Abstract
Objective and design
Immunoglobulin A nephropathy (IgAN) is a kidney disease characterized by the accumulation of IgA deposits in the glomeruli of the kidney, leading to inflammation and damage to the kidney. The inflammatory markers involved in IgAN remain to be defined. Gene expression analysis platforms, such as the NanoString nCounter system, are promising screening and diagnostic tools, especially in oncology. Still, their role as a diagnostic and prognostic tool in IgAN remains scarce. In this study, we aimed to validate the use of NanoString technology to identify potential inflammatory biomarkers involved in the progression of IgAN.
Subjects
A total of 30 patients with biopsy-proven IgAN and 7 cases of antineutrophil cytoplasmic antibody (ANCA)-associated pauci-immune glomerulonephritis were included for gene expression measurement. For the immunofluorescence validation experiments, a total of 6 IgAN patients and 3 controls were included.
Methods
Total RNA was extracted from formalin-fixed paraffin-embedded kidney biopsy specimens, and a customized 48-plex human gene CodeSet was used to study 29 genes implicated in different biological pathways. Comparisons in gene expression were made between IgAN and ANCA-associated pauci-immune glomerulonephritis patients to delineate an expression profile specific to IgAN. Gene expression was compared between patients with low and moderate risk of progression. Genes for which RNA expression was associated with disease progression were analyzed for protein expression by immunofluorescence and compared with controls.
Results
IgAN patients had a distinct gene expression profile with decreased expression in genes IL-6, INFG, and C1QB compared to ANCA patients. C3 and TNFRSF1B were identified as potential biomarkers for IgAN progression in patients early in their disease course. Protein expression for those 2 candidate genes was upregulated in IgAN patients compared to controls. Expression of genes implicated in fibrosis (PTEN, CASPASE 3, TGM2, TGFB1, IL2, and TNFRSF1B) was more pronounced in IgAN patients with severe fibrosis compared to those with none.
Conclusions
Our findings validate our NanoString mRNA profiling by examining protein expression levels of two candidate genes, C3 and TNFRSF1B, in IgAN patients and controls. We also identified several upregulated mRNA transcripts implicated in the development of fibrosis that may be considered fibrotic markers within IgAN patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunoglobulin A nephropathy (IgAN) is a complicated autoimmune condition where immunoglobulin A (IgA) is deposited in the kidney’s glomeruli, causing inflammation and ultimately leading to kidney failure [1]. IgAN is a leading cause of primary glomerulonephritis and chronic kidney disease [2]. Several clinical factors have been identified over the last decades to help predict disease progression, such as sustained proteinuria over a gram a day, baseline estimated glomerular filtration rate (eGFR), high blood pressure, and histopathologic findings from the Oxford classification [3]. In IgAN, the search for inflammatory biomarkers that can be utilized to track therapy effectiveness and foretell disease development remains unfinished [4]. No one biomarker can give a comprehensive picture of IgAN, so several biomarkers may be required to diagnose and treat the disease correctly. Although a number of clinical indicators have been found to help predict disease progression, specific biomarkers involved in the underlying inflammatory processes are not integrated into any prediction tool. Therefore, further research is needed to validate the clinical utility of inflammatory biomarker panels in IgAN.
State-of-the-art platforms for gene expression analysis, such as the NanoString nCounter system, are promising screening and diagnostic tools, especially in oncology. This innovative process, which directly measures individual micro ribonucleic acid (mRNA) transcripts without enzymatic steps, stands out from previous methods by its high sensitivity, precision, and efficiency [5]. Specifically, the NanoString nCounter system uses a digital molecular barcoding technology, which allows for high-throughput and sensitive detection of specific RNA molecules in a sample without amplification. It is often used in research to study gene expression, biomarker discovery, and pathway analysis. Increasingly used in several clinical settings, this technology could help identify biomarkers playing a role in various inflammatory pathologic processes.
