Abstract
Objectives
Contact dermatitis model involving repeated application of hapten is used as a tool to assess dermatitis, as characterized by thickening. Involvement of cell proliferation, elicited by repeated hapten-stimulation, in this swelling has been unclear. Curcumin is reported to reduce inflammation. We examined involvement of cell proliferation and the role of extracellular regulated kinase (ERK) in 2,4,6-trinitrochlorobenzene (TNCB) challenge-induced ear swelling. We also examined the effects of curcumin in this model.
Methods
Mice were sensitized with TNCB to the abdominal skin. Then, they were challenged with TNCB to the ear three times. The ERK activation inhibitor U0126 or curcumin was applied 30 min before each TNCB challenge.
Results
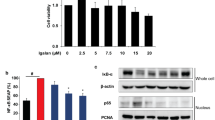
TNCB challenge-induced increased epidermal cell number and dermal thickening. Gene expressions of epithelial mitogen (EPGN), amphiregulin (AREG) and heparin-binding-epidermal growth factor (HB-EGF) were increased in the ears after the last TNCB challenge. Ki-67 immunoreactivity was increased in the dermis in TNCB-challenged ears. TNCB-induced swelling was inhibited by U0126 and curcumin. Curcumin also attenuated TNCB-induced ERK phosphorylation and expression of EPGN and AREG genes.
Conclusion
Ear swelling induced by TNCB challenge might be mediated, in part, by the EPGN- and AREG-ERK proliferation pathway and was inhibited by curcumin.








Similar content being viewed by others
References
Kimber I, Basketter DA, Gerberick GF, Dearman RJ. Allergic contact dermatitis. Int Immunopharmacol. 2002;2:201–11.
Coenraads PJ, Goncalo M. Skin diseases with high public health impact. Contact dermatitis. Eur J Dermatol. 2007;17:564–5.
Mittermann I, Aichberger KJ, Bunder R, Mothes N, Renz H, Valenta R. Autoimmunity and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2004;4:367–71.
Jin H, He R, Oyoshi M, Geha RS. Animal models of atopic dermatitis. J Invest Dermatol. 2009;129:31–40.
Christensen AD, Haase C. Immunological mechanisms of contact hypersensitivity in mice. APMIS. 2012;120:1–27.
Grabbe S, Schwarz T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol Today. 1998;19:37–44.
Kitagaki H, Ono N, Hayakawa K, Kitazawa T, Watanabe K, Shiohara T. Repeated elicitation of contact hypersensitivity induces a shift in cutaneous cytokine milieu from a T helper cell type 1 to a T helper cell type 2 profile. J Immunol. 1997;159:2484–91.
Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18.
Stokoe D, Macdonald SG, Cadwallader K, Symons M, Hancock JF. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994;264:1463–7.
Ghosh S, Banerjee S, Sil PC. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: a recent update. Food Chem Toxicol. 2015;83:111–24.
Matsumoto K, Lo MW, Hosoya T, Tashima K, Takayama H, Murayama T, et al. Experimental colitis alters expression of 5-HT receptors and transient receptor potential vanilloid 1 leading to visceral hypersensitivity in mice. Lab Invest. 2012;92:769–82.
Matsumoto K, Mizukoshi K, Oyobikawa M, Ohshima H, Tagami H. Establishment of an atopic dermatitis-like skin model in a hairless mouse by repeated elicitation of contact hypersensitivity that enables to conduct functional analyses of the stratum corneum with various non-invasive biophysical instruments. Skin Res Technol. 2004;10:122–9.
Man MQ, Hatano Y, Lee SH, Man M, Chang S, Feingold KR, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008;128:79–86.
Steelman LS, Chappell WH, Abrams SL, Kempf RC, Long J, Laidler P, et al. Roles of the Raf/MEK/ERK and PI3 K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY). 2011;3:192–222.
Ulrich P, Grenet O, Bluemel J, Vohr HW, Wiemann C, Grundler O, et al. Cytokine expression profiles during murine contact allergy: T helper 2 cytokines are expressed irrespective of the type of contact allergen. Arch Toxicol. 2001;75:470–9.
Korutla L, Kumar R. Inhibitory effect of curcumin on epidermal growth factor receptor kinase activity in A431 cells. Biochim Biophys Acta. 1994;1224:597–600.
Korutla L, Cheung JY, Mendelsohn J, Kumar R. Inhibition of ligand-induced activation of epidermal growth factor receptor tyrosine phosphorylation by curcumin. Carcinogenesis. 1995;16:1741–5.
Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane). J Biol Chem. 1995;270:24995–5000.
Shakhov AN, Kuprash DV, Azizov MM, Jongeneel CV, Nedospasov SA. Structural analysis of the rabbit TNF locus, containing the genes encoding TNF-beta (lymphotoxin) and TNF-alpha (tumor necrosis factor). Gene. 1990;95:215–21.
Hiscott J, Marois J, Garoufalis J, D’Addario M, Roulston A, Kwan I, et al. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol. 1993;13:6231–40.
Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990;10:2327–34.
Son YH, Jeong YT, Lee KA, Choi KH, Kim SM, Rhim BY, et al. Roles of MAPK and NF-kappaB in interleukin-6 induction by lipopolysaccharide in vascular smooth muscle cells. J Cardiovasc Pharmacol. 2008;51:71–7.
Lohoff M, Giaisi M, Kohler R, Casper B, Krammer PH, Li-Weber M. Early growth response protein-1 (Egr-1) is preferentially expressed in T helper type 2 (Th2) cells and is involved in acute transcription of the Th2 cytokine interleukin-4. J Biol Chem. 2010;285:1643–52.
Hinz M, Lemke P, Anagnostopoulos I, Hacker C, Krappmann D, Mathas S, et al. Nuclear factor kappaB-dependent gene expression profiling of Hodgkin’s disease tumor cells, pathogenetic significance, and link to constitutive signal transducer and activator of transcription 5a activity. J Exp Med. 2002;196:605–17.
Sica A, Dorman L, Viggiano V, Cippitelli M, Ghosh P, Rice N, et al. Interaction of NF-kappaB and NFAT with the interferon-gamma promoter. J Biol Chem. 1997;272:30412–20.
Nguyen DP, Li J, Yadav SS, Tewari AK. Recent insights into NF-kappaB signalling pathways and the link between inflammation and prostate cancer. BJU Int. 2014;114:168–76.
Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284:2–13.
Lawrence WT, Diegelmann RF. Growth factors in wound healing. Clin Dermatol. 1994;12:157–69.
Barrandon Y, Green H. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth factor. Cell. 1987;50:1131–7.
Pages G, Lenormand P, L’Allemain G, Chambard JC, Meloche S, Pouyssegur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA. 1993;90:8319–23.
Xie H, Pallero MA, Gupta K, Chang P, Ware MF, Witke W, et al. EGF receptor regulation of cell motility: EGF induces disassembly of focal adhesions independently of the motility-associated PLCgamma signaling pathway. J Cell Sci. 1998;111(Pt 5):615–24.
Nakagami H, Morishita R, Yamamoto K, Taniyama Y, Aoki M, Matsumoto K, et al. Mitogenic and antiapoptotic actions of hepatocyte growth factor through ERK, STAT3, and AKT in endothelial cells. Hypertension. 2001;37:581–6.
Yan S, Krebs S, Leister KJ, Wenner CE. Perturbation of EGF-activated MEK1 and PKB signal pathways by TGF-beta1 correlates with perturbation of EGF-induced cyclin D1 and DNA synthesis by TGF-beta1 in C3H 10T1/2 cells. J Cell Physiol. 2000;185:107–16.
Nguyen VN, Mirejovsky P, Mirejovsky T, Melinova L, Mandys V. Expression of cyclin D1, Ki-67 and PCNA in non-small cell lung cancer: prognostic significance and comparison with p53 and bcl-2. Acta Histochem. 2000;102:323–38.
Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12.
Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141–53.
Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75.
Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7.
Lee CC, Shiao HY, Wang WC, Hsieh HP. Small-molecule EGFR tyrosine kinase inhibitors for the treatment of cancer. Expert Opin Investig Drugs. 2014;23:1333–48.
Shehzad A, Wahid F, Lee YS. Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm (Weinheim). 2010;343:489–99.
Hidaka H, Ishiko T, Furuhashi T, Kamohara H, Suzuki S, Miyazaki M, et al. Curcumin inhibits interleukin 8 production and enhances interleukin 8 receptor expression on the cell surface: impact on human pancreatic carcinoma cell growth by autocrine regulation. Cancer. 2002;95:1206–14.
Cho JW, Lee KS, Kim CW. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int J Mol Med. 2007;19:469–74.
Hussain AR, Ahmed M, Al-Jomah NA, Khan AS, Manogaran P, Sultana M, et al. Curcumin suppresses constitutive activation of nuclear factor-kappa B and requires functional Bax to induce apoptosis in Burkitt’s lymphoma cell lines. Mol Cancer Ther. 2008;7:3318–29.
Sugimoto K, Hanai H, Tozawa K, Aoshi T, Uchijima M, Nagata T, et al. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology. 2002;123:1912–22.
Camacho-Barquero L, Villegas I, Sanchez-Calvo JM, Talero E, Sanchez-Fidalgo S, Motilva V, et al. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int Immunopharmacol. 2007;7:333–42.
Acknowledgements
We thank Ms. Yuka Inoue for technical assistance. This work was supported by Grants from the MEXT-Supported Program for the Strategic Research Foundation at Private Universities (2014–2018, S1411019) and KAKENHI (15K18880) by a Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: Yoshiya Tanaka.
Rights and permissions
About this article
Cite this article
Sakai, H., Sato, K., Sato, F. et al. Curcumin inhibits epigen and amphiregulin upregulated by 2,4,6-trinitrochlorobenzene associated with attenuation of skin swelling. Inflamm. Res. 66, 663–678 (2017). https://doi.org/10.1007/s00011-017-1048-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-017-1048-0