Abstract
Objective
To explore the molecular mechanism of γ-glutamylcysteine (γ-GC) in response to inflammation in vivo and in vitro on regulating the polarization of macrophages.
Methods
The expressions of gene or protein were assessed by qPCR and Western blot assays, respectively. Cell viability was investigated by CCK-8 assay. Eight-week-old male BALB/c mice were established to examine the therapeutic effects of γ-GC in vivo. The release of TNF-α and IL-4 was determined by ELISA assay. Macrophages polarization was identified by flow cytometry assay.
Results
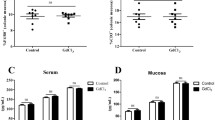
Our data showed that γ-GC treatment significantly improved the survival, weight loss, and colon tissue damage of IBD mice. Furthermore, we established M1- and M2-polarized macrophages, respectively, and our findings provided evidence that γ-GC switched M1/M2-polarized macrophages through activating AMPK/SIRT1 axis and inhibiting inflammation-related signaling pathway.
Conclusion
Collectively, both in vivo and in vitro experiments suggested that γ-GC has the potential to become a promising novel therapeutic dipeptide for the treatment of IBD, which provide new ideas for the treatment of inflammatory diseases in the future.









Similar content being viewed by others
Data availability
No data were used for the research described in the article.
References
Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–40. https://doi.org/10.1016/s0140-6736(07)60750-8.
Garber A, Regueiro M. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, etiopathogenesis, and management. Curr Gastroenterol Rep. 2019;21:31. https://doi.org/10.1007/s11894-019-0698-1.
Habibi F, Habibi ME, Gharavinia A, Mahdavi SB, Akbarpour MJ, Baghaei A, et al. Quality of life in inflammatory bowel disease patients: a cross-sectional study. J Res Med Sci. 2017;22:104. https://doi.org/10.4103/jrms.JRMS_975_16.
Singh S, George J, Boland BS, Vande Casteele N, Sandborn WJ. Primary non-response to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with inflammatory bowel diseases: a systematic review and meta-analysis. J Crohns Colitis. 2018;12:635–43. https://doi.org/10.1093/ecco-jcc/jjy004.
Cohen NA, Rubin DT. New targets in inflammatory bowel disease therapy: 2021. Curr Opin Gastroenterol. 2021;37:357–63. https://doi.org/10.1097/mog.0000000000000740.
Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. https://doi.org/10.1016/j.ejphar.2020.173090.
Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. 2018;19:1801. https://doi.org/10.3390/ijms19061801.
Gaojian T, Dingfei Q, Linwei L, Xiaowei W, Zheng Z, Wei L, et al. Parthenolide promotes the repair of spinal cord injury by modulating M1/M2 polarization via the NF-κB and STAT 1/3 signaling pathway. Cell Death Discov. 2020;6:97. https://doi.org/10.1038/s41420-020-00333-8.
Xu YW, Xing RX, Zhang WH, Li L, Wu Y, Hu J, et al. Toxoplasma ROP16(I/III) ameliorated inflammatory bowel diseases via inducing M2 phenotype of macrophages. World J Gastroenterol. 2019;25:6634–52. https://doi.org/10.3748/wjg.v25.i45.6634.
Song M, Kellum JA, Kaldas H, Fink MP. Evidence that glutathione depletion is a mechanism responsible for the anti-inflammatory effects of ethyl pyruvate in cultured lipopolysaccharide-stimulated RAW 264.7 cells. J Pharmacol Exp Ther. 2004;308:307–16. https://doi.org/10.1124/jpet.103.056622.
Anderson ME, Meister A. Transport and direct utilization of gamma-glutamylcyst(e)ine for glutathione synthesis. Proc Natl Acad Sci USA. 1983;80:707–11. https://doi.org/10.1073/pnas.80.3.707.
Zarka MH, Bridge WJ. Oral administration of γ-glutamylcysteine increases intracellular glutathione levels above homeostasis in a randomised human trial pilot study. Redox Biol. 2017;11:631–6. https://doi.org/10.1016/j.redox.2017.01.014.
Ferguson G, Bridge W. Glutamate cysteine ligase and the age-related decline in cellular glutathione: the therapeutic potential of γ-glutamylcysteine. Arch Biochem Biophys. 2016;593:12–23. https://doi.org/10.1016/j.abb.2016.01.017.
