Abstract
Objective
The treatment of eosinophilic chronic rhinosinusitis with nasal polyps (E-CRSwNP) remains a challenge due to its complex pathogenesis. Inositol polyphosphate-4-phosphatase type IA (INPP4A), a lipid phosphatase, has been implicated in allergic asthma. However, the expression and function of INPP4A in E-CRSwNP remain unclear. This study aims to investigate the role of INPP4A in macrophages in E-CRSwNP.
Methods
We assessed the expression of INPP4A in human and mouse nasal mucosal tissues via immunofluorescence staining. THP-1 cells were cultured and exposed to various cytokines to investigate the regulation of INPP4A expression and its functional role. Additionally, we established a murine nasal polyp (NP) model and administrated an INPP4A-overexpressing lentivirus evaluate its impact on NP.
Results
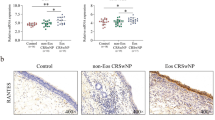
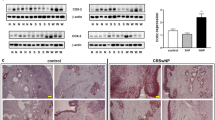
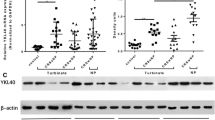
The percentage of INPP4A + CD68 + macrophages among total macrophages decreased in the E-CRSwNP group compared to the control and the non-eosinophilic CRSwNP (NE-CRSwNP) groups, exhibiting an inverse correlation with an increased percentage of CD206 + CD68 + M2 macrophages among total macrophages. Overexpression of INPP4A led to a reduced percentage of THP-1 cells polarizing towards the M2 phenotype, accompanied by decreased levels of associated chemotactic factors including CCL18, CCL22, CCL24, and CCL26. We also validated the involvement of the PI3K-AKT pathway in the function of INPP4A in vitro. Furthermore, INPP4A overexpression in the murine NP model resulted in the attenuation of eosinophilic inflammation in the nasal mucosa.
Conclusions
INPP4A deficiency promotes macrophage polarization towards the M2 phenotype, leading to the secretion of chemokines that recruit eosinophils and Th2 cells, thereby amplifying eosinophilic inflammation in E-CRSwNP. INPP4A may exert a suppressive role in eosinophilic inflammation and could potentially serve as a novel therapeutic strategy.









Similar content being viewed by others
Data availability
The data and materials used to support the findings of this study are available from the corresponding author upon request.
References
Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58((Suppl S29)):1–464. https://doi.org/10.4193/Rhin20.600.
Wang X, Zhang N, Bo M, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138(5):1344–53. https://doi.org/10.1016/j.jaci.2016.05.041.
Zhang Y, Gevaert E, Lou H, et al. Chronic rhinosinusitis in Asia. J Allergy Clin Immunol. 2017;140(5):1230–9. https://doi.org/10.1016/j.jaci.2017.09.009.
Kato A, Peters AT, Stevens WW, Schleimer RP, Tan BK, Kern RC. Endotypes of chronic rhinosinusitis: relationships to disease phenotypes, pathogenesis, clinical findings, and treatment approaches. Allergy. 2022;77(3):812–26. https://doi.org/10.1111/all.15074.
Liao B, Liu JX, Li ZY, et al. Multidimensional endotypes of chronic rhinosinusitis and their association with treatment outcomes. Allergy. 2018;73(7):1459–69. https://doi.org/10.1111/all.13411.
Yao Y, Wang ZC, Liu JX, et al. Increased expression of TIPE2 in alternatively activated macrophages is associated with eosinophilic inflammation and disease severity in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2017;7(10):963–72. https://doi.org/10.1002/alr.21984.
Krysko O, Holtappels G, Zhang N, et al. Alternatively activated macrophages and impaired phagocytosis of S. aureus in chronic rhinosinusitis. Allergy. 2011;66(3):396–403. https://doi.org/10.1111/j.1398-9995.2010.02498.x.
Saradna A, Do DC, Kumar S, Fu QL, Gao P. Macrophage polarization and allergic asthma. Transl Res. 2018;191:1–14. https://doi.org/10.1016/j.trsl.2017.09.002.
