Abstract
Objective
To investigate the role of IL-33 in gouty arthritis.
Material
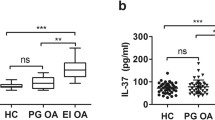
174 Balb/c (wild-type) and 54 ST2−/− mice were used in this study. In vitro experiments were conducted in bone marrow-derived macrophages (BMDMs). Synovial fluid samples from gouty arthritis (n = 7) and osteoarthritis (n = 8) hospital patients were used to measure IL-33 and sST2 levels.
Methods
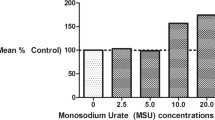
Gout was induced by injection of monosodium urate (MSU) crystals in the knee joint of mice. Pain was determined using the electronic von Frey and static weight bearing. Neutrophil recruitment was determined by H&E staining, Rosenfeld staining slides, and MPO activity. ELISA was used for cytokine and sST2 measurement. The priming effect of IL-33 was determined in BMDM.
Results
Synovial fluid of gout patients showed higher IL-33 levels and neutrophil counts than osteoarthritis patients. In mice, the absence of ST2 prevented mechanical pain, knee joint edema, neutrophil recruitment to the knee joint, and lowered IL-1β and superoxide anion levels. In macrophages, IL-33 enhanced the release of IL-1β and TNF-α, and BMDMs from ST2−/− showed reduced levels of these cytokines after stimulus with MSU crystals.
Conclusion
IL-33 mediates gout pain and inflammation by boosting macrophages production of cytokines upon MSU crystals stimulus.





Similar content being viewed by others
References
Rees F, Hui M, Doherty M. Optimizing current treatment of gout. Nat Rev Rheumatol. 2014;10(5):271–83.
Fattori V, Amaral FA, Verri WA Jr. Neutrophils and arthritis: Role in disease and pharmacological perspectives. Pharmacol Res. 2016;112:84–988.
Dalbeth, N.; Merriman, T. R.; Stamp, L. K., Gout. The Lancet2016, 388, (10055), 2039–2052.
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41.
Amaral FA, Costa VV, Tavares LD, Sachs D, Coelho FM, Fagundes CT, Soriani FM, Silveira TN, Cunha LD, Zamboni DS, Quesniaux V, Peres RS, Cunha TM, Cunha FQ, Ryffel B, Souza DG, Teixeira MM. NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout. Arthritis Rheum. 2012;64(2):474–84.
Popa-Nita O, Rollet-Labelle E, Thibault N, Gilbert C, Bourgoin SG, Naccache PH. Crystal-induced neutrophil activation. IX. Syk-dependent activation of class Ia phosphatidylinositol 3-kinase. J Leukoc Biol. 2007;82(3):763–7.
Martin WJ, Harper JL. Innate inflammation and resolution in acute gout. Immunol Cell Biol. 2010;88(1):15–9.
Galvao I, Vago JP, Barroso LC, Tavares LP, Queiroz-Junior CM, Costa VV, Carneiro FS, Ferreira TP, Silva PM, Amaral FA, Sousa LP, Teixeira MM. Annexin A1 promotes timely resolution of inflammation in murine gout. Eur J Immunol. 2017;47(3):585–96.
FitzGerald JD, Dalbeth N, Mikuls T, Brignardello-Petersen R, Guyatt G, Abeles AM, Gelber AC, Harrold LR, Khanna D, King C, Levy G, Libbey C, Mount D, Pillinger MH, Rosenthal A, Singh JA, Sims JE, Smith BJ, Wenger NS, Bae SS, Danve A, Khanna PP, Kim SC, Lenert A, Poon S, Qasim A, Sehra ST, Sharma TSK, Toprover M, Turgunbaev M, Zeng L, Zhang MA, Turner AS, Neogi T. 2020 American College of Rheumatology Guideline for the Management of Gout. Arthritis Care Res (Hoboken). 2020;72(6):744–60.
Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–90.
Fattori V, Hohmann MSN, Rossaneis AC, Manchope MF, Alves-Filho JC, Cunha TM, Cunha FQ, VerriJr WA. Targeting IL-33/ST2 signaling: regulation of immune function and analgesia. Expert Opin Ther Targets. 2017;21(12):1141–52.
Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol. 2016;17(2):122–31.
Arshad MI, Piquet-Pellorce C, Samson M. IL-33 and HMGB1 alarmins: sensors of cellular death and their involvement in liver pathology. Liver Int. 2012;32(8):1200–10.