Yang Y, Li L, Hang Q, Fang Y, Dong X, Cao P, et al. γ-glutamylcysteine exhibits anti-inflammatory effects by increasing cellular glutathione level. Redox Biol. 2019;20:157–66. https://doi.org/10.1016/j.redox.2018.09.019.
Lu S, Zhou J, Yang C, Zhang X, Shi Y, Liu J, et al. γ-Glutamylcysteine ameliorates D-gal-induced senescence in PC12 cells and mice via activating AMPK and SIRT1. Food Funct. 2022;13:7560–71. https://doi.org/10.1039/d2fo01246d.
Murano M, Maemura K, Hirata I, Toshina K, Nishikawa T, Hamamoto N, et al. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 2000;120:51–8. https://doi.org/10.1046/j.1365-2249.2000.01183.x.
Antoniou E, Margonis GA, Angelou A, Pikouli A, Argiri P, Karavokyros I, et al. The TNBS-induced colitis animal model: an overview. Ann Med Surg (Lond). 2016;11:9–15. https://doi.org/10.1016/j.amsu.2016.07.019.
Liu YJ, Tang B, Wang FC, Tang L, Lei YY, Luo Y, et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics. 2020;10:5225–41. https://doi.org/10.7150/thno.43716.
Na YR, Stakenborg M, Seok SH, Matteoli G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat Rev Gastroenterol Hepatol. 2019;16:531–43. https://doi.org/10.1038/s41575-019-0172-4.
Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520–9. https://doi.org/10.7150/ijbs.8879.
Zhou X, Li W, Wang S, Zhang P, Wang Q, Xiao J, et al. YAP aggravates inflammatory bowel disease by regulating M1/M2 macrophage polarization and gut microbial homeostasis. Cell Rep. 2019;27:1176-1189.e5. https://doi.org/10.1016/j.celrep.2019.03.028.
Jin S, Meng C, He Y, Wang X, Zhang Q, Wang Z, et al. Curcumin prevents osteocyte apoptosis by inhibiting M1-type macrophage polarization in mice model of glucocorticoid-associated osteonecrosis of the femoral head. J Orthop Res. 2020;38:2020–30. https://doi.org/10.1002/jor.24619.
Zhang H, Cao N, Yang Z, Fang X, Yang X, Li H, et al. Bilobalide alleviated dextran sulfate sodium-induced experimental colitis by inhibiting M1 macrophage polarization through the NF-κB signaling pathway. Front Pharmacol. 2020;11:718. https://doi.org/10.3389/fphar.2020.00718.
Xiong Y, Cui X, Zhou Y, Chai G, Jiang X, Ge G, et al. Dehydrocostus lactone inhibits BLM-induced pulmonary fibrosis and inflammation in mice via the JNK and p38 MAPK-mediated NF-κB signaling pathways. Int Immunopharmacol. 2021;98:107780. https://doi.org/10.1016/j.intimp.2021.107780.
Zhao XN, Li YN, Wang YT. Interleukin-4 regulates macrophage polarization via the MAPK signaling pathway to protect against atherosclerosis. Genet Mol Res. 2016. https://doi.org/10.4238/gmr.15017348.
Wang X, Zhang H, Guo R, Li X, Liu H, Wang Z, et al. MicroRNA-223 modulates the IL-4-medicated macrophage M2-type polarization to control the progress of sepsis. Int Immunopharmacol. 2021;96:107783. https://doi.org/10.1016/j.intimp.2021.107783.
Ohmori Y, Hamilton TA. IL-4-induced STAT6 suppresses IFN-gamma-stimulated STAT1-dependent transcription in mouse macrophages. J Immunol. 1997;159:5474–82.
Fu C, Jiang L, Hao S, Liu Z, Ding S, Zhang W, et al. Activation of the IL-4/STAT6 signaling pathway promotes lung cancer progression by increasing M2 myeloid cells. Front Immunol. 2019;10:2638. https://doi.org/10.3389/fimmu.2019.02638.
Rahabi M, Salon M, Bruno-Bonnet C, Prat M, Jacquemin G, Benmoussa K, et al. Bioactive fish collagen peptides weaken intestinal inflammation by orienting colonic macrophages phenotype through mannose receptor activation. Eur J Nutr. 2022;61:2051–66. https://doi.org/10.1007/s00394-021-02787-7.
