Abstract
Purpose
The purpose of this review is to discuss the role of γδ T cells played in humoral immune responses.
Background
The γδ T cell receptor (γδ TCR) recognizes antigens, including haptens and proteins, in an MHC-independent manner. The recognition of these antigens by γδ TCRs crosses antigen recognition by the B cell receptors (BCRs), suggesting that γδ T cells may be involved in the process of antigen recognition and activation of B cells. However, the role of γδ T cells in humoral immune responses is still less clear.
Methods
The kinds of literature about the γδ T cell-B cell interaction were searched on PubMed with search terms, such as γδ T cells, antibody, B cell responses, antigen recognition, and infection.
Results
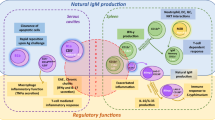
Accumulating evidence indicates that γδ T cells, independent of αβ T cells, participate in multiple steps of humoral immunity, including B cell maturation, activation and differentiation, antibody production and class switching. Mechanically, γδ T cells affect B cell function by directly interacting with B cells, secreting cytokines, or modulating αβ T cells.
Conclusion
In this review, we summarize current knowledge on how γδ T cells take part in the humoral immune response, which may assist future vaccine design.

Similar content being viewed by others
Data availability
The datasets generated during this review are available from the corresponding author upon reasonable request.
References
Craig NL. V(D)J recombination and transposition: closer than expected. Science. 1996;271:1512. https://doi.org/10.1126/science.271.5255.1512.
Papavasiliou F, Casellas R, Suh H, Qin XF, Besmer E, Pelanda R, Nemazee D, Rajewsky K, Nussenzweig MC. V(D)J recombination in mature B cells: a mechanism for altering antibody responses. Science. 1997;278:298–301. https://doi.org/10.1126/science.278.5336.298.
Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994;179:323–8. https://doi.org/10.1084/jem.179.1.323.
Chien YH, Konigshofer Y. Antigen recognition by gammadelta T cells. Immunol Rev. 2007;215:46–58. https://doi.org/10.1111/j.1600-065X.2006.00470.x.
Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, Chien YH. Antigen recognition determinants of gammadelta T cell receptors. Science. 2005;308:252–5. https://doi.org/10.1126/science.1106480.
Selin LK, Santolucito PA, Pinto AK, Szomolanyi-Tsuda E, Welsh RM. Innate immunity to viruses: control of vaccinia virus infection by gamma delta T cells. J Immunol. 2001;166:6784–94. https://doi.org/10.4049/jimmunol.166.11.6784.
Wang T, Gao Y, Scully E, Davis CT, Anderson JF, Welte T, Ledizet M, Koski R, Madri JA, Barrett A, et al. Gamma delta T cells facilitate adaptive immunity against West Nile virus infection in mice. J Immunol. 2006;177:1825–32. https://doi.org/10.4049/jimmunol.177.3.1825.
Zeng X, Wei YL, Huang J, Newell EW, Yu H, Kidd BA, Kuhns MS, Waters RW, Davis MM, Weaver CT, et al. gammadelta T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity. 2012;37:524–34. https://doi.org/10.1016/j.immuni.2012.06.011.
Zeng X, Meyer C, Huang J, Newell EW, Kidd BA, Wei YL, Chien YH. Gamma delta T cells recognize haptens and mount a hapten-specific response. Elife. 2014;3:e03609. https://doi.org/10.7554/eLife.03609.
Eberl M. Antigen recognition by human γδ T cells: one step closer to knowing. Immunol Cell Biol. 2020;98:351–4. https://doi.org/10.1111/imcb.12334.
Hayday AC. γδ T cell update: adaptate orchestrators of immune surveillance. J Immunol. 2019;203:311–20. https://doi.org/10.4049/jimmunol.1800934.
Castillo-González R, Cibrian D, Sánchez-Madrid F. Dissecting the complexity of γδ T-cell subsets in skin homeostasis, inflammation, and malignancy. J Allergy Clin Immunol. 2021;147:2030–42. https://doi.org/10.1016/j.jaci.2020.11.023.
Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J, et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35:596–610. https://doi.org/10.1016/j.immuni.2011.08.001.
Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–45. https://doi.org/10.1038/nri797.
Simonian PL, Roark CL, Wehrmann F, Lanham AM, Born WK, O’Brien RL, Fontenot AP. IL-17A-expressing T cells are essential for bacterial clearance in a murine model of hypersensitivity pneumonitis. J Immunol. 2009;182:6540–9. https://doi.org/10.4049/jimmunol.0900013.