This pilot study successfully validates the application of NanoString technology in identifying potential inflammatory targets related to different stages of IgAN disease progression. Our results revealed a distinctive gene expression profile in IgAN patients. Notably, we identified C3 and TNFRSF1B as promising biomarkers for monitoring IgAN progression, with their protein expression being significantly upregulated in IgAN patients compared to controls. Additionally, our investigation uncovered heightened expression of genes associated with fibrosis in IgAN patients with severe fibrosis compared to those without fibrosis. This finding suggests the potential utility of these mRNA transcripts as fibrotic markers for IgAN patients. The successful validation of NanoString mRNA profiling and the identification of relevant inflammatory and fibrotic markers may pave the way for improved diagnostic and prognostic tools in IgAN, enhancing our understanding of the disease’s underlying inflammatory processes.
Methods
Study design and population
All patients with biopsy-proven IgAN and antineutrophil cytoplasmic antibody (ANCA)-associated pauci-immune glomerulonephritis between 2005 and 2021 diagnosed at Maisonneuve-Rosemont Hospital, a single tertiary care hospital in Montreal, were considered for this retrospective study. Only patients with formalin-fixed paraffin-embedded (FFPE) biopsy samples available for gene expression analysis were included. Patients with known secondary causes of IgAN were excluded. For the immunofluorescence experiments, controls with no glomerular disease (after pathology review) were recruited among patients who underwent a nephrectomy in the context of a kidney lesion. All subjects provided written informed consent. This study was approved by our institution’s Research Ethics Committee (No MP-12-2014-525) in agreement with the Declaration of Helsinki.
Data collection
Clinical data were collected by reviewing medical records, including demographics, kidney presentation, comorbidities, history of familial kidney disease, and medications. Specifically, serum creatinine, eGFR, and proteinuria were noted at the time of the biopsy, at 6 months from biopsy time and then annually to the last available follow-up visit and/or initiation of renal replacement therapy. The Oxford MEST-C score [6] was retrieved from the biopsy report. Baseline eGFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation [7]. Urinary protein excretion was estimated using either total 24-h proteinuria (in g/day), spot urinary protein–creatinine ratio (in mg/mmol), or spot urinary albumin–creatinine ratio (in mg/mmol). For IgAN, the risk of a 50% decline in eGFR or progression to end-stage kidney disease (ESKD) 5 years after kidney biopsy was calculated using the New International Risk-Prediction Tool in IgAN [8], a prediction model that includes baseline clinical and pathological characteristics, demographics, and medication use. The studied primary outcomes were ESKD (eGFR < 15 ml/min/1.73m2, dialysis or kidney transplantation) or death.
Gene expression measurement
For our NanoString analysis, a total of 30 biopsy-confirmed IgA and 7 ANCA-positive pauci-immune glomerulonephritis patients were used. Total RNA from FFPE kidney biopsy specimens from patients with IgAN and ANCA-positive pauci-immune glomerulonephritis (control group) were extracted and purified using RNeasy FFPE kit (Qiagen) according to the manufacturer’s protocol. A total of 100 nanograms of RNA from each sample was hybridized to a customized 48-plex human gene CodeSet and processed on the nCounter platform. Purified RNA samples were analyzed using the nCounter gene expression system from NanoString Technology. A total of 29 genes implicated in different biological pathways were studied; those genes have been selected for their role in complement activation (C2, C3, C1QA, C1QB), apoptosis (BAX, BAD, Caspase 3), cell activation, and/or leucocytes stimulation (AGTR1, AGTR2, CXCL1, CD89S, ICAM1, MIF, PTEN, CD71, TGM2, TNFR1B, TNFRSF1B, TNFSF13, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, IFN-γ, TGF-β1, TNF). Housekeeping gene (ACTB, GAPDH, HPRT1, LDHA) normalization was performed to adjust counts of all probes relative to a probe that is not expected to vary between samples; the factor should be in the range between 0.1 and 10. All normalization procedures were performed according to the manufacturer’s protocol.