Li H, Marshall AJ. Phosphatidylinositol (3,4) bisphosphate-specific phosphatases and effector proteins: a distinct branch of PI3K signaling. Cell Signal. 2015;27(9):1789–98. https://doi.org/10.1016/j.cellsig.2015.05.013.
Khanna K, Chaudhuri R, Aich J, et al. Secretory inositol polyphosphate 4-phosphatase protects against airway inflammation and remodeling. Am J Respir Cell Mol Biol. 2019;60(4):399–412. https://doi.org/10.1165/rcmb.2017-0353OC.
Aich J, Mabalirajan U, Ahmad T, Agrawal A, Ghosh B. Loss-of-function of inositol polyphosphate-4-phosphatase reversibly increases the severity of allergic airway inflammation. Nat Commun. 2012;3:877. https://doi.org/10.1038/ncomms1880.
Tan H, Tong X, Gao Z, et al. The hMeDIP-Seq identified INPP4A as a novel biomarker for eosinophilic chronic rhinosinusitis with nasal polyps. Epigenomics. 2022. https://doi.org/10.2217/epi-2022-0053.
Lee M, Kim DW, Yoon H, et al. Sirtuin 1 attenuates nasal polypogenesis by suppressing epithelial-to-mesenchymal transition. J Allergy Clin Immunol. 2016;137(1):87-98.e7. https://doi.org/10.1016/j.jaci.2015.07.026.
Bae JS, Ryu G, Kim JH, et al. Effects of Wnt signaling on epithelial to mesenchymal transition in chronic rhinosinusitis with nasal polyp. Thorax. 2020;75(11):982–93. https://doi.org/10.1136/thoraxjnl-2019-213916.
Shi LL, Ma J, Deng YK, et al. Cold-inducible RNA-binding protein contributes to tissue remodeling in chronic rhinosinusitis with nasal polyps. Allergy. 2021;76(2):497–509. https://doi.org/10.1111/all.14287.
Schraivogel D, Gschwind AR, Milbank JH, et al. Targeted Perturb-seq enables genome-scale genetic screens in single cells. Nat Methods. 2020;17(6):629–35. https://doi.org/10.1038/s41592-020-0837-5.
Wen S, Li F, Tang Y, et al. MIR222HG attenuates macrophage M2 polarization and allergic inflammation in allergic rhinitis by targeting the miR146a-5p/TRAF6/NF-κB axis. Front Immunol. 2023;14:1168920. https://doi.org/10.3389/fimmu.2023.1168920.
Zhou H, Zhang W, Qin D, et al. Activation of NLRP3 inflammasome contributes to the inflammatory response to allergic rhinitis via macrophage pyroptosis. Int Immunopharmacol. 2022;110:109012. https://doi.org/10.1016/j.intimp.2022.109012.
Wang ZC, Yao Y, Wang N, et al. Deficiency in interleukin-10 production by M2 macrophages in eosinophilic chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2018;8(11):1323–33. https://doi.org/10.1002/alr.22218.
Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol (Baltimore, MD: 1950). 2017. https://doi.org/10.4049/jimmunol.1601515.
Zhuang C, Guo Z, Zhu J, et al. PTEN inhibitor attenuates cardiac fibrosis by regulating the M2 macrophage phenotype via the PI3K/AKT/TGF-β/Smad 2/3 signaling pathway. Int J Cardiol. 2022;356:88–96. https://doi.org/10.1016/j.ijcard.2022.04.007.
Wang W, Xu Y, Wang L, et al. Single-cell profiling identifies mechanisms of inflammatory heterogeneity in chronic rhinosinusitis. Nat Immunol. 2022;23(10):1484–94. https://doi.org/10.1038/s41590-022-01312-0.
Leite-Santos F, Tamashiro E, de Andrade Batista Murashima A, Anselmo-Lima WT, Valera FCP. Which are the best murine models to study Eosinophilic Chronic Rhinosinusitis? A contemporary review. Braz J Otorhinolaryngol. 2023;89(6):101328. https://doi.org/10.1016/j.bjorl.2023.101328.