Hoytema van Konijnenburg DP, Reis BS, Pedicord VA, Farache J, Victora GD, Mucida D. Intestinal epithelial and intraepithelial T cell crosstalk mediates a dynamic response to infection. Cell. 2017;171:783-794.e713. https://doi.org/10.1016/j.cell.2017.08.046.
Kaminski H, Marsères G, Cosentino A, Guerville F, Pitard V, Fournié JJ, Merville P, Déchanet-Merville J, Couzi L. Understanding human γδ T cell biology toward a better management of cytomegalovirus infection. Immunol Rev. 2020;298:264–88. https://doi.org/10.1111/imr.12922.
Willcox BE, Willcox CR. γδ TCR ligands: the quest to solve a 500-million-year-old mystery. Nat Immunol. 2019;20:121–8. https://doi.org/10.1038/s41590-018-0304-y.
Melandri D, Zlatareva I, Chaleil RAG, Dart RJ, Chancellor A, Nussbaumer O, Polyakova O, Roberts NA, Wesch D, Kabelitz D, et al. The γδTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat Immunol. 2018;19:1352–65. https://doi.org/10.1038/s41590-018-0253-5.
Crowley MP, Fahrer AM, Baumgarth N, Hampl J, Gutgemann I, Teyton L, Chien Y. A population of murine gammadelta T cells that recognize an inducible MHC class Ib molecule. Science. 2000;287:314–6. https://doi.org/10.1126/science.287.5451.314.
Wang X, Lin X, Zheng Z, Lu B, Wang J, Tan AH, Zhao M, Loh JT, Ng SW, Chen Q, et al. Host-derived lipids orchestrate pulmonary γδ T cell response to provide early protection against influenza virus infection. Nat Commun. 2021;12:1914. https://doi.org/10.1038/s41467-021-22242-9.
Bansal RR, Mackay CR, Moser B, Eberl M. IL-21 enhances the potential of human γδ T cells to provide B-cell help. Eur J Immunol. 2012;42:110–9. https://doi.org/10.1002/eji.201142017.
Mathew NR, Jayanthan JK, Smirnov IV, Robinson JL, Axelsson H, Nakka SS, Emmanouilidi A, Czarnewski P, Yewdell WT, Schön K, et al. Single-cell BCR and transcriptome analysis after influenza infection reveals spatiotemporal dynamics of antigen-specific B cells. Cell Rep. 2021;35:109286. https://doi.org/10.1016/j.celrep.2021.109286.
Qin XF, Reichlin A, Luo Y, Roeder RG, Nussenzweig MC. OCA-B integrates B cell antigen receptor-, CD40L- and IL 4-mediated signals for the germinal center pathway of B cell development. Embo J. 1998;17:5066–75. https://doi.org/10.1093/emboj/17.17.5066.
Yewdell WT, Smolkin RM, Belcheva KT, Mendoza A, Michaels AJ, Cols M, Angeletti D, Yewdell JW, Chaudhuri J. Temporal dynamics of persistent germinal centers and memory B cell differentiation following respiratory virus infection. Cell Rep. 2021;37:109961. https://doi.org/10.1016/j.celrep.2021.109961.
Sperling AI, Wortis HH. CD4-, CD8- gamma/delta cells from normal mice respond to a syngeneic B cell lymphoma and can induce its differentiation. Int Immunol. 1989;1:434–42. https://doi.org/10.1093/intimm/1.4.434.
Vidovic D, Dembic Z. Qa-1 restricted gamma delta T cells can help B cells. Curr Top Microbiol Immunol. 1991;173:239–44.
Zheng B, Marinova E, Han J, Tan TH, Han S. Cutting edge: gamma delta T cells provide help to B cells with altered clonotypes and are capable of inducing Ig gene hypermutation. J Immunol. 2003;171:4979–83. https://doi.org/10.4049/jimmunol.171.10.4979.
Su D, Shen M, Li X, Sun L. Roles of γδ T cells in the pathogenesis of autoimmune diseases. Clin Dev Immunol. 2013;2013:985753. https://doi.org/10.1155/2013/985753.
Pao W, Wen L, Smith AL, Gulbranson-Judge A, Zheng B, Kelsoe G, MacLennan IC, Owen MJ, Hayday AC. Gamma delta T cell help of B cells is induced by repeated parasitic infection, in the absence of other T cells. Curr Biol. 1996;6:1317–25. https://doi.org/10.1016/s0960-9822(02)70718-5.
Huang Y, Getahun A, Heiser RA, Detanico TO, Aviszus K, Kirchenbaum GA, Casper TL, Huang C, Aydintug MK, Carding SR, et al. γδ T cells shape preimmune peripheral B cell populations. J Immunol. 2016;196:217–31. https://doi.org/10.4049/jimmunol.1501064.