Data collection for gene expression was carried out in the nCounter Digital Analyzer. At the highest standard resolution, 555–1155 fields of view were collected per flow cell using a microscope objective and a CCD camera, yielding data of hundreds of thousands of target molecule counts. Every RNA target is identified by the color code generated by the ordered fluorescent segments present on the reporter probe. The expression level of each gene is determined by scoring the number of times its corresponding color code is detected. Data were imported into nSolver Analysis Software for downstream analysis.
Comparisons in gene expression were made between IgAN patients and those with another inflammatory glomerulonephritis (ANCA-associated pauci-immune glomerulonephritis) to depict an expression profile specific to IgAN. Subsequently, among individuals with IgAN, gene expression was compared between patients with a low chance of progression to ESKD (< 10%) and those at mild to moderate risk of progression (10–20%) with relatively minimal fibrosis on kidney biopsy. Genes for which RNA expression was associated with disease progression were analyzed in 6 patients for protein expression by immunofluorescence and compared with controls.
Histology
The same nephropathologist reviewed histologic slides for all patients with IgAN to provide MEST-C score: mesangial hypercellularity (with M0 and M1 corresponding to ≤ 50% and > 50% of glomeruli with hypercellularity, respectively), segmental glomerulosclerosis (with S0 if absent and S1 if present), endocapillary hypercellularity (with E0 if absent and E1 if present), tubular atrophy/interstitial fibrosis (with T0, T1, and T2 corresponding to ≤ 25%, 26%–50%, and > 50% of cortical area involvement, respectively), and cellular/fibro cellular crescents (with C0, C1, and C2 corresponding to their absence, presence in ≥1 and < 25% of glomeruli, and presence in ≥25% of the glomeruli, respectively). ANCA-associated pauci-immune glomerulonephritis was also graded based on its degree of tubular atrophy/interstitial fibrosis.
Immunofluorescence
The kidneys were fixed in 10% formaldehyde, dehydrated, and embedded in raffin. The tissue was sectioned (5 µM) and was subjected to antigen retrieval in citrate solution at pH 6. The sections were blocked with anti-donkey serum 5% and labeled with anti-Complement C3 (Catalog #PA1-29715 from Life Technologies/ThermoFisher) and TNFRSF1B (Catalog #MA5-31661 from Life Technologies/ThermoFisher). The slides were subsequently exposed to donkey anti-rabbit AF647-conjugated (1:400, Catalog # 711 605 152 Jackson ImmunoResearch Laboratories), donkey anti-mouse AF568 (Catalog # A10037 Life Technologies/ThermoFisher), and donkey anti-goat AF488 (Catalog # A11055 Life Technologies/ThermoFisher). Fluoroshield with DAPI (Millipore-Sigma) was used for nuclear staining and mounting. Slides were imaged using a Zeiss AxioObserver.Z1 inverted microscope coupled to an X-Cite 120LED Boost High-Power LED illumination system. Images for quantitative analysis were captured with a 20X objective and the number of positive cells was determined as the average of positive cells in at least 8 fields per kidney section.
Statistical analysis
Baseline characteristics, clinical and pathological parameters, and gene expression were compared between patients with IgAN and those with ANCA-associated pauci-immune glomerulonephritis (controls). Among patients with IgAN, gene expression was compared between progression score groups (< 10%, 10–20%, 21–40%, and > 40%), and more specifically between patients with scores < 10% and those with 10–20% to delineate markers of early disease. Wilcoxon or t-tests for continuous variables and the χ2 test or the Fisher exact tests were used for categorical variables. The Kruskal–Wallis test was also used for comparison between more than two groups. Differences were considered statistically significant if p-value < 0.05. Statistical analysis was performed by N.E. using SAS 9.4 (SAS Institute, Cary, NC).
