Abstract
Objectives
To investigate Toll-like receptor activation in human skin using tape stripping and imiquimod cream challenges in healthy volunteers.
Subjects, treatment and methods
Seventeen male Caucasian subjects underwent a baseline biopsy on their lower back prior to two tape stripping procedures 7 days apart. Subjects were then treated with 5 % imiquimod for 2 and 4 days on separate sites in the same area. Further biopsies were taken 22–24 h after each challenge and mRNA and microRNA extracted and expression values analysed using robust statistical and pathway analysis methods.
Results
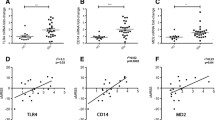
Fifteen of the 17 subjects completed the study according to protocol. No adverse events were associated with the procedures. A significant change (p < 0.05, fold change >1.5 or <−1.5) in mRNA expression of 7,996 genes was evident in biopsies taken at both time points post tape stripping, compared to baseline biopsy expression values. The induction of mRNAs involved in various pathways including adhesion and migration was evident. mRNA markers representing inflammatory cells [e.g., CD14, CD3E (p < 0.0001)] and mRNAs encoding genes regulated by type 1 interferon (IFN) [e.g., MX1, OAS1and CXCL10 (p < 0.0001)] were significantly up-regulated. IFNα and CXCL10 proteins were detectable in exudates released 1 and 4 h post tape stripping. A putative signalling network associating these transcripts and six microRNAs (hsa-miR, -31, -132, -155, 548c, 548n and 574) was identified using a meta-regulation network model. microRNAs not previously associated with IFN signalling have been identified. In contrast, only 223 known transcripts were significantly changed after imiquimod treatment, including CXCL10, and OAS1.
Conclusion
Results suggest that IFN signalling is important in these translational models and novel miRNA may be new targets in the treatment of IFN associated skin disease.






Similar content being viewed by others
References
Guiducci C, Tripodod C, Gong M, Sangaletti S, Colombo MP, Coffman RL, Barrat FJ. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207(13):2931–42.
Sun S, Rao NL, Venable J, Thurmond R, Karlsson L. TLR7/9 agonists as therapeutics for immune mediated inflammatory disorders. Inflamm Allergy Drug Targets. 2007;6:223–35.
Streilein JW, White Lonsberry LW, Bergstresser PR. Depletion of epidermal Langerhans cells and Ia immunogenicity from tape stripped mouse skin. J Exp Med. 1982;155:863–71.
Wood LC, Jakcson SM, Elias PM, Grunfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90:482–7.
Wenzel J, Worenkamper E, Freutel S, Henze S, Haller O, Bieber T, Tuting T. Enhanced type I interferon signalling promotes ThI-biased inflammation in cutaneous lupus erythematosus. J Path. 2005;205:435–42.
Nickoloff BJ, Naidu Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J Am Acad Derm. 1994;30(4):535–46.
Marks DJB, Radulovic M, McCartney S, Bloom S, Segal AW. Modified skin window technique for the extended characterisation of acute inflammation in humans. Inflamm Res. 2007;56(4):168–74.
Dickel H, Gambichler T, Kamphowe J, Altmeyer P, Skrygan M. Standardised tape stripping prior to patch testing induces up regulation of HSP90, HSP70, IL-33, TNFα and IL-8/CXCL8 mRNA: new insights into the involvement of ‘alarmins’. Contact Dermat. 2010;63:215–22.
Van der Fits L, Mourits S, Voerman JSA, Kant M, Boon L, Laman JD, Cornelissen F, Mus A-M, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL23/IL17 axis. J Immunol. 2009;182:5836–45.
Palamara F, Meindl S, Holemann M, Luhrs P, Stingl G, Sibilia M. Identification and characterisation of pDC-like cells in normal mouse skin and melanomas treated with imiquimod. J Immunol. 2004;173:3051–61.
Swindell WR, Johnston A, Carbajal S, Han G, Wohn C, Lu J, Xing X, Nair RP, Voorhees JJ, Elder JT, Wang X-J, Sano S, Prens EP, DiGiovanni J, Pittelkow MR, Ward NL, Gudjonsson JE. Genome-wide expression profiling of five mouse models identifies similarities and differences with human psoriasis. PLoS One. 2011;6(4):e18826.
Chan MP, Zimarowski MJ. Lupus erythematosus-like reaction in imiquimod-treated skin: a report of 2 cases. Am J Dermatopathol. 2011;33:523–7.
Schon MP, Schon M. Imiquimod: mode of action. Br J Derm. 2007;157(s2):8–13.
