, Cao B, Liang X, et al. Microenvironmental oxygen pressure orchestrates an anti- and pro-tumoral γδ T cell equilibrium via tumor-derived exosomes. Oncogene. 2019;38(15):2830–43. https://doi.org/10.1038/s41388-018-0627-z.
Atre N, Thomas L, Mistry R, et al. Role of nitric oxide in heat shock protein induced apoptosis of gammadeltaT cells. Int J Cancer. 2006;119(6):1368–76. https://doi.org/10.1002/ijc.21966.
Mensurado S, Blanco-Domínguez R, Silva-Santos B. The emerging roles of γδ T cells in cancer immunotherapy. Nat Rev Clin Oncol. 2023;20(3):178–91. https://doi.org/10.1038/s41571-022-00722-1.
Mensurado S, Blanco-Domínguez R, Silva-Santos B. The emerging roles of γδ T cells in cancer immunotherapy. Nat Rev Clin Oncol. 2023. https://doi.org/10.1038/s41571-022-00722-1.
Deng J, Yin H. Gamma delta (γδ) T cells in cancer immunotherapy; where it comes from, where it will go? Eur J Pharmacol. 2022. https://doi.org/10.1016/j.ejphar.2022.174803.
Kabelitz D, Serrano R, Kouakanou L, et al. Cancer immunotherapy with γδ T cells: many paths ahead of us. Cell Mol Immunol. 2020;17(9):925–39. https://doi.org/10.1038/s41423-020-0504-x.
Ou L, Wang H, Huang H, et al. Preclinical platforms to study therapeutic efficacy of human γδ T cells. Clin Transl Med. 2022;12(6):e814. https://doi.org/10.1002/ctm2.814.
Silva-Santos B, Mensurado S, Coffelt SB. γδ T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer. 2019;19(7):392–404. https://doi.org/10.1038/s41568-019-0153-5.
Bhat J, Placek K, Faissner S. Contemplating dichotomous nature of gamma delta T cells for immunotherapy. Front Immunol. 2022. https://doi.org/10.3389/fimmu.2022.894580.
Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017. https://doi.org/10.1038/nrdp.2017.38.
Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. https://doi.org/10.1038/nri3785.
Nielsen MM, Witherden DA, Havran WL. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol. 2017;17(12):733–45. https://doi.org/10.1038/nri.2017.101.
Wald S, Leibowitz A, Aizenbud Y, et al. γδT cells are essential for orthodontic tooth movement. J Dent Res. 2021;100(7):731–8. https://doi.org/10.1177/0022034520984774.
Nagai A, Takahashi K, Sato N, et al. Abnormal proportion of gamma delta T cells in peripheral blood is frequently detected in patients with periodontal disease. J Periodontol. 1993;64(10):963–7. https://doi.org/10.1902/jop.1993.64.10.963.
Chien YH, Zeng X, Prinz I. The natural and the inducible: interleukin (IL)-17-producing γδ T cells. Trends Immunol. 2013;34(4):151–4. https://doi.org/10.1016/j.it.2012.11.004.
Yu JJ, Ruddy MJ, Wong GC, et al. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood. 2007;109(9):3794–802. https://doi.org/10.1182/blood-2005-09-010116.
Ono T, Okamoto K, Nakashima T, et al. IL-17-producing γδ T cells enhance bone regeneration. Nat Commun. 2016. https://doi.org/10.1038/ncomms10928.
Awang RA, Lappin DF, Macpherson A, et al. Clinical associations between IL-17 family cytokines and periodontitis and potential differential roles for IL-17A and IL-17E in periodontal immunity. Inflamm Res. 2014;63(12):1001–12. https://doi.org/10.1007/s00011-014-0776-7.
Bunte K, Beikler T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20143394.
Krishnan S, Prise IE, Wemyss K, et al. Amphiregulin-producing γδ T cells are vital for safeguarding oral barrier immune homeostasis. Proc Natl Acad Sci U S A. 2018;115(42):10738–43. https://doi.org/10.1073/pnas.1802320115.