Results
Patient characteristics
Patients with IgAN with gene expression analyzed by NanoString were mostly Caucasians (76.7%) and females (73.3%), as shown in Table 1. Mean age at biopsy was 44.7 ± 15.3 years. Mean baseline eGFR and proteinuria were 60.9 ± 35.8 ml/min/1.73m2, and 3.2 ± 2.9 g/d respectively; most patients (96.5%) had a stage 0 or 1 of fibrosis at diagnosis. At baseline, 60% of patients had chronic kidney disease stage 3 or 4. Treatments were highly variable, but a significant proportion (80%) were on renin–angiotensin–aldosterone (RAA) system inhibitors, and 45% received glucocorticoids at some point in their disease course. Patients with ANCA-associated pauci-immune glomerulonephritis were all Caucasians (100%), with a majority of females (57.1%). Mean age at biopsy was 62.6 ± 9.4 years. At baseline, mean eGFR and proteinuria were 36.6 ± 33 ml/min/1.73m2 and 2.4 ± 2.2 g/d, respectively; all patients had stage 0 or 1 of fibrosis at diagnosis. Patients with ANCA-associated pauci-immune glomerulonephritis were significantly older and had more kidney impairment at baseline than patients with IgAN.
The 5-year risk of a 50% decline in eGFR or ESKD after biopsy was variable in IgAN patients: 9, 5, 8, and 6 patients (2 missing) had a 5-year risk of progression of < 10%, 10%–20%, 21%–40%, and > 40%, respectively. The median follow-up time for the IgAN group was 4.62 years (interquartile range 2.45 years). Among patients with IgAN, 8 patients (26.7%) reached ESKD and 3 (10%) died.
Gene expression profiles
IgAN patients had a different mRNA expression profile than patients with ANCA-associated pauci-immune glomerulonephritis. IL-6, INFG, and C1QB were significantly transcribed at higher levels in patients with ANCA-associated pauci-immune glomerulonephritis (Table 2). AGTR1 was more expressed in patients with IgAN compared to ANCA controls (p = 0.05).
The distribution of biomarkers in function of the IgA nephropathy prediction tool score range was analyzed. Markers known as CIQA, ICAM1, TNF, and TNFRSF1B did not show a statistically significant distribution, but their distribution difference was near the significance level (Table 3). As shown in Table 4, CIQB and TNFRSF1B expressions were higher among patients with an IgA prediction tool score range of 10–20% compared to a score < 10% (p-value = 0.01342 and p = 0.03337, respectively). C3 expression was also higher in patients with a score range of 10–20% (vs. with a score < 10%) with a p-value of 0.04911.
Immunofluorescence
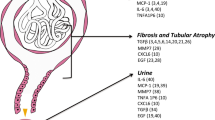
To ensure the accuracy and reliability of our findings obtained through NanoString mRNA profiling, we devised a validation strategy that involved the examination of protein expression levels of candidate genes. To conduct our protein expression analyses, we selected Complement C3 as a marker of mild to moderate risk of ESKD progression with relatively minimal fibrosis on kidney biopsy, and TNFRSF1B to serve as a marker expressed during stage 2 fibrosis. To this end, we obtained biopsies from patients diagnosed with IgA nephropathy that had high degree of fibrosis compared to the controls (Fig. 1A). Our findings revealed that both target genes identified in gene expression analysis (C3 and TNFRSF1B) exhibited a significant upregulation of protein expression in the biopsies obtained from patients with IgA nephropathy when compared to control tissue (Fig. 1B). Importantly, the expression patterns of Complement C3 and TNFRSF1B were distinct, further underscoring the significance of our observations. Complement C3 was found to be distributed around the tubular epithelial cells and glomerulus, with a modest signal, suggesting a possible role in the regulation of inflammation and immune responses in such regions. On the other hand, the signal for TNFRSF1B was more pronounced and concentrated in distinct regions that differed from those observed for Complement C3, highlighting its potential role in cellular proliferation and differentiation. Overall, our findings validate our NanoString mRNA profiling by examining protein expression levels of two candidate genes, C3 and TNFRSF1B, in IgAN patients and controls.
