Abstract
Objective and design
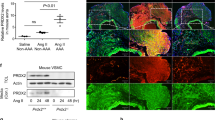
The age-associated increases in aseptic inflammation and necroptosis are closely related to the emergence of various age-associated diseases.
Methods
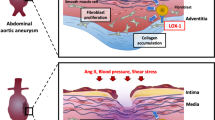
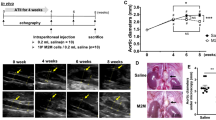
In this study, the role of HMGB1/TLR4-induced necroptosis in abdominal aortic aneurysm (AAA) formation was investigated. First, the levels of sterile inflammatory mediators (HMGB1, TLR4) and necroptosis markers were measured in the abdominal aortas of young and old C57BL/6JNifdc mice. We observed that sterile inflammatory mediators and necroptosis markers were greatly increased in the abdominal aortas of old mice. Then, angiotensin II (Ang II)-induced AAA model in APOE−/− mice was used in this study. Mice AAA models were treated with the RIP1 inhibitor necrostatin-1 (Nec-1) or the TLR4 inhibitor TAK-242, respectively.
Results
We found that HMGB1, TLR4, and necroptosis markers were elevated in old mice compared with those in young mice. Same elevation was also found in the development of AAA in APOE−/− mice. In addition, the necroptosis inhibitor Nec-1 alleviated Ang II-induced AAA development while downregulating the expression of HMGB1/TLR4. After blocking TLR4 with TAK-242, the expression of necroptosis markers decreased significantly, and the progression of AAA was also alleviated in APOE−/− mice.
Conclusions
Our results indicated that HMGB1/TLR4-mediated necroptosis enhances AAA development in the Ang II-induced AAA model in APOE−/− mice and that TLR4 might be a potential therapeutic target for AAA management.






Similar content being viewed by others
Data availability
The authors will provide the raw data of this study unreservedly.
References
Golledge J, et al. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26(12):2605–13.
Miyake T, Morishita R. Pharmacological treatment of abdominal aortic aneurysm. Cardiovasc Res. 2009;83(3):436–43.
Powell JT, Greenhalgh RM. Clinical practice. Small abdominal aortic aneurysms. N Engl J Med. 2003;348(19):1895–901.
Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371(22):2101–8.
Golledge J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol. 2019;16(4):225–42.
Rouer M, et al. Rapamycin limits the growth of established experimental abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2014;47(5):493–500.
Xu B, et al. Angiotensin-converting enzyme 2, coronavirus disease 2019, and abdominal aortic aneurysms. J Vasc Surg. 2021;74(5):1740–51.
Zhang T, et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med. 2016;22(2):175–82.
Wang Q, et al. Receptor-interacting protein kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation. Circ Res. 2015;116(4):600–11.
Karunakaran D, et al. Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci Adv. 2016;2(7):e1600224.
Wang H, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54(1):133–46.
He S, et al. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A. 2011;108(50):20054–9.
Fang H, et al. TLR4 is essential for dendritic cell activation and anti-tumor T-cell response enhancement by DAMPs released from chemically stressed cancer cells. Cell Mol Immunol. 2014;11(2):150–9.
Lin SY, et al. Necroptosis promotes autophagy-dependent upregulation of DAMP and results in immunosurveillance. Autophagy. 2018;14(5):778–95.
Bai L, et al. M2-like macrophages exert hepatoprotection in acute-on-chronic liver failure through inhibiting necroptosis-S100A9-necroinflammation axis. Cell Death Dis. 2021;12(1):93.
Hong YP, et al. High-fat diet aggravates acute pancreatitis via TLR4-mediated necroptosis and inflammation in rats. Oxid Med Cell Longev. 2020;2020:8172714.
Huang Z, et al. Necroptosis in microglia contributes to neuroinflammation and retinal degeneration through TLR4 activation. Cell Death Differ. 2018;25(1):180–9.
Wang Z, et al. Metformin represses the pathophysiology of AAA by suppressing the activation of PI3K/AKT/mTOR/autophagy pathway in ApoE(-/-) mice. Cell Biosci. 2019;9:68.
Wang Q, et al. Inhibition of receptor-interacting protein kinase 1 with necrostatin-1s ameliorates disease progression in elastase-induced mouse abdominal aortic aneurysm model. Sci Rep. 2017;7:42159.
Gu HF, et al. Chronic unpredictable mild stress promotes atherosclerosis via HMGB1/TLR4-mediated downregulation of PPARγ/LXRα/ABCA1 in ApoE(-/-) mice. Front Physiol. 2019;10:165.
Wheeler JB, et al. Relation of murine thoracic aortic structural and cellular changes with aging to passive and active mechanical properties. J Am Heart Assoc. 2015;4(3):e001744.
Johnston KW, et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1991;13(3):452–8.
Ullery BW, Hallett RL, Fleischmann D. Epidemiology and contemporary management of abdominal aortic aneurysms. Abdom Radiol (NY). 2018;43(5):1032–43.