Fattori V, Borghi SM, Verri WA. IL-33/ST2 signaling boosts inflammation and pain. Proc Natl Acad Sci U S A. 2017;114(47):E10034–5.
Verri WA Jr, Souto FO, Vieira SM, Almeida SC, Fukada SY, Xu D, Alves-Filho JC, Cunha TM, Guerrero AT, Mattos-Guimaraes RB, Oliveira FR, Teixeira MM, Silva JS, McInnes IB, Ferreira SH, Louzada-Junior P, Liew FY, Cunha FQ. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann Rheum Dis. 2010;69(9):1697–703.
Alves-Filho JC, Sonego F, Souto FO, Freitas A, Verri WA Jr, Auxiliadora-Martins M, Basile-Filho A, McKenzie AN, Xu D, Cunha FQ, Liew FY. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med. 2010;16(6):708–12.
Lan F, Yuan B, Liu T, Luo X, Huang P, Liu Y, Dai L, Yin H. Interleukin-33 facilitates neutrophil recruitment and bacterial clearance in S. aureus-caused peritonitis. Mol Immunol. 2016;72:74–80.
Le HT, Tran VG, Kim W, Kim J, Cho HR, Kwon B. IL-33 priming regulates multiple steps of the neutrophil-mediated anti-Candida albicans response by modulating TLR and dectin-1 signals. J Immunol. 2012;189(1):287–95.
Theoharides TC, Zhang B, Kempuraj D, Tagen M, Vasiadi M, Angelidou A, Alysandratos KD, Kalogeromitros D, Asadi S, Stavrianeas N, Peterson E, Leeman S, Conti P. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci USA. 2010;107(9):4448–53.
Taracanova A, Alevizos M, Karagkouni A, Weng Z, Norwitz E, Conti P, Leeman SE, Theoharides TC. SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors. Proc Natl Acad Sci USA. 2017;114(20):E4002–E40094009.
Xu D, Jiang HR, Kewin P, Li Y, Mu R, Fraser AR, Pitman N, Kurowska-Stolarska M, McKenzie AN, McInnes IB, Liew FY. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci USA. 2008;105(31):10913–8.
Verri WA Jr, Guerrero AT, Fukada SY, Valerio DA, Cunha TM, Xu D, Ferreira SH, Liew FY, Cunha FQ. IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. Proc Natl Acad Sci USA. 2008;105(7):2723–8.
Zarpelon AC, Cunha TM, Alves-Filho JC, Pinto LG, Ferreira SH, McInnes IB, Xu D, Liew FY, Cunha FQ, VerriJr WA. IL-33/ST2 signalling contributes to carrageenin-induced innate inflammation and inflammatory pain: role of cytokines, endothelin-1 and prostaglandin E2. Br J Pharmacol. 2013;169(1):90–101.
Magro DA, Hohmann MS, Mizokami SS, Cunha TM, Alves-Filho JC, Casagrande R, Ferreira SH, Liew FY, Cunha FQ, VerriJr WA. An interleukin-33/ST2 signaling deficiency reduces overt pain-like behaviors in mice. Braz J Med Biol Res. 2013;46(7):601–6.
Zhao J, Zhang H, Liu SB, Han P, Hu S, Li Q, Wang ZF, Mao-Ying QL, Chen HM, Jiang JW, Wu GC, Mi WL, Wang YQ. Spinal interleukin-33 and its receptor ST2 contribute to bone cancer-induced pain in mice. Neuroscience. 2013;253:172–82.
Liu S, Mi WL, Li Q, Zhang MT, Han P, Hu S, Mao-Ying QL, Wang YQ. Spinal IL-33/ST2 signaling contributes to neuropathic pain via neuronal CaMKII-CREB and astroglial JAK2-STAT3 cascades in mice. Anesthesiology. 2015;123(5):1154–69.
Han P, Zhao J, Liu SB, Yang CJ, Wang YQ, Wu GC, Xu DM, Mi WL. Interleukin-33 mediates formalin-induced inflammatory pain in mice. Neuroscience. 2013;241:59–66.
Han P, Liu S, Zhang M, Zhao J, Wang Y, Wu G, Mi W. Inhibition of spinal interlukin-33/ST2 signaling and downstream ERK and JNK pathways in electroacupuncture analgesia in formalin mice. PLoS One. 2015;10(6):e0129576.
