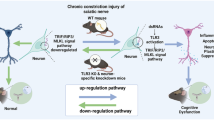
Abstract
Objective
Cognitive dysfunction is a common comorbidity in patients with chronic pain. Activation of Liver X receptors (LXRs) plays a potential role in improving cognitive disorders in central nervous diseases. In this study, we investigated the role of LXRs in cognitive deficits induced by neuropathic pain.
Methods
We established the spared nerve injury (SNI) model to investigate pain-induced memory dysfunction. Pharmacological activation of LXRs with T0901317 or inhibition with GSK2033 was applied. PI3K inhibitor LY294002 was administered to explore the underlying mechanism of LXRs. Changes in neuroinflammation, microglia polarization, and synaptic plasticity were assessed using biochemical technologies.
Results
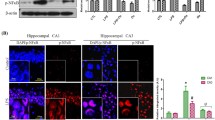
We found that SNI-induced cognitive impairment was associated with reduced LXRβ expression, increased M1-phenotype microglia, decreased synaptic proteins, and inhibition of PI3K/AKT signaling pathway in the hippocampus. Activation of LXRs using T0901317 effectively alleviated SNI-induced cognitive impairment. Additionally, T0901317 promoted the polarization of microglia from M1 to M2, reduced pro-inflammatory cytokines, and upregulated synaptic proteins in the hippocampus. However, administration of GSK2033 or LY294002 abolished these protective effects of T0901317 in SNI mice.
Conclusions
LXRs activation alleviates neuropathic pain-induced cognitive impairment by modulating microglia polarization, neuroinflammation, and synaptic plasticity, at least partly via activation of PI3K/AKT signaling in the hippocampus. LXRs may be promising targets for addressing pain-related cognitive deficits.











Similar content being viewed by others
Data availability
All data relevant to the study are included in the article or uploaded as supplementary information. Data is available upon request.
References
Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. 2019;123:e273–83.
Phelps CE, Navratilova E, Porreca F. Cognition in the chronic pain experience: preclinical insights. Trends Cogn Sci. 2021;25:365–76.
Zhao W, Zhao L, Chang X, Lu X, Tu Y. Elevated dementia risk, cognitive decline, and hippocampal atrophy in multisite chronic pain. Proc Natl Acad Sci USA. 2023;120:e2215192120.
Low LA. The impact of pain upon cognition: what have rodent studies told us? Pain. 2013;154:2603–5.
Fiore NT, Austin PJ. Are the emergence of affective disturbances in neuropathic pain states contingent on supraspinal neuroinflammation? Brain Behav Immun. 2016;56:397–411.
Ren W-J, Liu Y, Zhou L-J, Li W, Zhong Y, Pang R-P, et al. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents. Neuropsychopharmacology. 2011;36:979–92.
Gui W-S, Wei X, Mai C-L, Murugan M, Wu L-J, Xin W-J, et al. Interleukin-1β overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol Pain. 2016;12:174480691664678.
Saffarpour S, Janzadeh A, Rahimi B, Ramezani F, Nasirinezhad F. Chronic nanocurcumin treatment ameliorates pain-related behavior, improves spatial memory, and reduces hippocampal levels of IL-1β and TNFα in the chronic constriction injury model of neuropathic pain. Psychopharmacology. 2021;238:877–86.
Vasic V, Schmidt M. Resilience and vulnerability to pain and inflammation in the hippocampus. IJMS. 2017;18:739.
Liu Y, Zhou L-J, Wang J, Li D, Ren W-J, Peng J, et al. TNF-α differentially regulates synaptic plasticity in the hippocampus and spinal cord by microglia-dependent mechanisms after peripheral nerve injury. J Neurosci. 2017;37:871–81.
Wang Y, Leak RK, Cao G. Microglia-mediated neuroinflammation and neuroplasticity after stroke. Front Cell Neurosci. 2022;16:980722.
Cornell J, Salinas S, Huang H-Y, Zhou M. Microglia regulation of synaptic plasticity and learning and memory. Neural Regen Res. 2021;17:705–16.
Lecca D, Jung YJ, Scerba MT, Hwang I, Kim YK, Kim S, et al. Role of chronic neuroinflammation in neuroplasticity and cognitive function: a hypothesis. Alzheimers Dement. 2022;18:2327–40.
Hisaoka-Nakashima K, Ohata K, Yoshimoto N, Tokuda S, Yoshii N, Nakamura Y, et al. High-mobility group box 1-mediated hippocampal microglial activation induces cognitive impairment in mice with neuropathic pain. Exp Neurol. 2022;355:114146.