Kidney biopsies from IgA patients have increased fibrosis and immune markers. A Light microscopy of kidney biopsies stained for H&E shows increased fibrosis in IgA nephropathy patients compared to controls. B Immunofluorescence staining for Complement C3 and TNFRSF1B in IgA nephropathy patients compared to controls. Fold change of immunofluorescence, as represented in a bar graph, was performed using n = 6 IgA patients and n = 3 controls. *P < 0.05 determined by t-test. Scale bars: 100 μm
NanoString identified mRNA expressed targets as predictors of fibrosis
Fibroblasts are responsible for producing and depositing extracellular matrix components and may be stimulated by persistent inflammation and immunological responses, such as those during IgAN. The excessive production and accumulation of collagen and other matrix proteins lead to scar tissue formation, known as fibrosis. Therefore, we analyzed our IgAN patient samples for fibrosis formation and compared the severity of fibrosis with the NanoString mRNA transcript expression of our targets. From our histological slides, we observed fibrosis formation in IgAN patients that resulted in structural changes within the kidney, including thickening of the glomerular basement membrane, obliteration of the glomerular capillaries, and an increase in the volume of the interstitial compartment (Fig. 1A). From our NanoString mRNA expression profiles, we observed a significant increase in PTEN, CASPASE 3, TGM2, TGFB1, IL2, and TNFRSF1B from IgAN patients with level 2 fibrosis compared to those with none (Table 5). To gain insights into such significantly upregulated mRNA from IgA fibrotic patients, we compiled them into the STRING platform (https://string-db.org/) to analyze any potential biological processes and interactions in the form of an interaction network. Set at a high confidence of 0.7, our identified “fibrotic” genes did not show any strong correlation among themselves. However, when adding 5 more relevant nodes to the network, IL2RB, SLC9A3R1, TP53, TGFBR2, and XIAP, we were able to establish a more complete network of associating genes, with a total of 11 nodes and 14 edges (Fig. 2). The protein–protein interaction networks (PPI) enrichment p-value of 0.0393 indicates that the nodes are not random and that the observed number of edges is significant. When studying the various descriptions in the “Biology Process” category, we identified that such genes are primarily involved in apoptotic pathways, regulation of apoptosis, wound healing, response to growth factors, and regulation of lymphocyte activation, while the meaningful “Pathways” included Th17 cell differentiation, cellular senescence, MAPK signaling pathway, and PI3K-Akt signaling pathway. Therefore, our study identified several upregulated mRNA transcripts that were upregulated during the development of fibrosis and may be considered fibrotic markers within IgAN patients.
Interaction analysis of differentially expressed genes from kidneys of IgA patients. Using the STRING database, we determined the predicted interactome, biological processes (left), and signaling pathways (right) from significantly upregulated genes within IgA kidney biopsies compared to controls. Color codes of nodes are based on the annotation term for each “Biological Function” or “KEGG Pathway,” and interaction edges are based on a minimal confidence of 0.7 using sources of text mining, experiments, databases, and co-expressions
Discussion
Our pilot study aimed to validate the use of the NanoString technology in identifying potential inflammatory targets at different stages of IgAN disease progression. IgAN patients appear to have a distinct inflammatory profile from other inflammatory glomerulonephritis. We were able to identify C3 and TNFRSF1B as potential biomarkers of early inflammation in patients with a moderately increased risk of disease progression. When compared to controls and using an immunofluorescence technique, protein expression for those two markers was more pronounced, with distinct signal patterns. Those biomarkers might be implicated in the development of fibrosis when studying potential biological processes and interactions in the form of an interaction network.