Hendriks AGM, Keijsers RRMC, Seyger MMB, van Erp PEJ, van de Kerkhof PCM. Are newly discovered drivers of immune-mediated skin disorders expressed in normal skin regenerating from standardized surface injury? Dermatology. 2014;228(3):255–60.
Wenzel J, Tuting T. Identification of type I interferon-associated inflammation in the pathogenesis of cutaneous lupus erythematosus opens up options for novel therapeutic approaches. Exp Dermatol. 2007;16(5):454–63.
Ruggiero T, Trabucchi M, De Santa F, Zupo S, Harfe BD, McManus MT, Rosenfeld MG, Briata P, Gherzi R. LPS induces KH-type splicing regulatory protein-dependent processing of microRNA-155 precursors in macrophages. FASEB J. 2009;23(9):2898–908.
Lagos D, Pollara G, Henderson S, Gratrix F, Fabani M, Milne RS, Gotch F. Boshoff C (2010) miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat Cell Biol. 2010;12(5):513–9.
Xu L, Wen Z, Zhou Y, Lui Z, Li Q, Fei G, Lou J, Ren T. MicroRNA-7-regulated TLR9 signaling-enhanced growth and metastatic potential of human lung cancer cells by altering the phosphoinositide-3-kinase, regulatory subunit 3/Akt pathway. MBoC. 2013;24:42–55.
Li Q, Li X, Guo Z, Xu F, Xia J, Lui Z, Ren T. MicroRNA-574-5p was pivotal for TLR9 signaling enhanced tumor progression via down-regulating checkpoint suppressor 1 in human lung cancer. PLoS. 2012;7(11):e48278.
Elton TS, Selemon H, Elton S, Parinandi NL. Regulation of the MIR155 host gene in physiological and pathological processes. Gene. 2013;532:1–12.
Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, Margioris AN, Tsichlis PN, Tsatsanis C. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31(2):220–31.
O’Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci USA. 2009;106(17):7113–8.
Stanczyk J, Pedrioli DM, Brentano F, Sanchez-Pernaute O, Kolling C, Gay RE, Detmar M, Gay S, Kyburz D. Altered expression of microRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 2008;58(4):1001–9.
Wang G, Tam LS, Li EK, Kwan BC, Chow KM, Luk CC, Li PK, Szeto CC. Serum and urinary cell-free MiR-146a and MiR-155 in patients with systemic lupus erythematosus. J Rheumatol. 2010;37(12):2516–22.
Philippe L, Alsaleh G, Bahram S, Pfeffer S, Georgel P. The miR-17 ∼ 92 cluster: a key player in the control of inflammation during rheumatoid arthritis. Immunol Front. 2013. doi:10.3389/fimmu.2013.00070.
Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O’Leary JJ, Ruan Q, Johnson DS, Chen Y, O’Neill LAJ. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141–7.
Das A, Ganesh K, Khanna S, Sen CK, Roy S. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J immunol. 2014;192:1120–9.
Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo X, Li J, Haibo Z, Tang Y, Shen N. MicroRNA-21 and MicroRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J Immunol. 2011;184:6773–81.
Qi J, Qiao Y, Wang P, Li S, Zhao W, Gao C. microRNA-210 negatively regulates LPS-induced production of proinflammatory cytokines by targeting NF-κB1 in murine macrophages. FEBS Lett. 2012;586:1201–7.
Acknowledgments
The authors would gratefully like to acknowledge Anita Kapur for her operational leadership throughout the study. This study was funded by GlaxoSmithKline.
Conflict of interest
All authors were employees and share holders in GlaxoSmithKline at the time the work reported in this manuscript was performed. All authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest.
Ethical standards
The study reference number is EMI113437 and was approved by the IntegReview Ethical Review Board Austin, Texas on December 1st 2009. As this was a non interventional study a clinical trial application was not required at the time of study initiation. The study was conducted in accordance with all applicable regulatory requirements. The study was conducted in accordance with “good clinical practice” (GCP), all applicable subject privacy requirements, and the guiding principles of the 2008 Declaration of Helsinki. This includes, but is not limited to, the following: (1) IRB/IEC review and favourable opinion/approval to conduct the study and of any subsequent relevant amended documents, (2) written informed consent (and any amendments) to be obtained for each subject before participation in the study, (3) investigator reporting requirements (e.g., reporting of AEs/SAEs/protocol deviations to IRB/IEC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yoshiya Tanaka.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dickson, M.C., Ludbrook, V.J., Perry, H.C. et al. A model of skin inflammation in humans leads to a rapid and reproducible increase in the interferon response signature: a potential translational model for drug development. Inflamm. Res. 64, 171–183 (2015). https://doi.org/10.1007/s00011-015-0795-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-015-0795-z