Wang F, Ma J, Wang J, Chen M, Xia H, Yao S, et al. SIRT1 ameliorated septic associated-lung injury and macrophages apoptosis via inhibiting endoplasmic reticulum stress. Cell Signal. 2022;97:110398. https://doi.org/10.1016/j.cellsig.2022.110398.
Lok J, Leung W, Zhao S, Pallast S, van Leyen K, Guo S, et al. γ-glutamylcysteine ethyl ester protects cerebral endothelial cells during injury and decreases blood-brain barrier permeability after experimental brain trauma. J Neurochem. 2011;118:248–55. https://doi.org/10.1111/j.1471-4159.2011.07294.x.
Braidy N, Zarka M, Jugder BE, Welch J, Jayasena T, Chan DKY, et al. The precursor to glutathione (GSH), γ-glutamylcysteine (GGC), can ameliorate oxidative damage and neuroinflammation induced by Aβ(40) oligomers in human astrocytes. Front Aging Neurosci. 2019;11:177. https://doi.org/10.3389/fnagi.2019.00177.
Quintana-Cabrera R, Fernandez-Fernandez S, Bobo-Jimenez V, Escobar J, Sastre J, Almeida A, et al. γ-Glutamylcysteine detoxifies reactive oxygen species by acting as glutathione peroxidase-1 cofactor. Nat Commun. 2012;3:718. https://doi.org/10.1038/ncomms1722.
Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 2019;12:113–22. https://doi.org/10.25122/jml-2018-0075.
Ramos GP, Papadakis KA. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc. 2019;94:155–65. https://doi.org/10.1016/j.mayocp.2018.09.013.
Jiang Y, Jarr K, Layton C, Gardner CD, Ashouri JF, Abreu MT, et al. Therapeutic implications of diet in inflammatory bowel disease and related immune-mediated inflammatory diseases. Nutrients. 2021;13:890. https://doi.org/10.3390/nu13030890.
Cai Z, Wang S, Li J. Treatment of inflammatory bowel disease: a comprehensive review. Front Med (Lausanne). 2021;8:765474. https://doi.org/10.3389/fmed.2021.765474.
Na SY, Moon W. Perspectives on current and novel treatments for inflammatory bowel disease. Gut Liver. 2019;13:604–16. https://doi.org/10.5009/gnl19019.
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–40. https://doi.org/10.1002/jcp.26429.
Du Y, Rong L, Cong Y, Shen L, Zhang N, Wang B. Macrophage polarization: an effective approach to targeted therapy of inflammatory bowel disease. Expert Opin Ther Targets. 2021;25:191–209. https://doi.org/10.1080/14728222.2021.1901079.
Chandler SD, Zarka MH, Vinaya Babu SN, Suhas YS, Raghunatha Reddy KR, Bridge WJ. Safety assessment of gamma-glutamylcysteine sodium salt. Regul Toxicol Pharmacol. 2012;64:17–25. https://doi.org/10.1016/j.yrtph.2012.05.008.
Engevik MA, Herrmann B, Ruan W, Engevik AC, Engevik KA, Ihekweazu F, et al. Bifidobacterium dentium-derived y-glutamylcysteine suppresses ER-mediated goblet cell stress and reduces TNBS-driven colonic inflammation. Gut Microbes. 2021;13:1–21. https://doi.org/10.1080/19490976.2021.1902717.
López-Posadas R, Stürzl M, Atreya I, Neurath MF, Britzen-Laurent N. Interplay of GTPases and cytoskeleton in cellular barrier defects during gut inflammation. Front Immunol. 2017;8:1240. https://doi.org/10.3389/fimmu.2017.01240.
Mannino G, Caradonna F, Cruciata I, Lauria A, Perrone A, Gentile C. Melatonin reduces inflammatory response in human intestinal epithelial cells stimulated by interleukin-1β. J Pineal Res. 2019;67: e12598. https://doi.org/10.1111/jpi.12598.
Olesen CM, Coskun M, Peyrin-Biroulet L, Nielsen OH. Mechanisms behind efficacy of tumor necrosis factor inhibitors in inflammatory bowel diseases. Pharmacol Ther. 2016;159:110–9. https://doi.org/10.1016/j.pharmthera.2016.01.001.