Guida F, Iannotta M, Misso G, Ricciardi F, Boccella S, Tirino V, et al. Long-term neuropathic pain behaviors correlate with synaptic plasticity and limbic circuit alteration: a comparative observational study in mice. Pain. 2022;163:1590–602.
Tyrtyshnaia A, Manzhulo I. Neuropathic pain causes memory deficits and dendrite tree morphology changes in mouse hippocampus. J Pain Res. 2020;13:345–54.
Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, et al. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012;32:5747–56.
Tyrtyshnaia A, Bondar A, Konovalova S, Manzhulo I. Synaptamide improves cognitive functions and neuronal plasticity in neuropathic pain. Int J Mol Sci. 2021;22:12779.
Moutinho M, Codocedo JF, Puntambekar SS, Landreth GE. Nuclear receptors as therapeutic targets for neurodegenerative diseases: lost in translation. Annu Rev Pharmacol Toxicol. 2019;59:237–61.
Fitz NF, Nam KN, Koldamova R, Lefterov I. Therapeutic targeting of nuclear receptors, liver X and retinoid X receptors, for Alzheimer’s disease. Br J Pharmacol. 2019;176:3599–610.
Xu P, Li D, Tang X, Bao X, Huang J, Tang Y, et al. LXR agonists: new potential therapeutic drug for neurodegenerative diseases. Mol Neurobiol. 2013;48:715–28.
Wang B, Tontonoz P. Liver X receptors in lipid signalling and membrane homeostasis. Nat Rev Endocrinol. 2018;14:452–63.
Loane DJ, Washington PM, Vardanian L, Pocivavsek A, Hoe H-S, Duff KE, et al. Modulation of ABCA1 by an LXR agonist reduces β-amyloid levels and improves outcome after traumatic brain injury. J Neurotrauma. 2011;28:225–36.
Dai J, Xu S, Okada T, Liu Y, Zuo G, Tang J, et al. T0901317, an agonist of liver X receptors, attenuates neuronal apoptosis in early brain injury after subarachnoid hemorrhage in rats via liver X receptors/interferon regulatory factor/P53 upregulated modulator of apoptosis/dynamin-1-like protein pathway. Gebicki J, editor. Oxidat Med Cell Long. 2021;2021:1–16.
Repa JJ, Li H, Frank-Cannon TC, Valasek MA, Turley SD, Tansey MG, et al. Liver X receptor activation enhances cholesterol loss from the brain, decreases neuroinflammation, and increases survival of the NPC1 mouse. J Neurosci. 2007;27:14470–80.
Qiu C, Wang M, Yu W, Rong Z, Zheng H-S, Sun T, et al. Activation of the hippocampal LXRβ improves sleep-deprived cognitive impairment by inhibiting neuroinflammation. Mol Neurobiol. 2021;58:5272–88.
Xu X, Xiao X, Yan Y, Zhang T. Activation of liver X receptors prevents emotional and cognitive dysfunction by suppressing microglial M1-polarization and restoring synaptic plasticity in the hippocampus of mice. Brain Behav Immun. 2021;94:111–24.
Sun T, Li YJ, Tian QQ, Wu Q, Feng D, Xue Z, et al. Activation of liver X receptor beta-enhancing neurogenesis ameliorates cognitive impairment induced by chronic cerebral hypoperfusion. Exp Neurol. 2018;304:21–9.
Chen J, Zacharek A, Cui X, Shehadah A, Jiang H, Roberts C, et al. Treatment of stroke with a synthetic liver X receptor agonist, TO901317, promotes synaptic plasticity and axonal regeneration in mice. J Cereb Blood Flow Metab. 2010;30:102–9.
Cianciulli A, Porro C, Calvello R, Trotta T, Lofrumento DD, Panaro MA. Microglia mediated neuroinflammation: focus on PI3K modulation. Biomolecules. 2020;10:137.
Wang Y, Lin Y, Wang L, Zhan H, Luo X, Zeng Y, et al. TREM2 ameliorates neuroinflammatory response and cognitive impairment via PI3K/AKT/FoxO3a signaling pathway in Alzheimer’s disease mice. Aging (Albany). 2020;12:20862–79.
Han X, Cheng X, Xu J, Liu Y, Zhou J, Jiang L, et al. Activation of TREM2 attenuates neuroinflammation via PI3K/Akt signaling pathway to improve postoperative cognitive dysfunction in mice. Neuropharmacology. 2022;219:109231.