Hovav AH, Wilharm A, Barel O, et al. Development and function of γδT cells in the oral mucosa. J Dent Res. 2020;99(5):498–505. https://doi.org/10.1177/0022034520908839.
Barel O, Aizenbud Y, Tabib Y, et al. γδ T cells differentially regulate bone loss in periodontitis models. J Dent Res. 2022;101(4):428–36. https://doi.org/10.1177/00220345211042830.
Sandrock I, Reinhardt A, Ravens S, et al. Genetic models reveal origin, persistence and non-redundant functions of IL-17-producing γδ T cells. J Exp Med. 2018;215(12):3006–18. https://doi.org/10.1084/jem.20181439.
Treister N, Duncan C, Cutler C, et al. How we treat oral chronic graft-versus-host disease. Blood. 2012;120(17):3407–18. https://doi.org/10.1182/blood-2012-05-393389.
Bassim CW, Fassil H, Mays JW, et al. Oral disease profiles in chronic graft versus host disease. J Dent Res. 2015;94(4):547–54. https://doi.org/10.1177/0022034515570942.
Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–58. https://doi.org/10.1038/nri3212.
Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. https://doi.org/10.1016/j.bbmt.2014.12.001.
Pabst C, Schirutschke H, Ehninger G, et al. The graft content of donor T cells expressing gamma delta TCR+ and CD4+foxp3+ predicts the risk of acute graft versus host disease after transplantation of allogeneic peripheral blood stem cells from unrelated donors. Clin Cancer Res. 2007;13(10):2916–22. https://doi.org/10.1158/1078-0432.Ccr-06-2602.
Blazar BR, Taylor PA, Panoskaltsis-Mortari A, et al. Lethal murine graft-versus-host disease induced by donor gamma/delta expressing T cells with specificity for host nonclassical major histocompatibility complex class Ib antigens. Blood. 1996;87(2):827–37.
Huang Y, Cramer DE, Ray MB, et al. The role of alphabeta- and gammadelta-T cells in allogenic donor marrow on engraftment, chimerism, and graft-versus-host disease. Transplantation. 2001;72(12):1907–14. https://doi.org/10.1097/00007890-200112270-00007.
Wu N, Liu R, Liang S, et al. γδ T cells may aggravate acute graft-versus-host disease through CXCR4 signaling after allogeneic hematopoietic transplantation. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.687961.
Song Y, Zhu Y, Hu B, et al. Donor γδT cells promote GVL effect and mitigate aGVHD in allogeneic hematopoietic stem cell transplantation. Front Immunol. 2020. https://doi.org/10.3389/fimmu.2020.558143.
Drobyski WR, Majewski D, Hanson G (199) Graft-facilitating doses of ex vivo activated gammadelta T cells do not cause lethal murine graft-vs-host disease. Biol Blood Marrow Transplant 5(4): 222–30. https://doi.org/10.1053/bbmt.1999.v5.pm10465102
Kawasaki Y, Sato K, Hayakawa H, et al. Comprehensive analysis of the activation and proliferation kinetics and effector functions of human lymphocytes, and antigen presentation capacity of antigen-presenting cells in xenogeneic graft-versus-host disease. Biol Blood Marrow Transplant. 2018;24(8):1563–74. https://doi.org/10.1016/j.bbmt.2018.04.016.
Drobyski WR, Majewski D. Donor gamma delta T lymphocytes promote allogeneic engraftment across the major histocompatibility barrier in mice. Blood. 1997;89(3):1100–9.
Maeda Y, Reddy P, Lowler KP, et al. Critical role of host gammadelta T cells in experimental acute graft-versus-host disease. Blood. 2005;106(2):749–55. https://doi.org/10.1182/blood-2004-10-4087.
Anderson BE, McNiff JM, Matte C, et al. Recipient CD4+ T cells that survive irradiation regulate chronic graft-versus-host disease. Blood. 2004;104(5):1565–73. https://doi.org/10.1182/blood-2004-01-0328.