Ramella M, et al. Relevance of inflammation and matrix remodeling in abdominal aortic aneurysm (AAA) and popliteal artery aneurysm (PAA) progression. Am J Transl Res. 2018;10(10):3265–75.
Tan TW, et al. Outcomes of endovascular and open surgical repair of ruptured abdominal aortic aneurysms in elderly patients. J Vasc Surg. 2017;66(1):64–70.
Umebayashi R, Uchida HA, Wada J. Abdominal aortic aneurysm in aged population. Aging (Albany NY). 2018;10(12):3650–1.
Sakalihasan N, et al. Abdominal aortic aneurysms. Nat Rev Dis Primers. 2018;4(1):34.
Thompson SG, et al. Screening women aged 65 years or over for abdominal aortic aneurysm: a modelling study and health economic evaluation. Health Technol Assess. 2018;22(43):1–142.
Qiu H, et al. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res. 2010;107(5):615–9.
Zhang J, et al. Extracellular matrix disarray as a mechanism for greater abdominal versus thoracic aortic stiffness with aging in primates. Arterioscler Thromb Vasc Biol. 2016;36(4):700–6.
Rodriguez-Menocal L, et al. Macrophage-derived IL-18 and increased fibrinogen deposition are age-related inflammatory signatures of vascular remodeling. Am J Physiol Heart Circ Physiol. 2014;306(5):H641–53.
Albini PT, et al. Advanced atherosclerosis is associated with increased medial degeneration in sporadic ascending aortic aneurysms. Atherosclerosis. 2014;232(2):361–8.
Li M, Fukagawa NK. Age-related changes in redox signaling and VSMC function. Antioxid Redox Signal. 2010;12(5):641–55.
de Figueiredo Borges L, et al. Collagen is reduced and disrupted in human aneurysms and dissections of ascending aorta. Hum Pathol. 2008;39(3):437–43.
Groeneveld ME, et al. The potential role of neutrophil gelatinase-associated lipocalin in the development of abdominal aortic aneurysms. Ann Vasc Surg. 2019;57:210–9.
Brubaker AL, Palmer JL, Kovacs EJ. Age-related dysregulation of inflammation and innate immunity: lessons learned from rodent models. Aging Dis. 2011;2(5):346–60.
Deepa SS, et al. Necroptosis increases with age and is reduced by dietary restriction. Aging Cell. 2018;17(4):e12770.
Gupta SC, et al. Inflammation, a double-edge sword for cancer and other age-related diseases. Front Immunol. 2018;9:2160.
Joly L, et al. Influence of thoracic aortic inflammation and calcifications on arterial stiffness and cardiac function in older subjects. J Nutr Health Aging. 2016;20(3):347–54.
Wang S, Zhang Y. HMGB1 in inflammation and cancer. J Hematol Oncol. 2020;13(1):116.
Murakami Y, et al. Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsRNA-induced retinal degeneration. Cell Death Differ. 2014;21(2):270–7.
Imhof BA, Jemelin S, Emre Y. Toll-like receptors elicit different recruitment kinetics of monocytes and neutrophils in mouse acute inflammation. Eur J Immunol. 2017;47(6):1002–8.
Elzinga S, et al. Toll-like receptors and inflammation in metabolic neuropathy; a role in early versus late disease? Exp Neurol. 2019;320:112967.
Peek V, et al. Age-dependent changes of adipokine and cytokine secretion from rat adipose tissue by endogenous and exogenous toll-like receptor agonists. Front Immunol. 2020;11:1800.
He S, et al. HMGB1 released by irradiated tumor cells promotes living tumor cell proliferation via paracrine effect. Cell Death Dis. 2018;9(6):648.
Wang Y, et al. Necroptosis regulates tumor repopulation after radiotherapy via RIP1/RIP3/MLKL/JNK/IL8 pathway. J Exp Clin Cancer Res. 2019;38(1):461.
Matsunaga N, et al. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol. 2011;79(1):34–41.
Zhou T, et al. Identification of a novel class of RIP1/RIP3 dual inhibitors that impede cell death and inflammation in mouse abdominal aortic aneurysm models. Cell Death Dis. 2019;10(3):226.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) (Nos. 81970400).
Author information
Authors and Affiliations
Contributions
SB and LY: contributed equally to this work and shared co-first authors. SB and LY: carried out the experiments and drafted the article. DZ and LL: collected and analyzed the data. TW: performed ultrasound examinations. HY: contributed to the conception and design of this study, executed article revision. All authors read and agreed with the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All animal experimental procedures were agreed with Shandong Provincial Hospital’s Committee of Animal Protection and Use.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bian, S., Yang, L., Zhao, D. et al. HMGB1/TLR4 signaling pathway enhances abdominal aortic aneurysm progression in mice by upregulating necroptosis. Inflamm. Res. 72, 703–713 (2023). https://doi.org/10.1007/s00011-023-01694-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01694-3