Xin P, Xu X, Deng C, Liu S, Wang Y, Zhou X, et al. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharmacol. 2020;80:106210. https://doi.org/10.1016/j.intimp.2020.106210.
Yu T, Zuo Y, Cai R, Huang X, Wu S, Zhang C, et al. SENP1 regulates IFN-γ-STAT1 signaling through STAT3-SOCS3 negative feedback loop. J Mol Cell Biol. 2017;9:144–53. https://doi.org/10.1093/jmcb/mjw042.
Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. 2017;198:1006–14. https://doi.org/10.4049/jimmunol.1601515.
Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy–from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754:253–62. https://doi.org/10.1016/j.bbapap.2005.08.017.
Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192–7. https://doi.org/10.1016/j.cellsig.2013.11.004.
Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. https://doi.org/10.1016/j.immuni.2010.05.007.
Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–21. https://doi.org/10.1038/nri3520.
Park SJ, Lee KP, Kang S, Lee J, Sato K, Chung HY, et al. Sphingosine 1-phosphate induced anti-atherogenic and atheroprotective M2 macrophage polarization through IL-4. Cell Signal. 2014;26:2249–58. https://doi.org/10.1016/j.cellsig.2014.07.009.
Wellman AS, Metukuri MR, Kazgan N, Xu X, Xu Q, Ren NSX, et al. Intestinal epithelial sirtuin 1 regulates intestinal inflammation during aging in mice by altering the intestinal microbiota. Gastroenterology. 2017;153:772–86. https://doi.org/10.1053/j.gastro.2017.05.022.
Li L, Liu M, Cao M, He T, Bai X. Research progress on SIRT1 and sepsis. Histol Histopathol. 2019;34:1205–15. https://doi.org/10.14670/hh-18-146.
Rada P, Pardo V, Mobasher MA, García-Martínez I, Ruiz L, González-Rodríguez Á, et al. SIRT1 controls acetaminophen hepatotoxicity by modulating inflammation and oxidative stress. Antioxid Redox Signal. 2018;28:1187–208. https://doi.org/10.1089/ars.2017.7373.
Park SY, Lee SW, Lee SY, Hong KW, Bae SS, Kim K, et al. SIRT1/adenosine monophosphate-activated protein kinase α signaling enhances macrophage polarization to an anti-inflammatory phenotype in rheumatoid arthritis. Front Immunol. 2017;8:1135. https://doi.org/10.3389/fimmu.2017.01135.
Wang J, Ma S, Yu J, Zuo D, He X, Peng H, et al. MiR-9–5p promotes M1 cell polarization in osteoarthritis progression by regulating NF-κB and AMPK signaling pathways by targeting SIRT1. Int Immunopharmacol. 2021;101:108207. https://doi.org/10.1016/j.intimp.2021.108207.
Acknowledgements
This work was funded by the Natural Science Foundation of China (grant numbers 81771703, 81671565) and the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD).
Author information
Authors and Affiliations
Contributions
ZY, LL, and JZ designed the study. JZ, XY, and XB performed the mouse experiments. JZ, XY, and SL performed the cells experiments. JZ and ZY wrote the manuscript. ZY, LL, JZ, XY, XB, SL, XL, CY, and YS reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11_2023_1691_MOESM1_ESM.tif
Supplementary file1 Fig.S1 γ-GC regulated macrophages polarization via AMPK/SIRT1 signal pathway (A-H) Raw264.7 cells were stimulated with LPS (100 ng/mL) + IFN-γ (10 ng/mL) or γ-GC (80 μM) in the presence or absence of Compound C (2 μM) or/and EX527 (1 μM), then cell lysates were subjected to immunoblotting using the indicated antibodies. (I, J) Raw264.7 cells were stimulated with IL-4 (20 ng/mL) or γ-GC (80 μM) in the presence or absence of Compound C (2 μM) or/and EX527 (1 μM), then cell lysates were subjected to immunoblotting using the indicated antibodies. Data represent the mean ± SD of at least three independent experiments. **p < 0.01, ***p < 0.001 compared with the LPS+IFN-γ+γ-GC. (TIF 11673 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, J., Yan, X., Bi, X. et al. γ-Glutamylcysteine rescues mice from TNBS-driven inflammatory bowel disease through regulating macrophages polarization. Inflamm. Res. 72, 603–621 (2023). https://doi.org/10.1007/s00011-023-01691-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01691-6