Chen L, Song D, Chen B, Yang X, Cheng O. Activation of liver X receptor promotes hippocampal neurogenesis and improves long-term cognitive function recovery in acute cerebral ischemia-reperfusion mice. J Neurochem. 2020;154:205–17.
Lu L, Liu X, Fu J, Liang J, Hou Y, Dou H. sTREM-1 promotes the phagocytic function of microglia to induce hippocampus damage via the PI3K–AKT signaling pathway. Sci Rep. 2022;12:7047.
Zhang W, Xiong BR, Zhang LQ, Huang X, Zhou WC, Zou Q, et al. Disruption of the GABAergic system contributes to the development of perioperative neurocognitive disorders after anesthesia and surgery in aged mice. CNS Neurosci Ther. 2020;26:913–24.
Xiao JY, Xiong BR, Zhang W, Zhou WC, Yang H, Gao F, et al. PGE2-EP3 signaling exacerbates hippocampus-dependent cognitive impairment after laparotomy by reducing expression levels of hippocampal synaptic plasticity-related proteins in aged mice. CNS Neurosci Ther. 2018;24:917–29.
Zhang L-Q, Gao S-J, Sun J, Li D-Y, Wu J-Y, Song F-H, et al. DKK3 ameliorates neuropathic pain via inhibiting ASK-1/JNK/p-38-mediated microglia polarization and neuroinflammation. J Neuroinflammation. 2022;19:129.
Wu H, Zheng J, Xu S, Fang Y, Wu Y, Zeng J, et al. Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. J Neuroinflammation. 2021;18:2.
Yuan X, Han S, Manyande A, Gao F, Wang J, Zhang W, et al. Spinal voltage-gated potassium channel Kv1.3 contributes to neuropathic pain via the promotion of microglial M1 polarization and activation of the NLRP3 inflammasome. Eur J Pain. 2023;27:289–302.
Zhang LQ, Zhang W, Li T, Yang T, Yuan X, Zhou Y, et al. GLP-1R activation ameliorated novel-object recognition memory dysfunction via regulating hippocampal AMPK/NF-kappaB pathway in neuropathic pain mice. Neurobiol Learn Mem. 2021;182:107463.
Liu M-G, Chen J. Preclinical research on pain comorbidity with affective disorders and cognitive deficits: challenges and perspectives. Prog Neurobiol. 2014;116:13–32.
Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93:385–404.
McCarberg B, Peppin J. Pain pathways and nervous system plasticity: learning and memory in pain. Pain Med. 2019;20:2421–37.
Woodburn SC, Bollinger JL, Wohleb ES. The semantics of microglia activation: neuroinflammation, homeostasis, and stress. J Neuroinflammation. 2021;18:258.
Liu X-G. Normalization of neuroinflammation: a new strategy for treatment of persistent pain and memory/emotional deficits in chronic pain. JIR. 2022;15:5201–33.
Torta R, Ieraci V, Zizzi F. A review of the emotional aspects of neuropathic pain: from comorbidity to co-pathogenesis. Pain Ther. 2017;6:11–7.
Austin PJ, Fiore NT. Supraspinal neuroimmune crosstalk in chronic pain states. Curr Opin Physio. 2019;11:7–15.
Brooks TA, Hawkins BT, Huber JD, Egleton RD, Davis TP. Chronic inflammatory pain leads to increased blood–brain barrier permeability and tight junction protein alterations. Am J Physiol Heart Circul Physiol. 2005;289:H738–43.
Mai CL, Tan Z, Xu YN, Zhang JJ, Huang ZH, Wang D, et al. CXCL12-mediated monocyte transmigration into brain perivascular space leads to neuroinflammation and memory deficit in neuropathic pain. Theranostics. 2021;11:1059–78.
Ding X, Gao X, Wang Z, Jiang X, Lu S, Xu J, et al. Preoperative chronic and acute pain affects postoperative cognitive function mediated by neurotransmitters. J Mol Neurosci. 2021;71:515–26.
Hisaoka-Nakashima K, Moriwaki K, Yoshimoto N, Yoshii T, Nakamura Y, Ago Y, et al. Anti-interleukin-6 receptor antibody improves allodynia and cognitive impairment in mice with neuropathic pain following partial sciatic nerve ligation. Int Immunopharmacol. 2022;112:109219.
del Rey A, Yau H-J, Randolf A, Centeno MV, Wildmann J, Martina M, et al. Chronic neuropathic pain-like behavior correlates with IL-1β expression and disrupts cytokine interactions in the hippocampus. Pain. 2011;152:2827–35.