Ruiz-Miyazawa KW, Staurengo-Ferrari L, Mizokami SS, Domiciano TP, Vicentini F, Camilios-Neto D, Pavanelli WR, Pinge-Filho P, Amaral FA, Teixeira MM, Casagrande R, Verri WA Jr. Quercetin inhibits gout arthritis in mice: induction of an opioid-dependent regulation of inflammasome. Inflammopharmacology. 2017;25:555–70.
Guerrero AT, Verri WA Jr, Cunha TM, Silva TA, Rocha FA, Ferreira SH, Cunha FQ, Parada CA. Hypernociception elicited by tibio-tarsal joint flexion in mice: a novel experimental arthritis model for pharmacological screening. Pharmacol Biochem Behav. 2006;84(2):244–51.
Fattori V, Rasquel-Oliveira FS, Artero NA, Ferraz CR, Borghi SM, Casagrande R, VerriJr WA. Diosmin treats lipopolysaccharide-induced inflammatory pain and peritonitis by blocking NF-kappaB activation in mice. J Nat Prod. 2020;83(4):1018–26.
Fattori V, Pinho-Ribeiro FA, Staurengo-Ferrari L, Borghi SM, Rossaneis AC, Casagrande R, Verri WA Jr. The specialized pro-resolving lipid mediator Maresin-1 reduces inflammatory pain with a long-lasting analgesic effect. Br J Pharmacol. 2019;176(11):1728–44.
Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, Girard JP. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188(7):3488–95.
Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PLoS One. 2008;3(10):e3331.
Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10(2):103–10.
Staurengo-Ferrari L, Trevelin SC, Fattori V, Nascimento DC, de Lima KA, Pelayo JS, Figueiredo F, Casagrande R, Fukada SY, Teixeira MM, Cunha TM, Liew FY, Oliveira RD, Louzada-Junior P, Cunha FQ, Alves-Filho JC, Verri WA. Interleukin-33 receptor (ST2) Deficiency Improves the Outcome of Staphylococcus aureus-induced septic arthritis. Front Immunol. 2018;9:962.
Matsuyama Y, Okazaki H, Tamemoto H, Kimura H, Kamata Y, Nagatani K, Nagashima T, Hayakawa M, Iwamoto M, Yoshio T, Tominaga S, Minota S. Increased levels of interleukin 33 in sera and synovial fluid from patients with active rheumatoid arthritis. J Rheumatol. 2010;37(1):18–25.
Hodzic Z, Schill EM, Bolock AM, Good M. IL-33 and the intestine: the good, the bad, and the inflammatory. Cytokines. 2017;100:1–10.
Kochiashvili G, Kochiashvili D. Urinary IL-33 and galectin-3 increase in patients with interstitial cystitis/bladder pain syndrome (review). Georgian Med News. 2014;232–233:12–5.
Shang K, Wei Y, Su Q, Yu B, Tao Y, He Y, Wang Y, Shi G, Duan L. IL-33 ameliorates the development of MSU-induced inflammation through expanding MDSCs-like cells. Front Endocrinol (Lausanne). 2019;10:36.
Phelps, P.; McCarty, D. J., Jr., Crystal-induced inflammation in canine joints. II. Importance of polymorphonuclear leukocytes. J Exp Med1966, 124, (1), 115–26.
Chang YH, Garalla EJ. Suppression of urate crystal-induced canine joint inflammation by heterologous anti-polymorphonuclear leukocyte serum. Arthritis Rheum. 1968;11(2):145–50.
Mitroulis I, Kambas K, Ritis K. Neutrophils, IL-1beta, and gout: is there a link? Semin Immunopathol. 2013;35(4):501–12.
Fattori V, Zarpelon AC, Staurengo-Ferrari L, Borghi SM, Zaninelli TH, Da Costa FB, Alves-Filho JC, Cunha TM, Cunha FQ, Casagrande R, Arakawa NS, Verri WA Jr. Budlein A, a sesquiterpene lactone from viguiera robusta, alleviates pain and inflammation in a model of acute gout arthritis in mice. Front Pharmacol. 2018;9:1076.
Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, Drosos GI, Boumpas DT, Ritis K. Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PLoS One. 2011;6(12):e29318.
Amaral FA, Bastos LF, Oliveira TH, Dias AC, Oliveira VL, Tavares LD, Costa VV, Galvao I, Soriani FM, Szymkowski DE, Ryffel B, Souza DG, Teixeira MM. Transmembrane TNF-alpha is sufficient for articular inflammation and hypernociception in a mouse model of gout. Eur J Immunol. 2016;46(1):204–11.
