Abstract
Objective and design
The aim of this study is to elucidate TGF-β1 signaling pathways involved in COX-2 protein induction and modulation of TAU protein phosphorylation in cultured podocytes.
Materials, treatment and methods
In vitro cultured immortalized podocytes were stimulated with TGF-β1 in presence and absence of pharmacologic inhibitors for various signaling pathways and phosphatases. Then, COX-2 protein expression, as well as P38MAPK, AKT and TAU phosphorylation levels were evaluated by western blot analysis.
Results
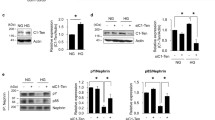
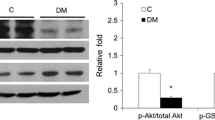
TGF-β1 induction of COX-2 protein levels was completely blocked by pharmacologic inhibitors of phosphatases, P38 MAPK, or NF-қB pathways. Time course experiments showed that TGF-β1 activated p38 MAPK after 5 min of stimulation. Interestingly, podocyte co-incubated with TGF-β1, high glucose and/or PGE2 showed strong increase in p38 MAPK and AKT phosphorylation as well as COX- 2 protein expression levels. Levels of phosphorylated AKT were further reduced and levels of phosphorylated p38 were increased when PGE2 was added to the culture media. Interestingly, selective phosphatases inhibitors completely abrogated PGE2-induced P38 MAPK and TAU phosphorylation. Also, inhibition of phosphatases reversed TGF-β1–induced COX-2 protein expression either alone or when incubated with high glucose or PGE2.
Conclusion
These data suggest TGF-β1 mediates its effect in podocyte through novel signaling mechanisms including phosphatases and TAU protein phosphorylation.






Similar content being viewed by others
References
Ritz E, Rychlik I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795–808.
Schrijvers BF, De Vriese AS, Flyvbjerg A. From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr Rev. 2004;25:971–1010.
Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–52.
Shankland SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–47.
Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA. 2000;97:8015–20.
Han DC, Hoffman BB, Hong SW, Guo J, Ziyadeh FN. Therapy with antisense TGF-beta1 oligodeoxynucleotides reduces kidney weight and matrix mRNAs in diabetic mice. Am J Physiol Renal Physiol. 2000;278:F628-34.
Chen S, Jim B, Ziyadeh FN. Diabetic nephropathy and transforming growth factor-beta: transforming our view of glomerulosclerosis and fibrosis build-up. Semin Nephrol 2003; 23:532–43.
Ziyadeh FN, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J Clin Invest 1994; 93:536–42.
Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1358–73.
Tsilibary EC. Microvascular basement membranes in diabetes mellitus. J Pathol 2003; 200:537–46.
Dessapt C, Baradez MO, Hayward A, Dei Cas A, Thomas SM, Viberti G, et al. Mechanical forces and TGFbeta1 reduce podocyte adhesion through alpha3beta1 integrin downregulation. Nephrol Dial Transpl. 2009;24:2645–55.
Wu DT, Bitzer M, Ju W, Mundel P, Bottinger EP. TGF-beta concentration specifies differential signaling profiles of growth arrest/differentiation and apoptosis in podocytes. J Am Soc Nephrol. 2005;16:3211–21.
Schiffer M, Bitzer M, Roberts IS, Kopp JB, ten Dijke P, Mundel P, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest 2001;108:807–16.
Xavier S, Niranjan T, Krick S, Zhang T, Ju W, Shaw AS, et al. TbetaRI independently activates Smad- and CD2AP-dependent pathways in podocytes. J Am Soc Nephrol. 2009;20:2127–37.
Kidger AM, Keyse SM. The regulation of oncogenic Ras/ERK signalling by dual-specificity mitogen activated protein kinase phosphatases (MKPs). Semin Cell Dev Biol 2016; 50:125–32.
Worby CA, Dixon JE. Pten Annu Rev Biochem 2014; 83:641–69.
Lin J, Shi Y, Peng H, Shen X, Thomas S, Wang Y, et al. Loss of PTEN promotes podocyte cytoskeletal rearrangement, aggravating diabetic nephropathy. J Pathol. 2015;236:30–40.
Santamaria B, Marquez E, Lay A, Carew RM, Gonzalez-Rodriguez A, Welsh GI, et al. IRS2 and PTEN are key molecules in controlling insulin sensitivity in podocytes. Biochim Biophys Acta. 2015;1853:3224–34.
Sun J, Li ZP, Zhang RQ, Zhang HM. Repression of miR-217 protects against high glucose-induced podocyte injury and insulin resistance by restoring PTEN-mediated autophagy pathway. Biochem Biophys Res Commun. 2017;483:318–24.
Jung KY, Chen K, Kretzler M, Wu C. TGF-beta1 regulates the PINCH-1-integrin-linked kinase-alpha-parvin complex in glomerular cells. J Am Soc Nephrol. 2007;18:66–73.
Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–91.
Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–20.
Yu L, Border WA, Huang Y, Noble NA. TGF-beta isoforms in renal fibrogenesis. Kidney Int 2003; 64:844–56.
Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 2000; 69:145–82.
Faour WH, He Y, He QW, de Ladurantaye M, Quintero M, Mancini A, et al. Prostaglandin E(2) regulates the level and stability of cyclooxygenase-2 mRNA through activation of p38 mitogen-activated protein kinase in interleukin-1 beta-treated human synovial fibroblasts. J Biol Chem. 2001;276:31720–31.
Tomasoni S, Noris M, Zappella S, Gotti E, Casiraghi F, Bonazzola S, et al. Upregulation of renal and systemic cyclooxygenase-2 in patients with active lupus nephritis. JASN. 1998;9:1202–12.
Weichert W, Paliege A, Provoost AP, Bachmann S. Upregulation of juxtaglomerular NOS1 and COX-2 precedes glomerulosclerosis in fawn-hooded hypertensive rats. Am J Physiol Renal Physiol. 2001;280:F706–14.
Takano T, Cybulsky AV. Complement C5b-9-mediated arachidonic acid metabolism in glomerular epithelial cells : role of cyclooxygenase-1 and -2. Am J Pathol. 2000;156:2091–101.
Wang JL, Cheng HF, Zhang MZ, McKanna JA, Harris RC. Selective increase of cyclooxygenase-2 expression in a model of renal ablation. Am J Physiol. 1998;275:F613–22.
Fujihara CK, Antunes GR, Mattar AL, Andreoli N, Malheiros DM, Noronha IL, et al. Cyclooxygenase-2 (COX-2) inhibition limits abnormal COX-2 expression and progressive injury in the remnant kidney. Kidney Int. 2003;64:2172–81.
Cheng H, Wang S, Jo Y-I, Hao C-M, Zhang M, Fan X, et al. Overexpression of cyclooxygenase-2 predisposes to podocyte injury. J Am Soc Nephrol. 2007;18:551–9.
Martineau LC, McVeigh LI, Jasmin BJ, Kennedy CR. p38 MAP kinase mediates mechanically induced COX-2 and PG EP4 receptor expression in podocytes: implications for the actin cytoskeleton. Am J Physiol Renal Physiol. 2004;286:F693–701.
Faour WH, Gomi K, Kennedy CR. PGE(2) induces COX-2 expression in podocytes via the EP(4) receptor through a PKA-independent mechanism. Cell Signal. 2008;20:2156–64.
Faour WH, Thibodeau JF, Kennedy CR. Mechanical stretch and prostaglandin E2 modulate critical signaling pathways in mouse podocytes. Cell Signal. 2010;22:1222–30.
Cheng H, Fan X, Moeckel GW, Harris RC. Podocyte COX-2 exacerbates diabetic nephropathy by increasing podocyte (pro)renin receptor expression. JASN. 2011;22:1240–51.
Wang L, Chang J-H, Paik S-Y, Tang Y, Eisner W, Spurney RF. Calcineurin (CN) activation promotes apoptosis of glomerular podocytes both in vitro and in vivo. Mol Endocrinol. 2011;25:1376–86.
Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–8.
Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev. 2008;4:39–45.
Stitt-Cavanagh EM, Faour WH, Takami K, Carter A, Vanderhyden B, Guan Y, et al. A maladaptive role for EP4 receptors in podocytes. JASN. 2010;21:1678–90.
Lasa M, Mahtani KR, Finch A, Brewer G, Saklatvala J, Clark AR. Regulation of cyclooxygenase 2 mRNA stability by the mitogen-activated protein kinase p38 signaling cascade. Mol Cell Biol. 2000;20:4265–74.
Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-ß and TNF-α, by human macrophages. J Immunol. 1998;160:3513–21.
Faour WH, Mancini A, He QW, Di Battista JA. T-cell-derived interleukin-17 regulates the level and stability of cyclooxygenase-2 (COX-2) mRNA through restricted activation of the p38 mitogen-activated protein kinase cascade: role of distal sequences in the 3′-untranslated region of COX-2 mRNA. J Biol Chem. 2003;278:26897–907.
Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, et al. AMP-activated protein kinase (AMPK) negatively regulates nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem. 2010;285:37503–12.
Acknowledgements
Dr Wissam H. Faour is a recipient of a grant from the Lebanese National Council for Scientific Research and an Assistant Professor of Pharmacology at the School of Medicine at the Lebanese American University. This project has been funded with support from the National Council for Scientific Research in Lebanon, Grant number: 01-08-2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No competing financial interests exist.
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Abdallah, M.S., Kennedy, C.R.J., Stephan, J.S. et al. Transforming growth factor-β1 and phosphatases modulate COX-2 protein expression and TAU phosphorylation in cultured immortalized podocytes. Inflamm. Res. 67, 191–201 (2018). https://doi.org/10.1007/s00011-017-1110-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-017-1110-y