Takabayashi T, Schleimer RP. Formation of nasal polyps: the roles of innate type 2 inflammation and deposition of fibrin. J Allergy Clin Immunol. 2020;145(3):740–50. https://doi.org/10.1016/j.jaci.2020.01.027.
Kato A, Schleimer RP, Bleier BS. Mechanisms and pathogenesis of chronic rhinosinusitis. J Allergy Clin Immunol. 2022. https://doi.org/10.1016/j.jaci.2022.02.016.
Zhou Y, Zhang T, Yan Y, et al. MicroRNA-223-3p regulates allergic inflammation by targeting INPP4A. Braz J Otorhinolaryngol. 2021;87(5):591–600. https://doi.org/10.1016/j.bjorl.2020.05.020.
Ho J, Earls P, Harvey RJ. Systemic biomarkers of eosinophilic chronic rhinosinusitis. Curr Opin Allergy Clin Immunol. 2020;20(1):23–9. https://doi.org/10.1097/ACI.0000000000000602.
Huang GJ, Chen ZQ, Fan ZJ, Li SH. The causal association between peripheral blood eosinophils and nasal polyps: a Mendelian randomization study. Eur Arch Otorhinolaryngol. 2023;280(9):4285–90. https://doi.org/10.1007/s00405-023-08129-z.
Drake VE, Rafaels N, Kim J. Peripheral blood eosinophilia correlates with hyperplastic nasal polyp growth. Int Forum Allergy Rhinol. 2016;6(9):926–34. https://doi.org/10.1002/alr.21793.
Haimerl P, Bernhardt U, Schindela S, et al. Inflammatory macrophage memory in nonsteroidal anti-inflammatory drug-exacerbated respiratory disease. J Allergy Clin Immunol. 2021;147(2):587–99. https://doi.org/10.1016/j.jaci.2020.04.064.
Zhong B, Du J, Liu F, et al. Activation of the mTOR/HIF-1α/VEGF axis promotes M1 macrophage polarization in non-eosinophilic chronic rhinosinusitis with nasal polyps. Allergy. 2022;77(2):643–6. https://doi.org/10.1111/all.15050.
Yu Z, Wang Y, Zhang J, et al. Expression of heme oxygenase-1 in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps: modulation by cytokines. Int Forum Allergy Rhinol. 2015;5(8):734–40. https://doi.org/10.1002/alr.21530.
Arranz A, Doxaki C, Vergadi E, et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci U S A. 2012;109(24):9517–22. https://doi.org/10.1073/pnas.1119038109.
Ma B, Athari SS, Mehrabi Nasab E, Zhao L. PI3K/AKT/mTOR and TLR4/MyD88/NF-κB signaling inhibitors attenuate pathological mechanisms of allergic asthma. Inflammation. 2021;44(5):1895–907. https://doi.org/10.1007/s10753-021-01466-3.
Athari SS. Targeting cell signaling in allergic asthma. Signal Transduct Target Ther. 2019;4:45. https://doi.org/10.1038/s41392-019-0079-0.
Byles V, Covarrubias AJ, Ben-Sahra I, et al. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun. 2013;4:2834. https://doi.org/10.1038/ncomms3834.
Covarrubias AJ, Aksoylar HI, Horng T. Control of macrophage metabolism and activation by mTOR and Akt signaling. Semin Immunol. 2015;27(4):286–96. https://doi.org/10.1016/j.smim.2015.08.001.
Weisser SB, McLarren KW, Voglmaier N, et al. Alternative activation of macrophages by IL-4 requires SHIP degradation. Eur J Immunol. 2011;41(6):1742–53. https://doi.org/10.1002/eji.201041105.
Wang X, Luo G, Zhang K, et al. Hypoxic Tumor-Derived exosomal miR-301a Mediates M2 macrophage polarization via PTEN/PI3Kγ to promote pancreatic cancer metastasis. Cancer Res. 2018;78(16):4586–98. https://doi.org/10.1158/0008-5472.CAN-17-3841.