Cunha TM, Verri WA Jr, Schivo IR, Napimoga MH, Parada CA, Poole S, Teixeira MM, Ferreira SH, Cunha FQ. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol. 2008;83(4):824–32.
Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–41.
Carreira EU, Carregaro V, Teixeira MM, Moriconi A, Aramini A, Verri WA Jr, Ferreira SH, Cunha FQ, Cunha TM. Neutrophils recruited by CXCR1/2 signalling mediate post-incisional pain. Eur J Pain. 2013;17(5):654–63.
Hattori H, Subramanian KK, Sakai J, Jia Y, Li Y, Porter TF, Loison F, Sarraj B, Kasorn A, Jo H, Blanchard C, Zirkle D, McDonald D, Pai SY, Serhan CN, Luo HR. Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc Natl Acad Sci USA. 2010;107(8):3546–51.
Zarpelon AC, Rodrigues FC, Lopes AH, Souza GR, Carvalho TT, Pinto LG, Xu D, Ferreira SH, Alves-Filho JC, McInnes IB, Ryffel B, Quesniaux VF, Reverchon F, Mortaud S, Menuet A, Liew FY, Cunha FQ, Cunha TM, VerriJr WA. Spinal cord oligodendrocyte-derived alarmin IL-33 mediates neuropathic pain. FASEB J. 2016;30(1):54–655.
Huang SJ, Yan JQ, Luo H, Zhou LY, Luo JG. IL-33/ST2 signaling contributes to radicular pain by modulating MAPK and NF-kappaB activation and inflammatory mediator expression in the spinal cord in rat models of noncompressive lumber disk herniation. J Neuroinflamm. 2018;15(1):12.
Liu B, Tai Y, Achanta S, Kaelberer MM, Caceres AI, Shao X, Fang J, Jordt SE. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc Natl Acad Sci USA. 2016;113(47):E7572–E75797579.
Chiu IM, Barrett LB, Williams EK, Strochlic DE, Lee S, Weyer AD, Lou S, Bryman GS, Roberson DP, Ghasemlou N, Piccoli C, Ahat E, Wang V, Cobos EJ, Stucky CL, Ma Q, Liberles SD, Woolf CJ. Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. Elife. 2014;19(3):e04660.
Acknowledgements
Grants that supported the present study were from the National Council for Scientific and Technological Development (CNPq, Brazil); Department of Science and Technology from the Science, Technology and Strategic Inputs Secretariat of the Ministry of Health (Decit/SCTIE/MS, Brazil) intermediated by CNPq with support of Araucária Foundation and State Health Secretariat, Paraná (SESA‐PR, Brazil; PPSUS Grant Agreement 041/2017, protocol 48.095); Funding Authority for Studies and Projects and State Secretariat of Science, Technology and Higher Education (MCTI/FINEP/CT‐INFRA‐PROINFRA, Brazil; Grant agreements 01.12.0294.00 and 01.13.0049.00); Programa de Apoio a Grupos de Excelência (PRONEX) grant supported by SETI/Araucária Foundation and MCTI/CNPq; and Paraná State Government (Agreement 014/2017, protocol 46.843); Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; finance code 001); National Institute of Science and Technology in Dengue and Host-microorganism Interaction (INCT dengue), which is a program sponsored by CNPq and the Minas Gerais Foundation for Science (FAPEMIG, Brazil); São Paulo Research Foundation under grant agreement 2013/08216-2 (Center for Research in Inflammatory Disease). We also thank the support of Central Multiusuário de Laboratórios de Pesquisa from Londrina State University (CMLP‐UEL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to be declared.
Ethical approval
All animal experiments were conducted in accordance with animal care and handling procedures of the International Association for Study of Pain (IASP) and with the approval Londrina State University Ethics Committee on Animal Research and Welfare named CEUA-UEL (Approval Number 2205.2016.39). All patients were recruited at the Division of Rheumatology, Hospital das Clínicas, Ribeirão Preto Medical School (HC-FMRP, São Paulo, Brazil), and presented clinical or laboratory variables that fulfilled the criteria for osteoarthritis or gouty arthritis. All patients were informed about the aims of the study and provided written consent before participating. The Human Ethics Committee of the FMRP approved this study (Approval Number 4971/2012).
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fattori, V., Staurengo-Ferrari, L., Zaninelli, T.H. et al. IL-33 enhances macrophage release of IL-1β and promotes pain and inflammation in gouty arthritis. Inflamm. Res. 69, 1271–1282 (2020). https://doi.org/10.1007/s00011-020-01399-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-020-01399-x