Interestingly, the tumor necrosis factor (TNF) receptor type 2 (TNFR2, also known as TNFRSF1B) is expressed both on activated effector T cells and regulatory cells, such as regulatory T cells (Tregs) and myeloid-derived suppressor cells. As such, it has contradictory pro-inflammation and anti-inflammation properties. Indeed, TNFRSF1B deficiency could, in some cases, worsen autoimmune diseases, and TNFRSF1B agonists might have opposite effects (reviewed in [9]). It has been suggested that patients with IgAN have a decreased Treg number [10,11,12] and suppressive potential [13]. However, we do not know if the TNFRSF1B expression we detected in the kidney was on Tregs, activated conventional T cells, or other cell types. In an epigenome-wide association study of the Framingham’s cohort, soluble TNFRSF1B (sTNFRSF1B) levels in the blood were associated with differentially methylated loci in active regulatory regions of genes previously identified in GWAS studies of IgAN patients, suggesting a possible role in the disease [14]. TNFRSF1B mRNA and protein levels is also elevated in IgAN patients [15], correlates with progression [16, 17], and could decrease with steroids [18]. However, the TNFRSF1B expression in the kidney has never been studied and does not appear to be expressed in IgA-dominant infection-related glomerulonephritis [19]. Complement C3 has a crucial role in IgAN, colocalizing with IgA in over 90% of patients [20]. The direct activation of C3 by IgA1-containing complexes forms a vital aspect of the immune pathogenesis in IgAN [21]. This observation aligns with and reinforces our study findings. Furthermore, our ICC co-staining of C3 and TNFRSF1B with fibrosis implies that the early RNA expression of C3 could serve as a prognostic marker of fibrosis, while TNFRSF1B RNA expression associates with the onset of fibrosis.
Fibrosis is a common phenomenon of IgAN, which we confirmed within our study using Jones’ staining. In order to find an association of our significantly upregulated genes with the onset of fibrosis, we compared the expression of such genes between IgAN patients with grade 2 fibrosis to those without fibrosis. As a result, we identified 6 genes that were significantly associated with fibrosis: PTEN, CASPASE 3, TGM2, TGFB1, IL2, and TNFRSF1B. Interestingly, such genes were predicted to be associated with the biological functions of wound healing, apoptosis, cellular response to growth factors, and regulation of immune systems.
We also predicted various relevant pathways belonging to our IgA nephropathic genes that are significantly upregulated during fibrotic tissue formation. These include Th17 cell differentiation, cellular senescence, and the MAPK and PI3K-Akt signaling pathways. It has been shown that the abnormal humoral immunity observed in IgAN may be mediated by the differentiation of T helper 17 cells [22] and kidney senescence is well documented to occur after injury [23]. It has been observed that Akt activation is increased in experimental tubulointerstitial fibrosis [24, 25]. Akt activation serves as a crucial component in multiple signaling pathways associated with kidney damage. Consequently, there is a belief that Akt plays a significant role in renal fibrosis. The MAPK pathway plays a pivotal role in regulating inflammatory and fibrotic responses within the kidney [26, 27]. Notably, one significant outcome of MAPK pathway activation is the induction of extracellular matrix protein synthesis, particularly collagen. The excessive deposition of collagen and other extracellular matrix components contributes to the progressive accumulation of fibrotic tissue in the kidney. Thus, the MAPK pathway serves as a key mediator in the development and progression of renal fibrosis by modulating various cellular and molecular events involved in fibrotic remodeling. These findings support the potential involvement of these pathways in driving the fibrotic process and provide valuable directions for further exploration.
Unfortunately, we failed to identify some biomarkers associated with a higher score at the IgA Nephropathy Prediction tool. Some biomarkers, known as C1QA, ICAM1, TNF, and TNFRSF1B, showed a distribution in function of the IgA prediction tool score range near a statistically significant level. Both ICAM1 and TNF were close to being associated with a score range of over 20% at the IgAN prediction score. High levels of ICAM-1 mRNA were associated with advanced histologic changes and lower kidney function in previous studies on IgAN [28]. TNF-alpha is also suspected to play an essential part in the pathogenesis of IgAN. Previous studies established a relation between high serum levels of TNF-a in patients with IgAN and MEST-C scores, higher proteinuria, and lower kidney function [29].