Weinstein JA, Zeng X, Chien YH, Quake SR. Correlation of gene expression and genome mutation in single B-cells. PLoS ONE. 2013;8:e67624. https://doi.org/10.1371/journal.pone.0067624.
Karunakaran MM, Willcox CR, Salim M, Paletta D, Fichtner AS, Noll A, Starick L, Nöhren A, Begley CR, Berwick KA, et al. Butyrophilin-2A1 directly binds germline-encoded regions of the Vγ9Vδ2 TCR and is essential for phosphoantigen sensing. Immunity. 2020;52:487-498.e486. https://doi.org/10.1016/j.immuni.2020.02.014.
Rigau M, Ostrouska S, Fulford TS, Johnson DN, Woods K, Ruan Z, McWilliam HEG, Hudson C, Tutuka C, Wheatley AK, et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science. 2020. https://doi.org/10.1126/science.aay5516.
Blazquez JL, Benyamine A, Pasero C, Olive D. New insights into the regulation of γδ T cells by BTN3A and other BTN/BTNL in tumor immunity. Front Immunol. 2018;9:1601. https://doi.org/10.3389/fimmu.2018.01601.
Russano AM, Agea E, Corazzi L, Postle AD, De Libero G, Porcelli S, de Benedictis FM, Spinozzi F. Recognition of pollen-derived phosphatidyl-ethanolamine by human CD1d-restricted gamma delta T cells. J Allergy Clin Immunol. 2006;117:1178–84. https://doi.org/10.1016/j.jaci.2006.01.001.
Delia D, Cattoretti G, Polli N, Fontanella E, Aiello A, Giardini R, Rilke F, Della Porta G. CD1c but neither CD1a nor CD1b molecules are expressed on normal, activated, and malignant human B cells: identification of a new B-cell subset. Blood. 1988;72:241–7.
Zheng Z, Venkatapathy S, Rao G, Harrington CA. Expression profiling of B cell chronic lymphocytic leukemia suggests deficient CD1-mediated immunity, polarized cytokine response, altered adhesion and increased intracellular protein transport and processing of leukemic cells. Leukemia. 2002;16:2429–37. https://doi.org/10.1038/sj.leu.2402711.
Brandes M, Willimann K, Lang AB, Nam KH, Jin C, Brenner MB, Morita CT, Moser B. Flexible migration program regulates gamma delta T-cell involvement in humoral immunity. Blood. 2003;102:3693–701. https://doi.org/10.1182/blood-2003-04-1016.
Petrasca A, Melo AM, Breen EP, Doherty DG. Human Vδ3(+) γδ T cells induce maturation and IgM secretion by B cells. Immunol Lett. 2018;196:126–34. https://doi.org/10.1016/j.imlet.2018.02.002.
Caccamo N, Battistini L, Bonneville M, Poccia F, Fournié JJ, Meraviglia S, Borsellino G, Kroczek RA, La Mendola C, Scotet E, et al. CXCR5 identifies a subset of Vgamma9Vdelta2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J Immunol. 2006;177:5290–5. https://doi.org/10.4049/jimmunol.177.8.5290.
Raju S, Kretzmer LZ, Koues OI, Payton JE, Oltz EM, Cashen A, Polic B, Schreiber RD, Shaw AS, Markiewicz MA. NKG2D-NKG2D ligand interaction inhibits the outgrowth of naturally arising low-grade B cell lymphoma in vivo. J Immunol. 2016;196:4805–13. https://doi.org/10.4049/jimmunol.1501982.
Nückel H, Switala M, Sellmann L, Horn PA, Dürig J, Dührsen U, Küppers R, Grosse-Wilde H, Rebmann V. The prognostic significance of soluble NKG2D ligands in B-cell chronic lymphocytic leukemia. Leukemia. 2010;24:1152–9. https://doi.org/10.1038/leu.2010.74.
Pistoia V, Tumino N, Vacca P, Veneziani I, Moretta A, Locatelli F, Moretta L. Human γδ T-cells: from surface receptors to the therapy of high-risk leukemias. Front Immunol. 2018;9:984. https://doi.org/10.3389/fimmu.2018.00984.
Dai YM, Liu HY, Liu YF, Zhang Y, He W. EBV transformation induces overexpression of hMSH2/3/6 on B lymphocytes and enhances γδT-cell-mediated cytotoxicity via TCR and NKG2D. Immunology. 2018;154:673–82. https://doi.org/10.1111/imm.12920.
Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. https://doi.org/10.1016/s1074-7613(02)00333-3.
Moser B, Eberl M. γδ T-APCs: a novel tool for immunotherapy? Cell Mol Life Sci. 2011;68:2443–52. https://doi.org/10.1007/s00018-011-0706-6.
Rezende RM, Lanser AJ, Rubino S, Kuhn C, Skillin N, Moreira TG, Liu S, Gabriely G, David BA, Menezes GB, et al. γδ T cells control humoral immune response by inducing T follicular helper cell differentiation. Nat Commun. 2018;9:3151. https://doi.org/10.1038/s41467-018-05487-9.
Koutsakos M, Wheatley AK, Loh L, Clemens EB, Sant S, Nüssing S, Fox A, Chung AW, Laurie KL, Hurt AC, et al. Circulating T(FH) cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Sci Transl Med. 2018. https://doi.org/10.1126/scitranslmed.aan8405.
Han HJ, Jang YS, Seo GY, Park SG, Kang SG, Yoon SI, Ko HJ, Lee GS, Kim PH. Murine γδ T cells render B cells refractory to commitment of IgA isotype switching. Immune Netw. 2018;18:e25. https://doi.org/10.4110/in.2018.18.e25.
Park K-H, Seo G-Y, Jang Y-S, Kim P-H. TGF-β and BAFF derived from CD4+CD25+Foxp3+ T cells mediate mouse IgA isotype switching. Genes Genom. 2012;34:619–25. https://doi.org/10.1007/s13258-012-0062-4.
Huang Y, Jin N, Roark CL, Aydintug MK, Wands JM, Huang H, O’Brien RL, Born WK. The influence of IgE-enhancing and IgE-suppressive gammadelta T cells changes with exposure to inhaled ovalbumin. J Immunol. 2009;183:849–55. https://doi.org/10.4049/jimmunol.0804104.
Cook L, Miyahara N, Jin N, Wands JM, Taube C, Roark CL, Potter TA, Gelfand EW, O’Brien RL, Born WK. Evidence that CD8+ dendritic cells enable the development of gammadelta T cells that modulate airway hyperresponsiveness. J Immunol. 2008;181:309–19. https://doi.org/10.4049/jimmunol.181.1.309.
Huang Y, Heiser RA, Detanico TO, Getahun A, Kirchenbaum GA, Casper TL, Aydintug MK, Carding SR, Ikuta K, Huang H, et al. γδ T cells affect IL-4 production and B-cell tolerance. Proc Natl Acad Sci USA. 2015;112:E39-48. https://doi.org/10.1073/pnas.1415107111.
Hidaka T, Kitani A, Hara M, Harigai M, Suzuki K, Kawaguchi Y, Ishizuka T, Kawagoe M, Nakamura H. IL-4 down-regulates the surface expression of CD5 on B cells and inhibits spontaneous immunoglobulin and IgM-rheumatoid factor production in patients with rheumatoid arthritis. Clin Exp Immunol. 1992;89:223–9. https://doi.org/10.1111/j.1365-2249.1992.tb06936.x.
Inoue SI, Niikura M, Asahi H, Kawakami Y, Kobayashi F. γδ T cells modulate humoral immunity against Plasmodium berghei infection. Immunology. 2018;155:519–32. https://doi.org/10.1111/imm.12997.
Pan T, Tan R, Li M, Liu Z, Wang X, Tian L, Liu J, Qu H. IL17-producing γδ T cells may enhance humoral immunity during pulmonary Pseudomonas aeruginosa infection in mice. Front Cell Infect Microbiol. 2016;6:170. https://doi.org/10.3389/fcimb.2016.00170.
Duan L, Liu D, Chen H, Mintz MA, Chou MY, Kotov DI, Xu Y, An J, Laidlaw BJ, Cyster JG. Follicular dendritic cells restrict interleukin-4 availability in germinal centers and foster memory B cell generation. Immunity. 2021;54:2256-2272.e2256. https://doi.org/10.1016/j.immuni.2021.08.028.
Finkelman FD, Katona IM, Mosmann TR, Coffman RL. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988;140:1022–7.
Stone SL, Peel JN, Scharer CD, Risley CA, Chisolm DA, Schultz MD, Yu B, Ballesteros-Tato A, Wojciechowski W, Mousseau B, et al. T-bet transcription factor promotes antibody-secreting cell differentiation by limiting the inflammatory effects of IFN-γ on B cells. Immunity. 2019;50:1172-1187.e1177. https://doi.org/10.1016/j.immuni.2019.04.004.