Cai Y, Shen X, Ding C, et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35(4):596–610. https://doi.org/10.1016/j.immuni.2011.08.001.
Ramírez-Valle F, Gray EE, Cyster JG. Inflammation induces dermal Vγ4+ γδT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proc Natl Acad Sci U S A. 2015;112(26):8046–51. https://doi.org/10.1073/pnas.1508990112.
Wu M, Yang J, Li X, et al. The role of γδ T cells in systemic lupus erythematosus. J Immunol Res. 2016. https://doi.org/10.1155/2016/2932531.
Bramanti TE, Dekker NP, Lozada-Nur F, et al. Heat shock (stress) proteins and gamma delta T lymphocytes in oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80(6):698–704. https://doi.org/10.1016/s1079-2104(05)80254-9.
Huang S, Tan YQ, Zhou G. Aberrant activation of the STING-TBK1 Pathway in γδ T cells regulates immune responses in oral lichen planus. Biomedicines. 2023. https://doi.org/10.3390/biomedicines11030955.
Yang JY, Wang F, Zhou G. Characterization and function of circulating mucosal-associated invariant T cells and γδT cells in oral lichen planus. J Oral Pathol Med. 2022;51(1):74–85. https://doi.org/10.1111/jop.13250.
Husein-Elahmed H, Steinhoff M. Potential role of Interleukin-17 in the pathogenesis of oral lichen planus: a systematic review with meta-analysis. J Eur Acad Dermatol Venereol. 2022;36(10):1735–44. https://doi.org/10.1111/jdv.18219.
Shao S, Tsoi LC, Sarkar MK, et al. IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci Transl Med. 2019. https://doi.org/10.1126/scitranslmed.aav7561.
Jones-Carson J, Vazquez-Torres A, van der Heyde HC, et al. Gamma delta T cell-induced nitric oxide production enhances resistance to mucosal candidiasis. Nat Med. 1995;1(6):552–7. https://doi.org/10.1038/nm0695-552.
Pavlova A, Sharafutdinov I. Recognition of Candida albicans and role of innate type 17 immunity in oral candidiasis. Microorganisms. 2020. https://doi.org/10.3390/microorganisms8091340.
Conti HR, Shen F, Nayyar N, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206(2):299–311. https://doi.org/10.1084/jem.20081463.
Conti HR, Bruno VM, Childs EE, et al. IL-17 receptor signaling in oral epithelial cells is critical for protection against oropharyngeal candidiasis. Cell Host Microbe. 2016;20(5):606–17. https://doi.org/10.1016/j.chom.2016.10.001.
Sparber F, Dolowschiak T, Mertens S, et al. Langerin+ DCs regulate innate IL-17 production in the oral mucosa during Candida albicans-mediated infection. PLoS Pathog. 2018;14(5):e1007069. https://doi.org/10.1371/journal.ppat.1007069.
Conti HR, Peterson AC, Brane L, et al. Oral-resident natural Th17 cells and γδ T cells control opportunistic Candida albicans infections. J Exp Med. 2014;211(10):2075–84. https://doi.org/10.1084/jem.20130877.
Martin B, Hirota K, Cua DJ, et al. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31(2):321–30. https://doi.org/10.1016/j.immuni.2009.06.020.
Maher CO, Dunne K, Comerford R, et al. Candida albicans stimulates IL-23 release by human dendritic cells and downstream IL-17 secretion by Vδ1 T cells. J Immunol. 2015;194(12):5953–60. https://doi.org/10.4049/jimmunol.1403066.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 82201068 and 82270983).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. XW was involved in writing—original draft preparation. YT and GZ reviewed and edited the manuscript. All authors gave their approval for the final manuscript to be published.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, XY., Tan, YQ. & Zhou, G. γδ T cells in oral diseases. Inflamm. Res. 73, 867–876 (2024). https://doi.org/10.1007/s00011-024-01870-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-024-01870-z