Our present study has some limitations. First, the small number of cases reduced the power of our study and reduced the chances of finding statistically significant differences in the distribution of the biomarkers analysis with the NanoString technology for the clinically relevant endpoints we tried to focus on. However, the purpose of the study was not to elaborate a prediction model using inflammatory biomarkers but to validate NanoString technology as a potential tool to delineate inflammatory processes in IgAN patients with ongoing kidney injury. Another limitation of our study is the limited number of candidate genes examined, mainly because of technical reasons. Nonetheless, we were able to focus on the main targets already described in the literature as involved in IgAN physiopathology.
In conclusion, our study validates the use of the NanoString technology as a promising tool for gene expression analysis in IgAN. Utilizing this innovative platform, we could simultaneously study multiple inflammatory biomarkers involved in IgAN and identify their activation patterns. We also validated the activation of genes known to contribute to kidney fibrosis, shedding light on the underlying mechanisms of disease progression. Although these findings confirm the usefulness of gene expression profiles in providing valuable tools for diagnosis and monitoring IgAN, further research is necessary to elucidate the clinical utility of gene expression analysis and its integration into predictive models and treatment strategies for IgAN. Further exploration of gene expression profiles holds promise for a better comprehension of disease and the development of targeted therapies to mitigate kidney fibrosis in IgAN patients.
Data availability
For access to original deidentified data, please contact louisphilippe. laurin@umontreal.ca.
References
Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402–14.
McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26:414–30.
Pattrapornpisut P, Avila-Casado C, Reich HN. IgA nephropathy: core curriculum 2021. Am J Kidney Dis. 2021;78:429–41.
Khalili M, Bonnefoy A, Genest DS, Quadri J, Rioux JP, Troyanov S. Clinical use of complement, inflammation, and fibrosis biomarkers in autoimmune glomerulonephritis. Kidney Int Rep. 2020;5:1690–9.
Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–25.
Haas M, Verhave JC, Liu ZH, Alpers CE, Barratt J, Becker JU, et al. A multicenter study of the predictive value of crescents in IgA nephropathy. J Am Soc Nephrol. 2017;28:691–701.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Barbour SJ, Coppo R, Zhang H, Liu ZH, Suzuki Y, Matsuzaki K, et al. Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Int Med. 2019;179:942–52.
Chen H, Kleinberger JW, Takane KK, Salim F, Fiaschi-Taesch N, Pappas K, et al. Augmented stat5 signaling bypasses multiple impediments to lactogen-mediated proliferation in human β-cells. Diabetes. 2015;64:3784–97.
Lin FJ, Jiang GR, Shan JP, Zhu C, Zou J, Wu XR. Imbalance of regulatory T cells to Th17 cells in IgA nephropathy. Scand J Clin Lab Invest. 2012;72:221–9.
Yang S, Chen B, Shi J, Chen F, Zhang J, Sun Z. Analysis of regulatory T cell subsets in the peripheral blood of immunoglobulin A nephropathy (IgAN) patients. Genet Mol Res. 2015;14:14088–92.
Jin L-W, Ye H-Y, Xu X-Y, Zheng Y, Chen Y. MiR-133a/133b inhibits Treg differentiation in IgA nephropathy through targeting FOXP3. Biomed Pharmacother. 2018;101:195–200.
Huang H, Peng Y, Long X-D, Liu Z, Wen X, Jia M, et al. Tonsillar CD4+ CD25+ regulatory T cells from IgA nephropathy patients have decreased immunosuppressive activity in experimental IgA nephropathy rats. Am J Nephrol. 2013;37:472–80.
Mendelson MM, Johannes R, Liu C, Huan T, Yao C, Miao X, et al. Epigenome-wide association study of soluble tumor necrosis factor receptor 2 levels in the framingham heart study. Front Pharmacol. 2018;9:207.
Miedziaszczyk M, Oko A, Wolc A, Woźniak A, Idasiak-Piechocka I. Assessment of serum concentration and urinary excretion of tumor necrosis factor receptor 1 and 2 and their potential as markers of immunoglobulin A nephropathy activity. Adv Clin Exp Med. 2023. https://doi.org/10.17219/acem/171000.