Wang X, Jiang Y, Li J, Wang Y, Tian Y, Guo Q, et al. DUSP1 promotes microglial polarization toward M2 phenotype in the medial prefrontal cortex of neuropathic pain rats via inhibition of MAPK pathway. ACS Chem Neurosci. 2021;12:966–78.
Kwon HS, Koh S-H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020;9:42.
Ransohoff RM. A polarizing question: Do M1 and M2 microglia exist? Nat Neurosci. 2016;19:987–91.
Tampellini D, Capetillo-Zarate E, Dumont M, Huang Z, Yu F, Lin MT, et al. Effects of synaptic modulation on β-amyloid, synaptophysin, and memory performance in Alzheimer’s disease transgenic mice. J Neurosci. 2010;30:14299–304.
Wang J, Chen H-S, Li H-H, Wang H-J, Zou R-S, Lu X-J, et al. Microglia-dependent excessive synaptic pruning leads to cortical underconnectivity and behavioral abnormality following chronic social defeat stress in mice. Brain Behav Immun. 2023;109:23–36.
Xiong B, Zhang W, Zhang L, Huang X, Zhou W, Zou Q, et al. Hippocampal glutamatergic synapses impairment mediated novel-object recognition dysfunction in rats with neuropathic pain. Pain. 2020;161:1824–36.
Zhai Q, Zhang Y, Ye M, Zhu S, Sun J, Wang Y, et al. Reducing complement activation during sleep deprivation yields cognitive improvement by dexmedetomidine. Br J Anaesth. 2023;131:542–55.
Xu F, Han L, Wang Y, Deng D, Ding Y, Zhao S, et al. Prolonged anesthesia induces neuroinflammation and complement-mediated microglial synaptic elimination involved in neurocognitive dysfunction and anxiety-like behaviors. BMC Med. 2023;21:7.
Egorova E, Starinets A, Tyrtyshnaia A, Ponomarenko A, Manzhulo I. Hippocampal neurogenesis in conditions of chronic stress induced by sciatic nerve injury in the rat. Cells Tissues Organs. 2019;207:58–68.
Fiore NT, Austin PJ. Glial-cytokine-neuronal adaptations in the ventral hippocampus of rats with affective behavioral changes following peripheral nerve injury. Neuroscience. 2018;390:119–40.
Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–7.
Zhang R, Dong Y, Liu Y, Moezzi D, Ghorbani S, Mirzaei R, et al. Enhanced liver X receptor signalling reduces brain injury and promotes tissue regeneration following experimental intracerebral haemorrhage: roles of microglia/macrophages. Stroke Vasc Neurol. 2023;2023:1.
Pfrieger FW. Role of cholesterol in synapse formation and function. Biochim Biophys Acta. 2003;1610:271–80.
Li YJ, Zhang K, Sun T, Wang J, Guo YY, Yang L, et al. Epigenetic suppression of liver X receptor beta in anterior cingulate cortex by HDAC5 drives CFA-induced chronic inflammatory pain. J Neuroinflammation. 2019;16:132.
Li X, Huang X, Feng Y, Wang Y, Guan J, Deng B, et al. Cylindrin from Imperata cylindrica inhibits M2 macrophage formation and attenuates renal fibrosis by downregulating the LXR-α/PI3K/AKT pathway. Eur J Pharmacol. 2023;950:175771.
Peng J, Pang J, Huang L, Enkhjargal B, Zhang T, Mo J, et al. LRP1 activation attenuates white matter injury by modulating microglial polarization through Shc1/PI3K/Akt pathway after subarachnoid hemorrhage in rats. Redox Biol. 2019;21:101121.
Wang G, Shi Y, Jiang X, Leak RK, Hu X, Wu Y, et al. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis. Proc Natl Acad Sci USA. 2015;112:2853–8.
Lee C-C, Huang C-C, Hsu K-S. Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology. 2011;61:867–79.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81974170) and the Natural Science Foundation of Hubei Province (2021CFB341).
Author information
Authors and Affiliations
Contributions
ZW, TXB, and HSY worked on the experiment design. HSY, YXM, and ZFT conducted behavioral tests and biochemical experiments. HSY, YXM, ZFT, and ZW worked on data collection/analysis. The manuscript was drafted by HSY, ZW, and TXB. AM, GF, and WJ participated in the revision of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: Thiago Cunha.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, S., Yuan, X., Zhao, F. et al. Activation of LXRs alleviates neuropathic pain-induced cognitive dysfunction by modulation of microglia polarization and synaptic plasticity via PI3K/AKT pathway. Inflamm. Res. 73, 157–174 (2024). https://doi.org/10.1007/s00011-023-01826-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01826-9