Cha H, Xie H, Jin C, Feng Y, Xie S, Xie A, Yang Q, Qi Y, Qiu H, Wu Q, et al. Adjustments of γδ T cells in the lung of Schistosoma japonicum-Infected C56BL/6 Mice. Front Immunol. 2020;11:1045. https://doi.org/10.3389/fimmu.2020.01045.
Huber S, Sartini D. T cells expressing the Vgamma1 T-cell receptor enhance virus-neutralizing antibody response during coxsackievirus B3 infection of BALB/c mice: differences in male and female mice. Viral Immunol. 2005;18:730–9. https://doi.org/10.1089/vim.2005.18.730.
Ullrich L, Lueder Y, Juergens AL, Wilharm A, Barros-Martins J, Bubke A, Demera A, Ikuta K, Patzer GE, Janssen A, et al. IL-4-producing Vγ1(+)/Vδ6(+) γδ T cells sustain germinal center reactions in Peyer’s patches of mice. Front Immunol. 2021;12:729607. https://doi.org/10.3389/fimmu.2021.729607.
Chu VT, Beller A, Rausch S, Strandmark J, Zänker M, Arbach O, Kruglov A, Berek C. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 2014;40:582–93. https://doi.org/10.1016/j.immuni.2014.02.014.
Lindner C, Wahl B, Föhse L, Suerbaum S, Macpherson AJ, Prinz I, Pabst O. Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. J Exp Med. 2012;209:365–77. https://doi.org/10.1084/jem.20111980.
Andreu-Ballester JC, Zamora V, Garcia-Ballesteros C, Benet-Campos C, Lopez-Chuliá F, Tormo-Calandín C, Cuéllar C. Anti-Anisakis sp. antibodies in serum of patients with sepsis and their relationship with γδ T cells and disease severity. Int J Parasitol. 2018;48:483–91. https://doi.org/10.1016/j.ijpara.2017.11.007.
Maloy KJ, Odermatt B, Hengartner H, Zinkernagel RM. Interferon gamma-producing gammadelta T cell-dependent antibody isotype switching in the absence of germinal center formation during virus infection. Proc Natl Acad Sci USA. 1998;95:1160–5. https://doi.org/10.1073/pnas.95.3.1160.
Xie S, Li J, Wang JH, Wu Q, Yang P, Hsu HC, Smythies LE, Mountz JD. IL-17 activates the canonical NF-kappaB signaling pathway in autoimmune B cells of BXD2 mice to upregulate the expression of regulators of G-protein signaling 16. J Immunol. 2010;184:2289–96. https://doi.org/10.4049/jimmunol.0903133.
Subbarayal B, Chauhan SK, Di Zazzo A, Dana R. IL-17 augments b cell activation in ocular surface autoimmunity. J Immunol. 2016;197:3464–70. https://doi.org/10.4049/jimmunol.1502641.
Gertner-Dardenne J, Bonnafous C, Bezombes C, Capietto AH, Scaglione V, Ingoure S, Cendron D, Gross E, Lepage JF, Quillet-Mary A, et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood. 2009;113:4875–84. https://doi.org/10.1182/blood-2008-08-172296.
Cheng ZF, Li HK, Yang HP, Lee CY, Tang SW, Lin YL, Hsiao SC. A novel endogenous CD16-expressing natural killer cell for cancer immunotherapy. Biochem Biophys Rep. 2021;26:100935. https://doi.org/10.1016/j.bbrep.2021.100935.
Junqueira C, Polidoro RB, Castro G, Absalon S, Liang Z, Sen Santara S, Crespo Â, Pereira DB, Gazzinelli RT, Dvorin JD, et al. γδ T cells suppress Plasmodium falciparum blood-stage infection by direct killing and phagocytosis. Nat Immunol. 2021;22:347–57. https://doi.org/10.1038/s41590-020-00847-4.
Couzi L, Pitard V, Sicard X, Garrigue I, Hawchar O, Merville P, Moreau JF, Déchanet-Merville J. Antibody-dependent anti-cytomegalovirus activity of human γδ T cells expressing CD16 (FcγRIIIa). Blood. 2012;119:1418–27. https://doi.org/10.1182/blood-2011-06-363655.
Funding
This work was supported by the National Natural Science Foundation of China (32070899, 31870899) to X.Z.
Author information
Authors and Affiliations
Contributions
LQ, YZ, and XZ drafted the main body of this manuscript. XZ takes primary responsibility for this paper as the corresponding author. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiu, L., Zhang, Y. & Zeng, X. The function of γδ T cells in humoral immune responses. Inflamm. Res. 72, 747–755 (2023). https://doi.org/10.1007/s00011-023-01704-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01704-4