Oh YJ, An JN, Kim CT, Yang SH, Lee H, Kim DK, et al. Circulating tumor necrosis factor α receptors predict the outcomes of human IgA nephropathy: a prospective cohort study. PLoS ONE. 2015;10:e0132826.
Sonoda Y, Gohda T, Suzuki Y, Omote K, Ishizaka M, Matsuoka J, et al. Circulating TNF receptors 1 and 2 are associated with the severity of renal interstitial fibrosis in IgA nephropathy. PLoS ONE. 2015;10:e0122212.
Murakoshi M, Gohda T, Sonoda Y, Suzuki H, Tomino Y, Horikoshi S, et al. Effect of tonsillectomy with steroid pulse therapy on circulating tumor necrosis factor receptors 1 and 2 in IgA nephropathy. Clin Exp Nephrol. 2017;21:1068–74.
Kung VL, Avasare R, Friedman MA, Koon SM, Neff TL, Protzek S, et al. Targeted transcriptional analysis of IgA vasculitis, IgA nephropathy, and IgA-dominant infection-related glomerulonephritis reveals both distinct and overlapping immune signatures. Kidney360. 2023;4:759–68.
Duval A, Caillard S, Fremeaux-Bacchi V. The complement system in IgAN: mechanistic context for therapeutic opportunities. Nephrol Dial Transplant. 2023;38:2685–93.
Maillard N, Wyatt RJ, Julian BA, Kiryluk K, Gharavi A, Fremeaux-Bacchi V, et al. Current understanding of the role of complement in IgA nephropathy. J Am Soc Nephrol. 2015;26:1503–12.
Qing J, Li C, Hu X, Song W, Tirichen H, Yaigoub H, et al. Differentiation of T Helper 17 cells may mediate the abnormal humoral immunity in iga nephropathy and inflammatory bowel disease based on shared genetic effects. Front Immunol. 2022;13: 916934.
Huang W, Hickson LJ, Eirin A, Kirkland JL, Lerman LO. Cellular senescence: the good, the bad and the unknown. Nat Rev Nephrol. 2022;18:611–27.
Lan A, Du J. Potential role of Akt signaling in chronic kidney disease. Nephrol Dial Transplant. 2015;30:385–94.
Kim IY, Park YK, Song SH, Seong EY, Lee DW, Bae SS, et al. Role of Akt1 in renal fibrosis and tubular dedifferentiation during the progression of acute kidney injury to chronic kidney disease. Korean J Intern Med. 2021;36:962–74.
Chen KH, Hsu HH, Yang HY, Tian YC, Ko YC, Yang CW, et al. Inhibition of spleen tyrosine kinase (syk) suppresses renal fibrosis through anti-inflammatory effects and down regulation of the MAPK-p38 pathway. Int J Biochem Cell Biol. 2016;74:135–44.
Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124:2299–306.
Arrizabalaga P, Sole M, Abellana R, de las Cuevas X, Soler J, Pascual J, et al. Tubular and interstitial expression of ICAM-1 as a marker of renal injury in IgA nephropathy. Am J Nephrol. 2003;23:121–8.
Li G, Wu W, Zhang X, Huang Y, Wen Y, Li X, et al. Serum levels of tumor necrosis factor alpha in patients with IgA nephropathy are closely associated with disease severity. BMC Nephrol. 2018;19:326.
Acknowledgements
The authors wish to thank Dr. Virginie Royal for providing the light microscopy biopsy images.
Funding
Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Contributions
Study design and conduct: L.P.L., Data collection: L.G., C.L., S.B., and N.H.. Data analysis and interpretation: C.L., N.E., C.G., and L.P.L. Drafting manuscript: C.L., C.G., and L.P.L. Revising manuscript content and approving the final version of manuscript: All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gaumond, L., Lamarche, C., Beauchemin, S. et al. Identification of inflammatory biomarkers in IgA nephropathy using the NanoString technology: a validation study in Caucasians. Inflamm. Res. 73, 447–457 (2024). https://doi.org/10.1007/s00011-023-01848-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01848-3