Liu L, Zhu X, Zhao T, Yu Y, Xue Y, Zou H. Sirt1 ameliorates monosodium urate crystal-induced inflammation by altering macrophage polarization via the PI3K/Akt/STAT6 pathway. Rheumatology (Oxford). 2019;58(9):1674–83. https://doi.org/10.1093/rheumatology/kez165.
Fang J, Ou Q, Wu B, et al. TcpC inhibits M1 but promotes M2 macrophage polarization via regulation of the MAPK/NF-κB and Akt/STAT6 pathways in urinary tract infection. Cells. 2022;11(17):2674. https://doi.org/10.3390/cells11172674.
Zhang A, Xu Y, Xu H, et al. Lactate-induced M2 polarization of tumor-associated macrophages promotes the invasion of pituitary adenoma by secreting CCL17. Theranostics. 2021;11(8):3839–52. https://doi.org/10.7150/thno.53749.
Mommert S, Schaper JT, Schaper-Gerhardt K, Gutzmer R, Werfel T. Histamine increases Th2 cytokine-induced CCL18 expression in human M2 macrophages. Int J Mol Sci. 2021;22(21):11648. https://doi.org/10.3390/ijms222111648.
Lee SH, Chaves MM, Kamenyeva O, et al. M2-like, IL-4/CCL24-mediated eosinophils in cutaneous leishmaniasis. Sci Immunol. 2020;5(46):eaaz4415. https://doi.org/10.1126/sciimmunol.aaz4415.
Lou H, Huang Y, Chen H, Wang Y, Zhang L, Wang C. M2 macrophages correlated with symptom severity and promote type 2 inflammation in allergic rhinitis. Allergy. 2019;74(11):2255–7. https://doi.org/10.1111/all.13852.
Vestergaard C, Deleuran M, Gesser B, Larsen CG. Thymus- and activation-regulated chemokine (TARC/CCL17) induces a Th2-dominated inflammatory reaction on intradermal injection in mice. Exp Dermatol. 2004;13(4):265–71. https://doi.org/10.1111/j.0906-6705.2004.00149.x.
Araujo-Pires AC, Vieira AE, Francisconi CF, et al. IL-4/CCL22/CCR4 axis controls regulatory T-cell migration that suppresses inflammatory bone loss in murine experimental periodontitis. J Bone Miner Res. 2015;30(3):412–22. https://doi.org/10.1002/jbmr.2376.
Pinho V, Oliveira SH, Souza DG, et al. The role of CCL22 (MDC) for the recruitment of eosinophils during allergic pleurisy in mice. J Leukoc Biol. 2003;73(3):356–62. https://doi.org/10.1189/jlb.0502243.
Kim B, Lee HJ, Im NR, et al. Decreased expression of CCL17 in the disrupted nasal polyp epithelium and its regulation by IL-4 and IL-5. PLoS ONE. 2018;13(5):e0197355. https://doi.org/10.1371/journal.pone.0197355.
Wang H, Hu DQ, Xiao Q, et al. Defective STING expression potentiates IL-13 signaling in epithelial cells in eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2021;147(5):1692–703. https://doi.org/10.1016/j.jaci.2020.12.623.
Peterson S, Poposki JA, Nagarkar DR, et al. Increased expression of CC chemokine ligand 18 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;129(1):119–27. https://doi.org/10.1016/j.jaci.2011.08.021.
Funding
National Natural Science Foundation of China (82271134, 81770986, 82071017); Fundamental Research Funds for the Central Universities (2042020kf1044, 2041022kf1113).
Author information
Authors and Affiliations
Contributions
YX provided funding, supervision, writing-review and editing; YYX designed the study,performed experiments, analysed data and drafted original article; TXT, PQL, JYH, SYC,DL, TG,YLX and DG assessed, recorded the patients health information and collected the samples of participants; All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Y., Tong, X., Liu, P. et al. Deficiency of INPP4A promotes M2 macrophage polarization in eosinophilic chronic rhinosinusitis with nasal polyps. Inflamm. Res. 73, 581–595 (2024). https://doi.org/10.1007/s00011-024-01855-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-024-01855-y